Abstract
Depression, anxiety and apathy are common mood disturbances in Parkinson's disease (PD) but their pathophysiology is unclear. Advanced neuroimaging has been increasingly used to unravel neural substrates linked to these disturbances. A systematic review is provided of neuroimaging findings in depression, anxiety and apathy in PD. A PubMed, MEDLINE and EMBASE search of peer‐reviewed original research articles on these mood disturbances in PD identified 38 studies on depression, eight on anxiety and 14 on apathy in PD. Most of the imaging studies used either position emission tomography or single‐photon emission computed tomography techniques. These studies generally suggest increased neural activity in the prefrontal regions and decreased functional connectivity between the prefrontal−limbic networks in depressed patients. Functional imaging studies revealed an inverse correlation between dopaminergic density in the caudate and putamen with the severity of anxiety in PD. There was no consistent correlation between dopaminergic density of thalamus and anxiety. Studies demonstrated both positive and inverse correlations between apathy and metabolism or activity in the striatum, amygdalar, prefrontal, temporal and parietal regions. The clinical variability of study subjects and differences in image pre‐processing and analytical strategies may contribute to discrepant findings in these studies. Both nigrostriatal and extra‐nigrostriatal pathways (in particular the frontal region and its connecting areas) are affected in mood disorders in PD. Identifying the relative contributions of these neural pathways in PD patients with overlapping motor and mood symptoms could provide new pathophysiological clues for the development of better therapeutic targets for affected patients.
Keywords: anxiety, apathy, depression, frontostriatal pathway, neuroimaging, Parkinson's disease
Introduction
Parkinson's disease (PD) involves the degeneration of dopaminergic neurons in the substantia nigra pars compacta that leads to clinical manifestations of resting tremor, bradykinesia, postural instability and rigidity. There is increasing evidence to suggest that PD is not simply a pure motor disorder but rather a systemic disease with variegated non‐motor symptoms, such as mood disturbances 1. Depression is a prevalent mood disturbance in PD, occurring in around 2.7%–90% of patients 2, and affects quality of life 3.
Anxiety and apathy are also frequent symptoms in patients with PD 4, 5. In fact, studies have shown that depression often coexists with anxiety 6 and apathy 7. The clinical presentations of depression share some similarities with anxiety and apathy, such as fatigue, agitation, psychomotor retardation, lack of facial expression and difficulties concentrating 8. It has been suggested that depression and anxiety may be two related but separable entities since they are associated with different demographic and clinical features in PD 9, 10. Likewise, a recent meta‐analysis study showed that half of PD patients with apathy did not suffer from concomitant depression, thereby implying that apathy may be a separate clinical entity 7. Clinical observations and behavioural studies seem to suggest distinct aetiologies involving considerable overlap between these mood disturbances. However, the exact mechanism of these three mood disturbances in PD remains to be elucidated.
Advanced neuroimaging techniques have been increasingly employed to explore the neural substrates of mood disturbances in PD. However, the exact neuropathology of these three common mood disturbances in PD remains to be elucidated. To address current gaps in knowledge, a concise summary is provided of neuroimaging studies on mood disturbances in PD, highlighting new insights from these studies and drawing attention to the potential limitations and future research prospects.
Methods
MEDLINE, PubMed (alone or via MeSH) and EMBASE searches for peer‐reviewed original research on humans with search terms ‘depression’, ‘anxiety’, ‘apathy’, ‘mood symptoms’, ‘Parkinson's disease’, ‘Parkinson disease’, ‘neuroimaging’, ‘magnetic resonance imaging (MRI)’, ‘functional MRI’, ‘diffusion tensor imaging (DTI)’, ‘resting state functional MRI’, ‘single‐photon emission computed tomography (SPECT)’, ‘positron emission tomography (PET)’ and ‘transcranial sonography (TCS)’ identified 38 studies on depression, 8 on anxiety and 14 on apathy in PD (Fig. 1). Suitable references included in these studies were also extracted. The inclusion criteria were studies involving PD patients who did not have any other neurological conditions (e.g. stroke and dementia), using at least one of the aforementioned neuroimaging modalities, measuring at least one of the mood symptoms, and reporting the neural substrates of at least one of the mood symptoms in PD. Studies focusing on the pre‐to‐post intervention changes of mood symptoms but providing no information about the neural substrates of mood symptoms using one of the neuroimaging modalities were not included in our review.
Neuroimaging of depression in PD
Of the 38 studies on depression, 33 reported findings from one single imaging modality: 19 used either PET [11, 12, 13,15, 16, 17, 18, 19] or SPECT 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 techniques, four used T1‐weighted imaging 31, 32, 33, three used DTI 34, 35, 36, six used resting state functional MRI (RS‐FMRI) 37, 38, 39, 40, 41, 42 and two used TCS methods 43, 44. The remaining four of the 38 studies reported findings from structural T1‐weighted imaging plus another imaging method, including PET 14, DTI 45, task FMRI 46 and RS‐FMRI 47, respectively. One study performed both TCS and T2‐weighted MRI 48. Whilst most studies excluded participants with cognitive impairment (e.g. dementia), one study 12 included PD patients with dementia and two studies did not specify whether their participants had cognitive decline 29, 30. Approximately half of these studies scanned PD patients in the ‘on‐medication’ state, whilst the other half of the studies acquired imaging data in the ‘off‐medication’ state and four studies did not specify the state of PD patients during imaging acquisition. As for the use of antidepressants, some studies requested patients to discontinue antidepressants before MRI scanning whilst other studies involved both on‐ and off‐medication patients. In addition, some studies did not indicate the patients’ severity or types of depression. For studies that reported depression types or severity, heterogeneous depression features of patients were noted, such that two studies only recruited patients with minor depression 24, 39, another three studies only included patients with major depression 17, 25, 38 and four studies excluded patients with depression [18,19,26,28], whilst most studies had patients with various severities of depression (e.g. 13, 20, 23). See Table 1 and Table S1 for the details of these studies.
Table 1.
Neuroimaging studies in depression in Parkinson's disease (PD)
| Study | Subjects | Sex (M/F) | H and Y (Mean ± SD) | Duration of PD (years) | UPDRS‐III | Imaging modality | Imaging analytical method | Diagnosis/measurement | Key findings |
|---|---|---|---|---|---|---|---|---|---|
| Ballanger et al. (2012) 11 |
4 dPDs 8 PDs 7 HCs |
4/0 6/2 5/2 |
No data |
4.5 ± 3.3 7.0 ± 3.0 |
27.3 ± 11.6 25.9 ± 4.5 |
[18F]MPPF PET | WB | DSM‐IV/BDI, MADRS |
HCs > dPDs: [18F]MPPF uptake in left dorsal anterior cingulate and orbitofrontal cortices, and right hippocampus and temporal cortex HCs > PDs: Bi. inferior frontal cortex and right ventral striatum and insula PDs > dPDs: Left hippocampus and superior temporal and orbitofrontal cortices and right insula |
| Bohnen et al. (2007) 12 |
12 PDs 6 PD‐Ds 10 HCs |
12/0 6/0 10/0 |
No data |
5.7 ± 4.1 3.8 ± 2.3 |
No data | [11C]PMP PET | ROI | CSDD | Inverse correlation between cortical cholinergic activity and depressive symptoms |
| Boileau et al. (2008) 13 |
7 dPDs 7 HCs |
4/3 4/3 |
1.71 ± 0.49 | 4 ± 2.9 | 21.1 ± 0.34 | [11C]DASB PET | ROI | DSM‐IV/ HAM‐D, IDS |
dPDs > HCs: [11C]DASB binding in dorsolateral and prefrontal cortices Positive correlation between [11C]DASB binding in the orbitofrontal cortex and depression |
| Ceravolo et al. (2013) 20 | 44 PDs | No data | No data | 1.14 ± 0.98 | 17.9 ± 7.7 | [123I]FP‐CIT SPECT | ROI | DSM‐IV‐TR/ HAM‐D, HAM‐A, BDI |
More depressed PDs > less depressed PDs: DAT binding in bi. caudate and putamen Positive correlation between depression and DAT binding bi. caudate and putamen |
| Felicio et al. (2010) 21 |
10 PDs 10 dPDs |
5/5 6/4 |
2.5 ± 0.4 2.2 ± 0.5 |
4.7 ± 2.2 4.0 ± 2.4 |
29.5 ± 10.6 26.3 ± 10.0 |
99mTc‐TRODAT‐1 SPECT | ROI | BDI | dPDs > PDs: DAT density in left caudate and right putamen |
| Guttman et al. (2007) 19 |
9 PDs 13 HCs |
7/2 5/8 |
2.56 ± 0.53 | 12.22 ± 3.73 | No data | [11C]DASB PET | ROI | HAM‐D, IDS | Inverse correlations between depression scores and [11C]DASB binding in putamen and insular and occipital cortices |
| Hesse et al. (2009) 22 |
110 PDs 20 dPDs 18 HCs |
59/51 13/17 8/10 |
1.6 ± 1.6 2.3 ± 0.9 |
3.1 ± 4.9 4.4 ± 4.5 |
28.6 ± 13.1 31.4 ± 13.7 |
[123I]FP‐CIT SPECT | ROI | DSM‐IV/BDI |
PDs > dPDs: [123I]FP‐CIT binding in striatum, thalamus, midbrain/brainstem HCs > dPDs without SSRI treatment: [123I]FP‐CIT binding in thalamus and midbrain |
| Huang et al. (2013) 14 |
26 PDs 12 HCs |
16/10 7/5 |
1–2.5 | 5.5 ± 0.7 | No data | [18F]FDG‐PET, T1‐weighted |
ROI (PET) WB (T1) |
BDI, BAI, AES |
Positive correlation between depression and metabolic elevations bi. amygdala No volumetric difference between HCs and PDs |
| Imamura et al. (2011) 23 |
22 PDs 16 minor dPDs 14 major dPDs 9 HCs |
10/12 7/9 12/24/5 |
2.98 ± 0.85 3.53 ± 0.96 3.32 ± 0.87 |
6.33 ± 4.81 6.77 ± 5.87 4.3 ± 3.4 |
22.3 ± 17.4 33.4 ± 18.5 26.7 ± 13.5 |
123I‐IMP SPECT | ROI | DSM‐IV/BDI | HCs > dPDs: rCBF in bi. PCC, hippocampus, cuneus, superior parietal and primary visual areas |
| Matsui et al. (2006) 24 |
18 PDs 22 dPDs |
5/17 2/20 |
3.2 ± 0.4 3.3 ± 0.5 |
8.8 ± 5.8 10.5 ± 6.8 |
28.9 ± 15.7 38.4 ± 15.3 |
123I‐IMP SPECT | ROI | DSM‐IV/ HAM‐D | PDs > dPDs: Perfusion in left superior and inferior frontal gyri |
| Mayberg et al. (1990) 15 |
4 PDs 5 dPDs 6 HCs |
4/0 3/2 4/2 |
≤3 |
8.5 ± 4 7.2 ± 2 |
No data | [18F]FDG‐PET | ROI | DSM‐III/ HAM‐D |
HCs and PDs > dPDs: Metabolic activity in caudate and orbital‐inferior frontal area Inverse correlation between metabolism in orbital‐inferior frontal area and depression scores |
| Mentis et al. (2002) 16 |
15 PDs 14 HCs |
No data | 3.3 ± 0.9 | No data | No data | [18F]FDG‐PET | WB | BDI |
In PDs, inverse correlation between depression and metabolism in lateral/medial frontal, ACC, orbitofrontal cortex Positive correlation between depression and metabolism in cerebellum |
| Pålhagen et al. (2009) 25 |
11 dPDs 14 PDs 12 MDs |
6/5 8/6 7/5 |
2.2 ± 0.4 1.8 ± 0.4 |
9.7 ± 4.7 6.9 ± 2.4 |
24.9 ± 10.8 22.6 ± 10.1 |
HMPAO SPECT | ROI | DSM‐III–IV/ HAM‐D, MADRS |
dPDs vs. MDs: ↑ rCBF in right frontal and left frontoparietal regions; ↓ rCBF in right preoccipital region dPDs + PDs vs. MDs: ↓ rCBF in bi. preoccipital regions and occipital lobe dPDs vs. PDs: ↑ rCBF in bi. frontoparietal areas, right dorsolateral and left anterior frontal areas dPDs + MDs vs. PDs: ↑ rCBF in right dorsolateral frontal area |
| Politis et al. (2010) 18 |
10 dPDs 24 PDs 10 HCs |
6/4 20/4 8/2 |
3.1 ± 0.6 2.5 ± 0.8 |
10.3 ± 9.1 8.6 ± 4.5 |
81.9 ± 16.3 61.2 ± 18.5 |
[11C]DASB PET | ROI | DSM‐IV/ HAM‐D, BDI |
dPDs and HCs > PDs: Serotonergic binding in amygdala, hypothalamus, caudal raphe nuclei and PCC HCs > dPDs: Serotonergic binding in ACC, caudate nucleus, insula, prefrontal cortex, putamen, rostral raphe nuclei, thalamus and ventral striatum |
| Rektorova et al. (2008) 26 | 20 PDs | 10/10 | No data | 5.6 ± 3.5 | 20.9 ± 10.8 | [123I]FP‐CIT SPECT | ROI | MADRS |
Inverse correlation between depression scores and DAT availability in bi. striatum and putamen, left striatum and putamen → depression |
| Remy et al. (2005) 17 |
8 dPDs 12 PDs 7 HCs |
5/3 9/3 |
1–3.5 1–3.5 |
3.1 ± 1.8 4.9 ± 2.6 |
24.3 ± 11.2 23.3 ± 6.7 |
[11C]RTI‐32 PET | ROI | BDI, AES, STAS |
HCs > PDs + dPDs: Binding value in bi. caudate, putamen, ventral striatum and substantia nigra HCs > dPDs: Binding value in ACC, thalamus PDs > dPDs: Binding value in bi. thalamus and locus coeruleus ACC, right amygdala and left ventral striatum Inverse correlation between depression and binding value in left ventral striatum |
| Vriend et al. (2014) 27 | 100 PDs | 61/39 | 2.08 ± 0.64 | 3.5 ± 4.4 | 23.6 ± 11.5 | [123I]FP‐CIT SPECT | ROI | BDI | Inverse correlation between depression and DAT binding |
| Weintraub et al. (2005) 28 |
76 PDs 46 HCs |
57/19 24/22 |
No data | 7.5 ± 5.5 | No data | 99mTc‐ TRODAT‐1 SPECT | ROI | POMS, STAS | Inverse correlation between depression and DAT availability in left anterior putamen |
| Wu et al. (2011) 29 |
17 PDs 13 MDs 10 HCs |
9/8 7/6 5/5 |
No data | 1.17 ± 0.5 | No data | 99mTc‐ TRODAT‐1 SPECT | ROI | CCMD‐3, DSM‐IV/ HAM‐D | HCs and MDs > PDs: Dopaminergic availability in bi. striatum |
| Qamhawi et al. (2015) 30 |
345 PDs 56 SWEDDs 185 HCs |
231/114 40/16 126/59 |
1.5 ± 0.5 1.4 ± 0.5 |
0.53 ± 0.5 |
19.6 ± 8.9 12.5 ± 6.8 |
[123I]FP‐CIT SPECT | ROI | GDS | No significant relationship between depression and raphe specific binding ratio |
| Becker et al. (1997) 43 |
30 HCs 13 dPDs 17 PDs |
24/6 11/2 14/3 |
2–4 (PDs + dPDs) |
9.7 ± 5.8 (PDs + dPDs) |
27.7 ± 13.1 | TCS | ROI |
CGI HAM‐D |
HCs + PDs > dPDs: Raphe echogenicity Inverse correlation between raphe echogenicity and depression severity |
| Berg et al. (1999) 48 |
31 patients (20dPDs 11 PDs) |
16/15 | 2.58 ± 0.76 | 6.6 (1–17) | No UPDRS, but CURS = 35.1 (16–50) |
TCS T2‐weighted |
ROI | DSM‐IV/ HAM‐D, BDI |
dPDs > PDs: More hyperintense in mesencephalic midline Higher T2 values in pontine midline, but lower in mesencephalic midline Decreased echogenicity in mesencephalic midline |
| Walter et al. (2007) 44 |
55 HCs 55 MDs 45 PDs 45 dPDs |
27/28 11/44 23/22 23/22 |
No data |
7.4 ± 6.4 8.1 ± 6.4 |
32.4 ± 20.7 34.1 ± 18.5 |
TCS | ROI | DSM‐IV/BDI, HAM‐D |
HCs + PDs > MDs + dPDs: Echogenicity in brainstem dPDs: Hyperechogenicity in substantia nigra, hypoechogenicity in brainstem raphe |
| Cardoso et al. (2009) 46 |
20 dPDs 16 PDs |
20/0 16/0 |
2.5 ± 0.6 2.3 ± 0.3 |
11.2 ± 6.9 10.2 ± 4.3 |
36.7 ± 12.2 32.8 ± 8.74 |
T1‐weighted task FMRI |
ROI (T1) WB (FMRI) |
DSM‐IV/ HAM‐D |
Structural (volume): dPDs > PDs: Bi. mediodorsal thalamic nucleis Functional (activity): PDs > dPDs: Bi. dorsomedial prefrontal cortices and middle cingulate gyri; left mediodorsal thalamus |
| Feldmann et al. (2008) 31 |
23 dPDs 27 PDs 16 HCs |
dPDs + PDs: 30/20 9/7 |
2.7 ± 0.4 2.7 ± 0.4 |
9.9 ± 5.2 11.2 ± 5.9 |
35.1 ± 9.3 33.6 ± 8.8 |
T1‐weighted | WB | MADRS |
No difference between HCs and PDs and between HCs and dPDs PDs > dPDs: Left orbitofrontal inferior gyrus and rectus; right temporal superior pole and rectus Inverse relationship between depression scores and grey matter volumes in bi. orbitofrontal and right temporal and limbic regions in dPDs |
| Kostić et al. (2010) 32 |
24 PDs 16 dPDs 26 HCs |
13/11 8/8 14/12 |
2 (1–3) 2 (1–3) |
5 (1–19) 6 (1–14) |
19 (10–35) 23 (4–36) |
T1‐weighted |
WB | HAM‐D |
HCs > PDs + dPDs: GM in right ACC and insula; left middle frontal and angular PDs > dPDs: WM in right ACC and inferior orbitofrontal region PDs + dPDs: inverse correlation between WM volume in right inferior orbitofrontal region and depression scores |
| van Mierlo et al. (2015) 33 |
67 PDs |
43/24 | 2.1 ± 0.6 | 2.95 ± 3.39 | 23.27 ± 10.56 | T1‐weighted | WB, followed by ROI | BDI | Inverse correlation between depression and bi. hippocampus and right amygdalar volume |
| Huang et al. (2014) 34 |
15 dPDs 15 PDs |
9/6 9/6 |
2.7 ± 0.8 2.5 ± 1 |
5.3 ± 4.8 4.2 ± 4.0 |
54.6 ± 23.7 45.3 ± 26.1 |
DTI | WB | HAM‐D |
PDs > dPDs: FA in left uncinate fasciculus, superior and inferior longitudinal fasciculi, anterior thalamic radiation and forceps minor dPDs: Inverse correlation between depression and FA in left deep temporal cortex No difference or correlation was found in mean diffusivity |
| Li et al. (2010) 35 |
14 dPDs 18 PDs |
4/10 10/8 |
1.96 ± 0.99 1.83 ± 0.75 |
6.29 ± 5.51 5.67 ± 2.57 |
39.04 ± 22.28 33.83 ± 15.09 |
DTI | ROI | DSM‐IV/ HAM‐D |
PDs > dPDs: FA in bi. mediodorsal thalamic areas Inverse correlations between depression and FA in bi. mediodorsal thalamic areas |
| Matsui et al. (2007) 36 |
14 dPDs 14 PDs |
2/12 4/10 |
3.1 ± 0.4 3.1 ± 0.4 |
8.8 ± 5.2 7.4 ± 5.1 |
34.9 ± 14.7 27.4 ± 14.1 |
DTI | ROI | HAM‐D |
PDs > dPDs: FA in bi. ACC No difference or correlation was found in mean diffusivity |
| Surdhar et al. (2012) 45 |
6 dPDs6 PDs 6 HCs |
5/1 5/1 5/1 |
No data | No data |
16.67 ± 11.6 11.83 ± 2.5 |
T1‐weighted DTI |
ROI | GDS |
No FA difference in corpus callosum and uncinate fasciculus HCs > dPDs: GM volume in bi. amygdala |
| Huang et al. (2015) 47 |
19 dPDs 28 HCs |
10/9 9/19 |
2.6 ± 0.7 2.3 ± 1.0 |
5.2 ± 4.8 4.6 ± 5.0 |
57.7 ± 22.4 50.8 ± 22.4 |
T1‐weighted RS‐FMRI |
ROI | HAM‐D |
Structural (volume): No difference between groups in amygdala Functional (activity): dPDs > HCs and PDs: Left amygdala regional cerebral functional activity PDs + dPDs: Positive correlation between depression scores and left amygdala PDs > dPDs: Connectivity between right amygdala and frontoparietal areas dPDs: Inverse correlation between functional connectivity of right amygdala with right middle frontal gyrus and depression |
| Hu et al. (2015) 37 |
20 dPDs 39 PDs 41 HCs |
9/11 26/13 20/21 |
1.4 ± 0.6 1.72 ± 0.64 |
5.35 ± 2.82 6.5 ± 3.54 |
27.65 ± 13.17 28.21 ± 13.17 |
RS‐FMRI | ROI | DSM‐V/ HAM‐D |
dPDs > PDs: Connectivity between left amygdala and bi. mediodorsal thalamus and between right amygdala and left superior temporal and calcarine gyri dPDs vs. HCs: ↑ connectivity between left amygdala and bi. mediodorsal thalamus; ↓ connectivity between left amygdala and left putamen, inferior frontal gyrus and right cerebellum; ↓ connectivity between right amygdala and left rectus, inferior orbitofrontal gyrus and right putamen |
| Lou et al. (2015) 38 |
17 dPDs 17 PDs 17 HCs |
8/9 9/8 9/8 |
2.71 ± 0.25 2.62 ± 0.28 |
No data |
44.06 ± 12.34 40.47 ± 9.19 |
RS‐FMRI | WB | DSM‐IV/ HAM‐D |
PDs > dPDs: Connectivity in left dorsolateral prefrontal cortex and right superior temporal gyrus dPDs > PDs: Connectivity in right PCC HCs > dPDs: Connectivity in bi. frontal, temporal and parietal regions and left insula Inverse correlation between depression and PCC |
| Luo et al. (2014) 42 |
29 dPDs 30 PDs 30 HCs |
14/15 15/15 15/15 |
1.79 ± 0.62 1.73 ± 0.58 |
1.98 ± 1.64 2.12 ± 1.30 |
28.34 ± 16.9 26.83 ± 12.44 |
RS‐FMRI | WB followed by ROI | DSM‐IV/ HAM‐D |
dPDs > PDs and HCs: Regional cerebral function in left orbitofrontal area PDs + dPDs: Positive correlation between depression and regional cerebral function in left orbitofrontal cortex HCs > PDs > dPDs: Connectivity in the prefrontal−limbic network |
| Sheng et al. (2014) 39 |
20 dPDs 21 PDs 25 HCs |
13/8 13/7 16/9 |
2.1 ± 0.75 1.95 ± 0.63 |
3.4 ± 1.7 4.0 ± 2.4 |
39.4 ± 10.8 43.8 ± 8.2 |
RS‐FMRI | WB followed by ROI | DSM‐IV/ HAM‐D |
dPDs vs. PDs: Regional activity: ↑ in left middle frontal gyrus and right inferior frontal gyrus; ↓ in left amygdala and bi. lingual gyri dPDs vs. PDs: Connectivity: ↓ within prefrontal−limbic system and ↑ in prefrontal cortex and lingual gyrus |
| Skidmore et al. (2013) 40 | 15 PDs | 12/3 | No data | No data | 37 ± 13 | RS‐FMRI | WB | HAM‐D, LARS |
Positive correlation between depression and regional cerebral function in bi. cunei and cerebellums, right subgenual cingulate, lateral geniculate and mesial frontal gyrus Depression was predicted by functional activity signal in right subgenual cingulate |
| Wen et al. (2013) 41 |
17 dPDs 16 PDs 21 HCs |
7/10 8/8 13/8 |
2.1 ± 1.9 1.5 ± 1 |
6.4 ± 5.4 5.6 ± 7.4 |
42. ±46 33.8 ± 24.2 |
RS‐FMRI | WB | DSM‐IV/ HAM‐D |
dPDs vs. PDs: ↑ Regional cerebral function in bi. (mostly right) frontal areas, including ACC and dorsolateral prefrontal cortex and left temporal lobe; ↓ regional cerebral function in bi. cerebellum dPDs vs. HCs: ↑ Bi. temporal and parietal regions and left inferior frontal gyrus; ↓ bi. caudate and precuneus, right frontal, superior temporal and thalamic areas Positive correlation between depression and regional cerebral function in the dorsolateral prefrontal cortex |
ACC, anterior cingulated cortex; AES, Apathy Evaluation Scale; ALFF, amplitude of low frequency function; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; bi., bilateral; CCMD‐3, Chinese Classification of Mental Disorders version 3; CGI, clinical global impression; CSDD, Cornell Scale for Depression in Dementia; CURS, Columbia University Rating Scale; DAT, dopamine transporter; dPDs, PD patients with depression; DSM‐IV, ‐IV‐TR, ‐III, and ‐V, Diagnostic and Statistical Manual of Mental Disorders, 4th, 4th‐TR, 3rd, and 5th editions; DTI, diffusion tensor imaging; FA, fractional anisotropy; GDS, Geriatric Depression Scale; GM, grey matter; HAM‐A, Hamilton Anxiety Scale; HAM‐D, Hamilton Depression Scale; H and Y, Hoehn and Yahr scale; HCs, healthy controls; IDS, Inventory of Depressive Symptomatology; LARS, Lille Apathy Rating Scale; MADRS, Montgomery Asberg Depression Rating Scale; MD, patients with major depression; PCC, posterior cingulate cortex; PDs, patients with PD alone; PD‐Ds, patients with PD and dementia; PET, positron emission tomography; POMS, Profile of Mood States; rCBF, regional cerebral blood flow; ROI, region of interest; RS‐FMRI, resting state functional magnetic resonance imaging; SPECT, single‐photon emission computed tomography; STAS, State–Trait Anxiety Scale; SWEDD, patients with scans without evident dopaminergic deficits; TCS, transcranial sonography; UPDRS‐III, Unified Parkinson's Disease Rating Scale III; WB, whole brain; WM, white matter.
The PET or SPECT studies of depression in PD examined neural metabolic activity in the resting state using various radiotracers. The majority reported reduced neural metabolic activity in PD patients with depression (dPDs) in comparison with patients with PD alone or healthy controls (HCs) or found inverse correlations between depression and neural metabolism. The abnormal regions were predominantly in the frontal lobe and striatum as well as the subcortical or limbic regions including thalamus, amygdala, hippocampus, anterior cingulate cortex and insula. A few studies reported an increase of neural metabolism in the prefrontal and subcortical regions (i.e. caudate and putamen, and amygdala), compared to HCs or PD patients without depression. Some found positive correlations between depression and neural metabolic activity in these regions 13, 14, 17, 19, 20. One study with a large sample size of early PD patients did not find any correlation between depression severity and neural metabolism of the raphe nucleus 30.
Of the seven studies using T1‐weighted imaging, only one reported bilaterally increased thalamic grey matter (GM) volume in dPD patients compared to PD patients without depression 47, and three did not find any GM volumetric differences between HCs and dPDs 14, 31, 48, whereas three studies showed that dPD patients had decreased GM volumes in the prefrontal, parietal and insular regions as well as the limbic system (anterior cingulate cortices and amygdala) 31, 32, 46 compared to HCs or non‐depressed PD patients and further demonstrated inverse correlations between depression and brain volumes in the prefrontal and limbic regions 32, 33.
A study using TCS and T2‐weighted imaging showed decreased echogenicity but increased hyperintensity in the mesencephalic midline structure in depressed PD patients, thereby suggesting an alteration in the basal limbic system in dPD 44. The other two studies applying TCS demonstrated hypoechogenicity in the brainstem raphe in dPD patients as opposed to HCs and non‐depressed PD patients 43, 48. Using DTI techniques, three out of four studies reported compromised white matter connectivity indexed by decreased fractional anisotropy (FA) in various tracts, including the bilateral anterior cingulate cortex and thalamus and multiple tracts connecting to the left frontal and deep temporal lobes 34, 35, 36. However, one study reported a non‐significant group difference in the FA of the corpus callosum and uncinate fasciculus, a tract that interconnects the amygdala, hippocampus, temporal pole and the orbitofrontal cortex 45.
Compared to DTI findings, RS‐FMRI studies of depression in PD revealed both increased and decreased resting state neural activities in dPD patients, compared to non‐depressed PD patients and HCs. In dPD patients, increased functional connectivity between spatially discrete regions in the resting state was observed in the subcortical areas, such as the connectivity of the left amygdala with the bilateral mediodorsal thalamus 37, whilst decreased connectivity was seen in the connection between cortico‐limbic 37 or cortico‐cortico networks 38. In addition, depression was inversely correlated with functional connectivity between the amygdala and prefrontal and posterior cingulate cortices 38, 47, whilst it was positively correlated with regional neural activities in the left amygdala, right cingulate and thalamus, and bilateral cerebellum prefrontal cortices 40, 41, 42, 47. With regard to regional neural functional activity during the resting state, dPD patients showed regionally increased activity mainly in the prefrontal and limbic regions 39, 41, 42, 47, but also in the temporal and parietal regions 41. In essence, these studies generally suggest that there is a regionally increased neural activity in the prefrontal regions and decreased functional connectivity between the prefrontal−limbic networks in dPD patients. These changes correlated with depression severity.
These studies using different neuroimaging modalities suggest a wide spectrum of neural involvement. The implication of an extra‐nigrostriatal pathway in the prefrontal, temporal and limbic cortices implies that other neurotransmitters, such as serotonin and noradrenaline, are also implicated in depression in PD 49, 50, 51. This view is supported by studies using PET or SPECT to show that cortical cholinergic activity inversely correlated with depression 12 and changes of serotonin or noradrenaline in PD patients with depression, compared to HCs or PD patients without depression 11, 13, 16, 17, 18, in addition to dopaminergic alterations 19, 20, 21, 22, 26, 27. Although it is challenging to identify a unifying model of neuropathology of depression in PD, these observations suggest that depression is unlikely to be the result of a single brain region or neurotransmitter system but involves the dysregulation of cortico‐limbic networks in addition to that of the nigrostriatal pathway.
Challenges and limitations
Comparisons between studies are difficult since imaging modalities and/or techniques are not uniform. Most were focused on PET or SPECT, but more recent studies have used non‐invasive modalities, such as RS‐FMRI, to explore the alterations of neural functional activity; other non‐invasive imaging techniques, such as DTI, are limited. The inclusion versus exclusion of patients in the on‐medication state and different severities of depression may restrain cross‐study comparisons. For studies using PET or SPECT, diverse radiotracers were used to explore the associations of various neurotransmitters, including serotonergic, cholinergic and dopaminergic transporters, and general glucose metabolism with depression. Although [123I]FP‐CIT was a widely used radiotracer for measuring dopaminergic dysfunction 20, 22, 26, 27, it was also used for determining serotonin level 30 in depression in PD. Although findings from studies using different radiotracers to target different neurotransmitters suggest the involvement of multiple neurotransmitters and the extra‐nigrostriatal pathway in depression in PD, divergent radiotracers may be related to controversial results 20.
The majority of studies adopted region of interest (ROI) strategies to study various regions and few examined the whole brain; this may add to selection bias of the brain regions. The methods to evaluate depression are not comparable. Half of the studies only used scales (mostly one scale) to determine the severity of depression, whilst others followed the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM‐IV‐TR) criteria for depression. The most common scale used for measuring depression severity was the Hamilton Depression Scale (HAM‐D), but other scales (e.g. Beck Depression Inventory) were also used.
Different imaging modalities may provide different aspects of neural alterations associated with depression in PD, but studies using DTI and FMRI (both task and resting state based) are still scarce. Differences in analytical strategies (ROI versus voxel‐wise whole brain) and in the varied inclusion and exclusion criteria (e.g. on or off antiparkinsonian or antidepressant medications, minor or major depression) may preclude cross‐study comparisons.
Neuroimaging of anxiety in PD
Compared to depression, only eight neuroimaging studies of anxiety in PD were identified. Most studies on anxiety used either PET or SPECT techniques 14, 17, 20, 28, 51, 52, and only three studies used T1‐weighted imaging 14, 53, 54, whilst no study used any other imaging modalities. For studies using either PET or SPECT, five of them used dopamine radiotracers 17, 20, 28, 51, 52, one study examined general glucose metabolism 14, whilst one study additionally explored noradrenaline in addition to dopamine 17. All eight studies adopted ROI approaches with the caudate and putamen being the main foci, except for one study that additionally examined structural changes in the whole brain that were associated with anxiety in PD 14. Additional inclusion of the prefrontal areas, such as the orbitofrontal cortex and inferior frontal gyrus, as the ROIs was found in two studies 17, 53. The amygdala and hippocampus as ROIs were studied in three 17, 53, 54 and two 53, 54 studies, respectively, and the thalamus in two studies 17, 53. Most of the PET/SPECT studies 14, 17, 28, 51 showed an inverse correlation between dopaminergic density in the caudate and putamen with the severity of anxiety in PD, whilst the study using T1‐weighted imaging showed no significant anxiety‐associated structural changes in these two key regions in PD 53. Two studies demonstrated an inverse correlation between anxiety symptoms and brain volume 54 and dopaminergic density 17 of the amygdala, although this was not replicated in another study 53. T1‐weighted imaging studies did not reveal a significant role of the hippocampal areas in anxiety in PD 53, 54. There was no consistent correlation of the thalamus with anxiety based on brain volume and dopaminergic density 17, 53. Table 2 gives details of the studies.
Table 2.
Neuroimaging studies in anxiety in Parkinson's disease (PD)
| Study | Subjects | Sex (M/F) | H and Y (Mean ± SD) | Duration of PD (years) | UPDRS‐III | Imaging modality | Imaging analytical method | Diagnosis/measurement | Key findings |
|---|---|---|---|---|---|---|---|---|---|
| Ceravolo et al. (2013) 20 | 44 PDs | No data | No data | 1.14 ± 0.98 | 17.9 ± 7.7 | [123I]FP‐CIT SPECT | ROI | DSM‐IV‐TR/ HAM‐D, HAM‐A, BDI | Positive correlation between anxiety and DAT binding bi. caudate and putamen |
| Erro et al. (2012) 51 |
9 anxPDs 25 PDs |
4/5 18/7 |
No data |
14.9 ± 3.5 16.2 ± 3.1 |
15.5 ± 5.7 13.3 ± 6.1 |
[123I]FP‐CIT SPECT | ROI | HADS‐A |
PDs > anxPDs: DAT density in bi. caudate and left putamen Inverse correlation between anxiety and DAT density in right caudate |
| Huang et al. (2013) 14 |
26 PDs 12 HCs |
16/10 7/5 |
1–2.5 | 5.5 ± 0.7 | No data | [18F]FDG‐PET, T1‐weighted |
ROI (PET) WB (T1) |
BDI, BAI, AES |
Inverse correlation between anxiety and metabolic elevations in bi. caudate No volumetric difference between HCs and PDs |
| Moriyama et al. (2011) 52 |
12 sad‐PDs 20 PDs |
9/3 15/5 |
2.6 ± 0.9 2.6 ± 0.5 |
7.1 ± 3.8 9.0 ± 6.2 |
34.7 ± 16.1 31.7 ± 12.2 |
99mTc‐TRODAT‐1 SPECT | ROI | DSM‐IV‐TR/BSPS | DAT binding potential was positively correlated with anxiety for bi. putamen and left putamen |
| Remy et al. (2005) 17 |
8 dPDs 12 PDs 7 HCs |
5/3 9/3 |
1–3.5 1–3.5 |
3.1 ± 1.8 4.9 ± 2.6 |
24.3 ± 11.2 23.3 ± 6.7 |
[11C]RTI‐32 PET | ROI | BDI, AES, STAS | Inverse correlation between anxiety and binding value in left ventral striatum, caudate, locus coeruleus, inferior thalamic region and bi. amygdala and medial thalamus |
| Weintraub et al. (2005) 28 |
76 PDs 46 HCs |
57/19 24/22 |
No data | 7.5 ± 5.5 | No data | 99mTc‐TRODAT‐1 SPECT | ROI | POMS, STAS | Inverse correlation between anxiety and DAT availability in left anterior putamen |
| Tinaz et al. (2011) 53 |
15 PDs 15 HCs |
No data | 2.17 ± 0.3 | 6 ± 2.8 | 31.53 ± 5.84 | T1‐weighted | ROI | STAS | No significant correlation between anxiety and brain volumetric features |
| Vriend et al. (2015) 54 | 110 PDs | 66/44 | 2.08 ± 0.58 | 3.3 ± 3.6 | 24.9 ± 10.4 | T1‐weighted | ROI | BAI, BDI | Inverse correlation between affective symptoms of anxiety and volume of left amygdala |
AES, Apathy Evaluation Scale; anxPDs, PD patients with anxiety; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; bi., bilateral; BSPS, Brief Social Phobia Scale; DAT, dopamine transporter; dPDs, PD patients with depression; DSM‐IV‐TR, Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision; HADS‐A, Hospital Anxiety Depression Scale − Anxiety Subscale; HAM‐A, Hamilton Anxiety Scale; HAM‐D, Hamilton Depression Scale; HCs, healthy controls; PDs, patients with PD disease alone; PET, positron emission tomography; POMS, Profile of Mood States; ROI, region of interest; sad‐PDs, PD patients with social anxiety disorder; SPECT, single‐photon emission computed tomography; STAS, State–Trait Anxiety Scale; UPDRS‐III, Unified Parkinson's Disease Rating Scale III; WB, whole brain.
Amongst the aforementioned studies, none of them included patients with cognitive symptoms (e.g. dementia); one study 52 recruited PD patients with social anxiety disorder, one study mentioned that some of the patients had social anxiety or panic attacks 20, whilst the remaining studies did not specify the anxiety type(s) of patients; likewise, only one study excluded patients with depression, a common comorbidity of anxiety 51. Two studies 28, 54 included patients in the on‐medication state (i.e. patients were on antiparkinsonian medication during MRI scanning), one study 53 did not clarify the medication state of their patients, whilst the remaining studies included patients in the off‐medication state. Patients in three of the eight studies used either antidepressant or anxiolytic medication during the study period 20, 53, 54, and there were three studies that did not indicate whether patients used antidepressant or anxiolytic medication 14, 17, 52. See Table S2 for the clinical characteristics of studies on anxiety in PD.
Challenges and limitations
Although anxiety is a common mood complaint in PD, it is apparent that there are insufficient neuroimaging studies examining the underlying neuropathology of anxiety in PD. Whilst limited studies hamper our understanding of the pathogenesis of anxiety in PD, some methodological issues in the existing studies may also contribute to the difficulty in drawing meaningful conclusions. Only two of the eight studies applied strict diagnostic criteria (e.g. DSM‐IV‐TR criteria) in addition to the employment of anxiety scales, whilst the remaining six studies used one single scale to evaluate anxiety symptoms. Interestingly, only the two studies using both diagnostic criteria and scales to improve diagnostic accuracy demonstrated a positive correlation between anxiety and dopaminergic density in caudate or putamen 20, 52. Therefore, varied measurements of anxiety may, at least in part, explain the conflicting findings. Also, only two studies 20, 52 specified the anxiety types of their patients and one study 28 excluded patients with anxiety. Although all studies excluded patients with cognitive impairment, most studies did not control for depression and did not provide specific information about patients’ anxiety symptoms/types. Although most studies included PD patients in the off‐medication state, some studies either involved patients in the on‐medication state or did not indicate the medication state of their patients. The wide variety in anxiety measurements, symptoms and severity, the use of antiparkinsonian, antidepressant or anxiolytic medications, and the confounding effects of depression add to the difficulty of drawing conclusions on the neuropathology of anxiety in PD.
Further, most studies included rather small samples, which may lack enough statistical power to truly detect the neural substrates of anxiety. With regard to neuroimaging methodologies, the application of ROI methods with focus on putamen and caudate cannot provide comprehensive examinations of the pathogenesis of anxiety. On the contrary, results from such an approach may be compounded by the involvement of these regions in the pathogenesis of motor dysfunction. For studies using PET or SPECT, other neurotransmitters associated with anxiety in PD, such as acetylcholine, norepinephrine and serotonin, were under‐studied given that most of these studies adopted radiotracers to examine dopaminergic changes. To date, only PET/SPECT and T1 structural MRI have been used in anxiety in PD. The application of other imaging modalities, such as DTI and FMRI, which are able to provide information about the pathology at the level of neural networks rather than at the level of individual regions may provide further insights.
Neuroimaging of apathy in PD
There were 14 neuroimaging studies of apathy in PD (Table 3). Similar to the studies of depression and anxiety in PD, most imaging studies of apathy in PD utilized either PET or SPECT techniques 14, 17, 55, 56, 57, 58, 59, 60, 61 and focused on measuring cerebral glucose metabolism with the exception of two PET studies 17, 60 and one SPECT study 61 examining dopaminergic changes associated with apathy. Of the 14 studies, five involved T1‐weighted imaging 14, 62, 63, 64, 65, two used RS‐FMRI 40, 64 and one study performed DTI in addition to T1‐weighted imaging 65. Approximately half of the studies used ROI methods with caudate, putamen and limbic regions being the most common ROIs. All 14 studies excluded patients with cognitive symptoms (e.g. dementia) and seven of them included patients who remained on antiparkinsonian medication during MRI scanning. Additionally, three of the 14 studies recruited patients who were on mood stabilizers. Amongst the 14 studies, five studies clearly excluded patients with depression 55, 57, 58, 59, 61, whilst seven studies did not 14, 17, 60, 62, 63, 64, 65 and two did not indicate whether patients with depression were excluded from the studies 40, 56.
Table 3.
Neuroimaging studies of apathy in PD
| Study | Subjects | Sex (M/F) | H and Y (Mean ± SD) | Duration of PD (years) | UPDRS‐III | Imaging modality | Imaging analytical method | Diagnosis/measurement | Key findings |
|---|---|---|---|---|---|---|---|---|---|
| Huang et al. (2013) 14 |
26 PDs 12 HCs |
16/10 7/5 |
1–2.5 | 5.5 ± 0.7 | No data | [18F]FDG‐PET, T1‐weighted |
ROI (PET) WB (T1) |
BDI, BAI, AES |
Positive correlation between apathy and bi. ACC and orbitofrontal lobes Inverse correlation between apathy and right inferior parietal and left superior temporal gyri No volumetric difference between HCs and PDs |
| Lawrence et al. (2011) 55 |
10 low aPDs 10 high aPDs |
No data |
2.35 ± 0.6 2.7 ± 0.8 |
No data |
25.6 ± 11.0 28.2 ± 11.4 |
H2 15O PET FMRI | ROI | AS | ↓ Activity in left amygdala and striatum, bi. vmPFC, and midbrain in high aPDs |
| Le Jeune et al. (2009) 56, a | 12 PDs | 8/4 |
1.6 ± 1.2 (off) 0.9 ± 1.0 (on) |
No data |
14.3 ± 0.9 (off) 5.5 ± 3.4 (on) |
[18F]FDG‐PET | WB | AES, MADRS, AMDP‐AT |
Positive correlation between apathy and metabolism in right, frontal and parietal regions and left fusiform gyrus Inverse correlation between apathy and metabolism in bi. cingulate and left middle frontal gyri |
| Remy et al. (2005) 17 |
8 dPDs 12 PDs 7 HCs |
5/3 9/3 |
1–3.5 1–3.5 |
3.1 ± 1.8 4.9 ± 2.6 |
24.3 ± 11.2 23.3 ± 6.7 |
[11C]RTI‐32 PET | ROI | BDI, AES, STAS | Inverse correlation between apathy and binding value in bi. ventral striatum |
| Robert et al. (2012) 57 | 45 PDs (no depression) | No data | No data | 11.3 ± 4.1 |
8.4 ± 5.9 (ON) 29.9 ± 12.2 (OFF) |
[18F]FDG ‐PET | WB | AES, MADRS |
Positive correlations between apathy and cerebral metabolism in right inferior frontal gyrus, middle frontal gyrus, cuneus and insula Inverse correlations between apathy and cerebellar metabolism in bi. posterior cerebellums |
| Robert et al. (2014) 58 | 36 PDs (no depression) | No data | No data | No data | 7.9 ± 5.4 | [18F]FDG ‐PET | WB | AES, MADRS | Positive correlation between apathy and increased metabolism in left PCC |
| Robert et al. (2014) 59 | 44 PDs (no depression) | No data | No data | 11.4 ± 4.1 |
7.5 ± 5.2 (ON)b
32.6 ± 12.8 (OFF) |
[18F]FDG ‐PET | ROI | AES, MADRS, AMDP‐AT | Inverse correlation between metabolism in right ventral striatum and postoperative increased AES scores |
| Thobois et al. (2010) 60 |
12 aPDs 13 PDs |
6/6 10/3 |
No data |
10.3 ± 2.2 10.5 ± 3.0 |
14.5 ± 9.9 10.3 ± 5.9 |
[11C]‐raclopride PET | WB, ROI | BDI, BAI, SAS | aPDs > PDs: Binding value in bi. orbitofrontal and temporal cortices, and PCC; left dorsolateral prefrontal cortex, striatum and thalamus; right amygdala |
| Santangelo et al. (2015) 61 |
14 aPDs 14 PDs |
4/10 4/10 |
No data |
13.5 ± 6.1 12.8 ± 5.8 |
14.27 ± 7.4 14.1 ± 8.9 |
[123I]‐FP‐CIT SPECT | ROI | AES |
PDs > aPDs: dopamine in right caudate Inverse correlation between dopamine density in right caudate and apathy |
| Isella et al. (2002) 62 |
33 PDs 25 HCs |
18/15 12/13 |
No data | 4.9 ± 3.86 | 31.9 ± 15.69 | T1‐weighted | ROI | AES, GDS | PDs: Apathy was positively correlated with bi. temporal atrophy but inversely correlated with thickness of the right mesial temporal lobe |
| Reijnders et al. (2010) 63 | 60 PDs | No data | 1.5–3 | 6.6 ± 4.3 | 17.3 ± 4.9 | T1‐weighted | WB | AES, LARS, HAM‐D, NPI | Inverse correlation between apathy and grey matter density in bi. precentral gyri, inferior frontal and parietal gyri, and insula, right precuneus and PCC |
| Baggio et al. (2015) 64 |
25 aPDs 37 PDs 31 HCs |
20/5 17/20 15/16 |
1.84 ± 0.69 1.70 ± 0.57 |
7.24 ± 4.13 7.54 ± 5.52 |
15.56 ± 7.94 15.37 ± 8.48 |
RS‐FMRI T1‐weighted |
ROI | AS, BDI |
HCs and PDs > aPDs: Functional connectivity in left limbic striatal and frontal networks PDs + aPDs: Apathy was inversely correlated with connectivities between left limbic striatal and left frontal areas and between left frontal subdivisions No group differences or correlations with apathy or GM volume or subcortical volume/ shape |
| Skidmore et al. (2013) 40 | 15 PDs | 12/3 | No data | No data | 37 ± 13 | RS‐FMRI | WB | HAM‐D, LARS |
Apathy was positively correlated with regional cerebral functional signal in right middle orbital gyrus and bi. subgenual cingulate, but inversely correlated with left SMA, IPL, fusiform gyrus, and bi. cerebellums Apathy was predicted by regional cerebral functional signal in left SMA |
| Carriere et al. (2014) 65 |
10 HCs 10 aPDs 10 PDs |
4/6 6/4 6/4 |
No data |
11.9 ± 6.5 11.9 ± 3.2 |
28.1 ± 10.8 11.9 ± 3.2 |
T1‐weighted DTI |
T1: ROI DTI: WB |
LARS |
PDs + HCs > aPDs: Left nucleus accumbens HCs > aPDs: Bi. nucleus accumbens PDs > aPDs: Dorsolateral part of left caudate Positive correlation between apathy and atrophy in left nucleus accumbens No difference in FA |
ACC, anterior cingulated cortex; AES, Apathy Evaluation Scale; ALFF, amplitude of low frequency function; AMDP‐AT, Association for Methodology and Documentation in Psychiatry − Anxiety; aPDs, PD patients with apathy; AS, Apathy Scale; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; bi., bilateral; dPDs, PD patients with depression; DTI, diffusion tensor imaging; FA, fractional anisotropy; GDS, Geriatric Depression Scale; GM, grey matter; HAM‐D, Hamilton Depression Scale; HCs, healthy controls; IPL, inferior parietal lobule; LARS, Lille Apathy Rating Scale; MADRS, Montgomery Asberg Depression Rating Scale; NPI, Neuropsychiatric Inventory; PCC, posterior cingulate cortex; PDs, patients with PD alone; PET, positron emission tomography; ROI, region of interest; RS‐FMRI, resting state functional magnetic resonance imaging; SAS, Starkstein Apathy Scale; SMA, supplementary motor area; SPECT, single‐photon emission computed tomography; STAS, State‐Trait Anxiety Scale; UPDRS‐III, Unified Parkinson's Disease Rating Scale III; vmPFC, ventromedial prefrontal cortex; WB, whole brain.
Only the postoperative AES scores are used here as apathy significantly increased after subthalamic nucleus deep brain stimulation;
Only pre‐operative UPDRS‐III scores are shown here.
Most studies demonstrated an inverse correlation between apathy and cerebral metabolism in the striatum, cerebellum, and prefrontal, temporal, parietal and limbic lobes 14, 17, 56, 57, 59, 61. However, positive correlations between apathy and prefrontal, temporal, parietal and limbic areas were also noted in some studies 14, 56, 57, 58. Studies using T1‐weighted imaging reported reduced GM thickness in the temporal lobes 62 or increased atrophy in the frontal and parietal lobes (e.g. bilateral precentral gyri, right precuneus and posterior cingulate cortex), insula 63 and left nucleus accumbens 65 to be correlated with apathy, although this was not replicated in other studies 14, 64. The only study using DTI to examine the neural substrates of apathy in PD did not observe any difference in white matter integrity between patients with apathy and those without apathy and HCs 65. For spontaneous neural activity measured with RS‐FMRI, the study by Baggio and colleagues showed that PD patients with apathy had decreased functional connectivity between the left striatal and frontal areas, and amongst PD patients apathy was inversely correlated with functional connectivity between the subdivisions of the left frontal lobe 64. Meanwhile, another RS‐FMRI work indicated that apathy was positively correlated with regional cerebral functional activity in the right orbital gyrus and bilateral cingulate areas, but inversely correlated with the left parietal lobe (supplementary motor area and inferior parietal lobule) and bilateral cerebellum 40.
Challenges and limitations
These studies highlighted frontal, limbic and striatal involvement in apathy in PD. However, inconsistent findings of an increased or a decreased cerebral metabolic/functional activity was found in various brain regions. Apathy either was associated with a GM decrease or had no relationship with GM changes. There are several explanations for the inconclusive results. First, there were differences in the analytical methods. Most studies used ROI methods, whilst few examined the whole brain. Amongst studies using ROI methods, the selection of ROIs was not necessarily identical; hence the results can be different. Secondly, unlike depression and anxiety, a well‐accepted standardized diagnostic system for apathy has not been established. Different studies therefore adopted different scales to measure apathy severity and all studies but one 63 used only one scale, presenting a potential issue with measurement validity and reliability. Also, differences in the medication state during MRI scanning and clinical characteristics (e.g. symptom severity and whether other mood symptoms were controlled for) may contribute to inconclusive findings. Thirdly, most studies only had one single imaging modality, making it difficult to directly examine the associated structural and functional changes in the same samples. Finally, given the limited studies, there is a great need for more research, especially studies incorporating other imaging modalities, such as DTI, to improve our understanding of the neuropathology of PD‐related apathy.
Summary
Although all of the three mood disturbances are common complications in PD, most neuroimaging works have focused on depression, suggesting that anxiety and apathy may be under‐recognized mood complaints in PD. In early untreated PD, anxiety and apathy are common 66, implying that these two mood disturbances are also part of a spectrum of ‘hypodopaminergy’ in the same way as depression. Amongst the imaging studies of the three mood disturbances, the majority used one single imaging modality and ROI analytical strategies. For depression and anxiety, most studies adopted scales for evaluating the severity of mood symptoms, whilst few studies additionally applied standardized diagnostic criteria (e.g. DSM) to ensure the diagnoses. For apathy, the standardized diagnostic system has not been well established, leaving researchers to completely rely on scales to determine the severity. As some mood scales are based on participants’ self‐report, bias can be introduced to the study due to subjective perception. Other contributing factors to the discrepant findings across studies included variability in patients’ demographics, medication conditions and symptomatology (e.g. comorbidities of different mood symptoms) as well as differences in image pre‐processing and statistical analysis.
Involvement of the nigrostriatal pathway
The striatal areas, including putamen and caudate, were the most studied substrates in PD‐related mood disturbances. The existing imaging works have underlined the involvement of these areas in depression, anxiety and apathy. Both increased and decreased neural activity of putamen and caudate have been identified in patients with depression, anxiety or apathy compared to non‐depressed PD or HCs. Divergent positive and negative relationships between mood severity and the activity changes of the regions were reported. Compared to functional imaging studies, none of the existing structural imaging studies reported any significant macrostructural or microstructural changes associated with PD‐related mood disturbances. Whether this implies greater sensitivity of neural functional changes or more susceptibility of neural functional activity as opposed to structural changes in the nigrostriatal pathway remains unclear, especially given that there is a lack of sufficient studies using either T1 MRI or DTI.
Involvement of the extra‐nigrostriatal pathway
Whilst the striatum was the focus of most imaging studies in PD‐related mood disturbances, it does not necessarily mean that the extra‐nigrostriatal areas are not affected. In fact, our literature review identifies several areas that are beyond the nigrostriatal pathway. The reported extra‐nigrostriatal areas are mainly in the frontal territory or its connecting areas, such as the orbitofrontal and ventromedial prefrontal cortices, cingulate cortex, amygdala and uncinate fasciculus. However, although the cause−effect relationship between mood severity and neural functional and structural features cannot be concluded, it should be clear that the frontal area and its connecting areas are implicated in PD‐related mood disturbances.
Is the frontostriatal pathway a key component of a neural model for mood disturbances?
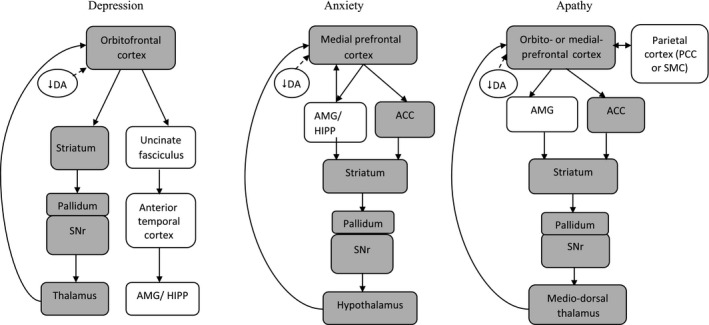
The aetiology of each of the mood disturbances is likely to be multifactorial 67, 68, 69. The mechanisms of these mood disturbances are not fully elucidated, especially for anxiety and apathy. Nevertheless, existing studies suggest that the frontal and striatal areas are most robustly implicated in PD patients with symptoms of depression, anxiety or apathy. The frontostriatal pathway has been demonstrated to be the significant neural system affected by geriatric depression, anxiety and apathy 70, 71, 72. Arguably this pathway (Fig. 2) may also be applicable to PD‐related mood disturbances.
Figure 2.
Simplified schemes of the depression, anxiety and apathy networks in PD. Generally, as PD pathology defined by dopaminergic deficiency progresses, the implicated neural areas that are associated with mood symptoms involve the prefrontal cortex including orbitofrontal cortex and medial prefrontal cortex and then extend to some regions in the anterior temporal and parietal lobes, and subcortical areas. Amongst the three mood symptoms, the shared brain regions are mainly within the frontostriatal pathway. Blocks in grey refer to the frontostriatal pathway; ACC, anterior cingulate cortex; AMG, amygdala; DA, dopamine; HIPP, hippocampus; PCC, posterior cingulate cortex; SMC, supplementary motor cortex; SNr, substantia nigra.
Figure 1.
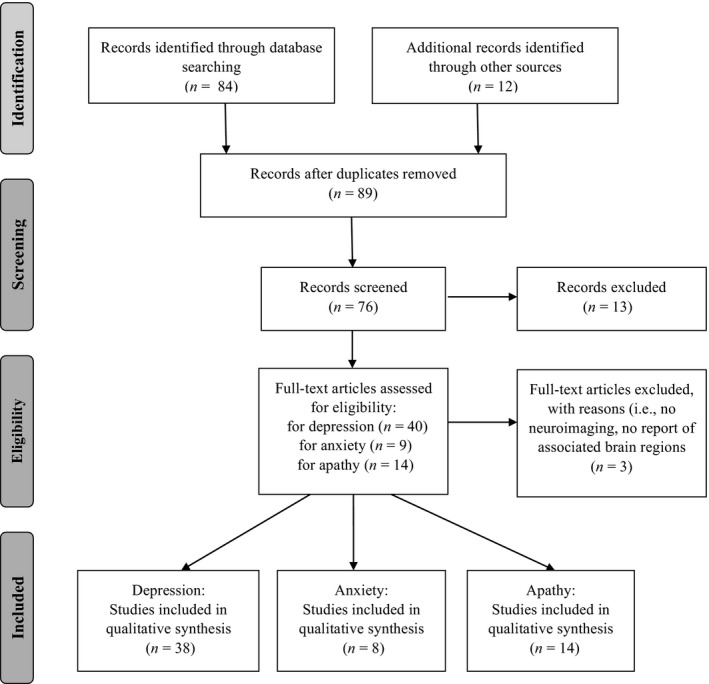
Flow diagram of study selection.
Conclusions
Published neuroimaging studies have predominantly focused on neuropathology of depression in PD, whilst little is known about the neural substrates of anxiety and apathy. Most imaging studies on mood disturbances in PD used ROI approaches with the striatum as the main study region. This approach may limit our understanding of the extra‐nigrostriatal involvement in mood disturbances. Nonetheless, there is evidence suggesting that both nigrostriatal and extra‐nigrostriatal pathways (in particular the frontal region and its connecting areas) are dysregulated. The frontostriatal pathway may be an attractive neural model for understanding mood disturbances in PD. Future studies using a multimodal imaging approach, unbiased whole‐brain analytical methods, and taking into account the comorbidity effects when examining these mood symptoms will be required to elucidate the exact mechanisms. Identifying the relative contributions of these pathways in PD patients with overlapping motor and mood symptoms could provide new pathophysiological clues for the development of better therapeutic targets for affected patients.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.
Supporting information
Table S1. Clinical characteristics of neuroimaging studies in depression in PD.
Table S2. Clinical characteristics of neuroimaging studies in anxiety in PD.
Table S3. Clinical characteristics of neuroimaging studies in apathy in PD.
Acknowledgement
National Medical Research Council (STaR award to EK‐ T and Translational Clinical Research program award in Parkinson's disease).
References
- 1. Gallagher D, Schrag A. Psychosis, apathy, depression and anxiety in Parkinson's disease. Neurobiol Dis 2012; 46: 581–589. [DOI] [PubMed] [Google Scholar]
- 2. Reijnders J, Ehrt U, Weber W, Aarsland D, Leentjens A. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord 2008; 23: 183–189. [DOI] [PubMed] [Google Scholar]
- 3. Karlsen K, Tandberg E, Arsland D, Larsen J. Health related quality of life in Parkinson's disease: a prospective longitudinal study. J Neurol Neurosurg Psychiatry 2000; 69: 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pontone G, Williams J, Anderson K, et al Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson's disease. Mov Disord 2009; 24: 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pont‐Sunyer C, Hotter A, Gaig C, et al The onset of nonmotor symptoms in Parkinson's disease (the ONSET PD study). Mov Disord 2015; 30: 229–237. [DOI] [PubMed] [Google Scholar]
- 6. Richard I. Anxiety disorders in Parkinson's disease. Adv Neurol 2005; 96: 42–55. [PubMed] [Google Scholar]
- 7. den Brok M, van Dalen J, van Gool W, Moll van Charante E, de Bie R, Richard E. Apathy in Parkinson's disease: a systematic review and meta‐analysis. Mov Disord 2015; 30: 79–769. [DOI] [PubMed] [Google Scholar]
- 8. Leentjens A. Depression in Parkinson's disease: conceptual issues and clinical challenges. J Geriatr Psychiatry Neurol 2004; 17: 120–126. [DOI] [PubMed] [Google Scholar]
- 9. Marinus J, Leentjens A, Visser M, Stiggelbout A, van Hilten J. Evaluation of the hospital anxiety and depression scale in patients with Parkinson's disease. Clin Neuropharmacol 2002; 25: 318–324. [DOI] [PubMed] [Google Scholar]
- 10. Kano O, Ikeda K, Cridebring D, Takazawa T, Yoshii Y, Iwasaki Y. Neurobiology of depression and anxiety in Parkinson's disease. Parkinsons Dis 2011; 2011: 143547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ballanger B, Klinger H, Eche J, et al Role of serotonergic 1A receptor dysfunction in depression associated with Parkinson's disease. Mov Disord 2012; 27: 84–89. [DOI] [PubMed] [Google Scholar]
- 12. Bohnen N, Kaufer D, Hendrickson R, Constantine G, Mathis C, Moore R. Cortical cholinergic denervation is associated with depressive symptoms in Parkinson's disease and parkinsonian dementia. J Neurol Neurosurg Psychiatry 2007; 78: 641–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boileau I, Warsh J, Guttman M, et al Elevated serotonin transporter binding in depressed patients with Parkinson's disease: a preliminary PET study with [11C]DASB. Mov Disord 2008; 23: 1776–1780. [DOI] [PubMed] [Google Scholar]
- 14. Huang C, Ravdin L, Nirenberg M, et al Neuroimaging markers of motor and nonmotor features of Parkinson's disease: an 18F fluorodeoxyglucose positron emission computed tomography study. Dement Geriatr Cogn Disord 2013; 35: 183–196. [DOI] [PubMed] [Google Scholar]
- 15. Mayberg H, Starkstein S, Sadzot B, et al Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson's disease. Ann Neurol 1990; 28: 57–64. [DOI] [PubMed] [Google Scholar]
- 16. Mentis MJ, McIntosh AR, Perrine K, et al Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson's disease. Am J Psychiatry 2002; 159: 746–754. [DOI] [PubMed] [Google Scholar]
- 17. Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005; 128: 1314–1322. [DOI] [PubMed] [Google Scholar]
- 18. Politis M, Wu K, Loane C, et al Depressive symptoms in PD correlate with higher 5‐HTT binding in raphe and limbic structures. Neurology 2010; 75: 1920–1927. [DOI] [PubMed] [Google Scholar]
- 19. Guttman M, Boileau I, Warsh J, et al Brain serotonin transporter binding in non‐depressed patients with Parkinson's disease. Eur J Neurol 2007; 14: 523–528. [DOI] [PubMed] [Google Scholar]
- 20. Ceravolo R, Frosini D, Poletti M, et al Mild affective symptoms in de novo Parkinson's disease patients: relationship with dopaminergic dysfunction. Eur J Neurol 2013; 20: 480–485. [DOI] [PubMed] [Google Scholar]
- 21. Felicio A, Moriyama T, Godeiro‐Junior C, et al Higher dopamine transporter density in Parkinson's disease patients with depression. Psychopharmacology 2010; 211: 27–31. [DOI] [PubMed] [Google Scholar]
- 22. Hesse S, Meyer PM, Strecker K, et al Monoamine transporter availability in Parkinson's disease patients with or without depression. Eur J Nucl Med Mol Imaging 2009; 36: 428–435. [DOI] [PubMed] [Google Scholar]
- 23. Imamura K, Okayasu N, Nagatsu T. The relationship between depression and regional cerebral blood flow in Parkinson's disease and the effect of selegiline treatment. Acta Neurol Scand 2011; 124: 28–39. [DOI] [PubMed] [Google Scholar]
- 24. Matsui H, Nishinaka K, Oda M, Komatsu K, Kubori T, Udaka F. Minor depression and brain perfusion images in Parkinson's disease. Mov Disord 2006; 21: 1169–1174. [DOI] [PubMed] [Google Scholar]
- 25. Pålhagen S, Ekberg S, Wålinder J, Granérus A, Granerus G. HMPAO SPECT in Parkinson's disease (PD) with major depression (MD) before and after antidepressant treatment. J Neurol 2009; 256: 1510–1518. [DOI] [PubMed] [Google Scholar]
- 26. Rektorova I, Srovnalova H, Kubikova R, Prasek J. Striatal dopamine transporter imaging correlates with depressive symptoms and Tower of London task performance in Parkinson's disease. Mov Disord 2008; 23: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 27. Vriend C, Raijmakers P, Veltman DJ, et al Depressive symptoms in Parkinson's disease are related to reduced [123I]FP‐CIT binding in the caudate nucleus. J Neurol Neurosurg Psychiatry 2014; 85: 159–164. [DOI] [PubMed] [Google Scholar]
- 28. Weintraub D, Newberg A, Cary M, et al Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson's disease. J Nucl Med 2005; 46: 227–232. [PubMed] [Google Scholar]
- 29. Wu H, Lou C, Huang Z, Shi G. SPECT imaging of dopamine transporters with (99m)Tc‐TRODAT‐1 in major depression and Parkinson's disease. J Neuropsychiatry Clin Neurosci 2011; 23: 63–67. [DOI] [PubMed] [Google Scholar]
- 30. Qamhawi Z, Towey D, Shah B, et al Clinical correlates of raphe serotonergic dysfunction in early Parkinson's disease. Brain 2015; 138: 2964–2973. [DOI] [PubMed] [Google Scholar]
- 31. Feldmann A, Illes Z, Kosztolanyi P, et al Morphometric changes of gray matter in Parkinson's disease with depression: a voxel‐based morphometry study. Mov Disord 2008; 23: 42–46. [DOI] [PubMed] [Google Scholar]
- 32. Kostić V, Agosta F, Petrović I, et al Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology 2010; 75: 857–863. [DOI] [PubMed] [Google Scholar]
- 33. van Mierlo T, Chung C, Foncke E, Berendse H, van den Heuvel O. Depressive symptoms in Parkinson's disease are related to decreased hippocampus and amygdala volume. Mov Disord 2015; 30: 245–252. [DOI] [PubMed] [Google Scholar]
- 34. Huang P, Xu X, Gu Q, et al Disrupted white matter integrity in depressed versus non‐depressed Parkinson's disease patients: a tract‐based spatial statistics study. J Neurol Sci 2014; 346: 145–148. [DOI] [PubMed] [Google Scholar]
- 35. Li W, Liu J, Skidmore F, Liu Y, Tian J, Li K. White matter microstructure changes in the thalamus in Parkinson disease with depression: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol 2010; 31: 1861–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsui H, Nishinaka K, Oda M, et al Depression in Parkinson's disease diffusion tensor imaging study. J Neurol 2007; 254: 1170–1173. [DOI] [PubMed] [Google Scholar]
- 37. Hu X, Song X, Yuan Y, et al Abnormal functional connectivity of the amygdala is associated with depression in Parkinson's disease. Mov Disord 2015; 30: 238–244. [DOI] [PubMed] [Google Scholar]
- 38. Lou Y, Huang P, Li D, et al Altered brain network centrality in depressed Parkinson's disease patients. Mov Disord 2015; 30: 1777–1784. [DOI] [PubMed] [Google Scholar]
- 39. Sheng K, Fang W, Su M, et al Altered spontaneous brain activity in patients with Parkinson's disease accompanied by depressive symptoms, as revealed by regional homogeneity and functional connectivity in the prefrontal‐limbic system. PLoS One 2014; 9: e84705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skidmore F, Yang M, Baxter L, et al Apathy, depression, and motor symptoms have distinct and separable resting activity patterns in idiopathic Parkinson disease. NeuroImage 2013; 81: 484–495. [DOI] [PubMed] [Google Scholar]
- 41. Wen X, Wu X, Liu J, Li K, Yao L. Abnormal baseline brain activity in non‐depressed Parkinson's disease and depressed Parkinson's disease: a resting‐state functional magnetic resonance imaging study. PLoS One 2013; 8: e63691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luo C, Chen Q, Song W, et al Resting‐state fMRI study on drug‐naive patients with Parkinson's disease and with depression. J Neurol Neurosurg Psychiatry 2014; 85: 675–683. [DOI] [PubMed] [Google Scholar]
- 43. Becker T, Becker G, Seufert J, et al Parkinson's disease and depression: evidence for an alteration of the basal limbic system detected by transcranial sonography. J Neurol Neurosurg Psychiatry 1997; 63: 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walter U, Hoeppner J, Prudente‐Morrissey L, et al Parkinson's disease‐like midbrain sonography abnormalities are frequent in depressive disorders. Brain 2007; 130: 1799–1807. [DOI] [PubMed] [Google Scholar]
- 45. Surdhar I, Gee M, Bouchard T, Coupland N, Malykhin N, Camicioli R. Intact limbic‐prefrontal connections and reduced amygdala volumes in Parkinson's disease with mild depressive symptoms. Parkinsonism Relat Disord 2012; 18: 809–813. [DOI] [PubMed] [Google Scholar]
- 46. Cardoso E, Maia F, Fregni F, et al Depression in Parkinson's disease: convergence from voxel‐based morphometry and functional magnetic resonance imaging in the limbic thalamus. NeuroImage 2009; 47: 467–472. [DOI] [PubMed] [Google Scholar]
- 47. Huang P, Xuan M, Gu Q, et al Abnormal amygdala function in Parkinson's disease patients and its relationship to depression. J Affect Disord 2015; 183: 263–268. [DOI] [PubMed] [Google Scholar]
- 48. Berg D, Supprian T, Hofmann E, et al Depression in Parkinson's disease: brainstem midline alteration on transcranial sonography and magnetic resonance imaging. J Neurol 1999; 246: 1186–1193. [DOI] [PubMed] [Google Scholar]
- 49. Barone P. Neurotransmission in Parkinson's disease: beyond dopamine. Eur J Neurol 2010; 17: 364–376. [DOI] [PubMed] [Google Scholar]
- 50. Aarsland D, Påhlhagen S, Ballard C, Ehrt U, Svenningsson P. Depression in Parkinson disease − epidemiology, mechanisms and management. Nat Rev Neurol 2012; 8: 35–47. [DOI] [PubMed] [Google Scholar]
- 51. Erro R, Pappatà S, Amboni M, et al Anxiety is associated with striatal dopamine transporter availability in newly diagnosed untreated Parkinson's disease patients. Parkinsonism Relat Disord 2012; 18: 1034–1038. [DOI] [PubMed] [Google Scholar]
- 52. Moriyama T, Felicio A, Chagas M, et al Increased dopamine transporter density in Parkinson's disease patients with social anxiety disorder. J Neurol Sci 2011; 310: 53–57. [DOI] [PubMed] [Google Scholar]
- 53. Tinaz S, Courtney M, Stern C. Focal cortical and subcortical atrophy in early Parkinson's disease. Mov Disord 2011; 26: 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vriend C, Boedhoe P, Rutten S, Berendse H, van der Werf Y, van den Heuvel O. A smaller amygdala is associated with anxiety in Parkinson's disease: a combined FreeSurfer−VBM study. J Neurol Neurosurg Psychiatry 2015; pii: jnnp‐2015–jnnp310383. [DOI] [PubMed] [Google Scholar]
- 55. Lawrence A, Goerendt I, Brooks D. Apathy blunts neural response to money in Parkinson's disease. Soc Neurosci 2011; 6: 653–662. [DOI] [PubMed] [Google Scholar]
- 56. Le Jeune F, Drapier D, Bourguignon A, et al Subthalamic nucleus stimulation in Parkinson disease induces apathy: a PET study. Neurology 2009; 73: 1746–1751. [DOI] [PubMed] [Google Scholar]
- 57. Robert G, Le Jeune F, Lozachmeur C, et al Apathy in patients with Parkinson disease without dementia or depression: a PET study. Neurology 2012; 79: 1155–1160. [DOI] [PubMed] [Google Scholar]
- 58. Robert G, Le Jeune F, Dondaine T, et al Apathy and impaired emotional facial recognition networks overlap in Parkinson's disease: a PET study with conjunction analyses. J Neurol Neurosurg Psychiatry 2014; 85: 1153–1158. [DOI] [PubMed] [Google Scholar]
- 59. Robert GH, Le Jeune F, Lozachmeur C, et al Preoperative factors of apathy in subthalamic stimulated Parkinson disease: a PET study. Neurology 2014; 83: 1620–1626. [DOI] [PubMed] [Google Scholar]
- 60. Thobois S, Ardouin C, Lhommee E, et al Non‐motor dopamine withdrawal syndrome after surgery for Parkinson's disease: predictors and underlying mesolimbic denervation. Brain 2010; 133: 1111–1127. [DOI] [PubMed] [Google Scholar]
- 61. Santangelo G, Vitale C, Picillo M, et al Apathy and striatal dopamine transporter levels in de‐novo, untreated Parkinson's disease patients. Parkinsonism Relat Disord 2015; 21: 489–493. [DOI] [PubMed] [Google Scholar]
- 62. Isella V, Melzi P, Grimaldi M, et al Clinical, neuropsychological, and morphometric correlates of apathy in Parkinson's disease. Mov Disord 2002; 17: 366–371. [DOI] [PubMed] [Google Scholar]
- 63. Reijnders J, Scholtissen B, Weber W, Aalten P, Verhey F, Leentjens A. Neuroanatomical correlates of apathy in Parkinson's disease: a magnetic resonance imaging study using voxel‐based morphometry. Mov Disord 2010; 25: 2318–2325. [DOI] [PubMed] [Google Scholar]
- 64. Baggio H, Segura B, Garrido‐Millan J, et al Resting‐state frontostriatal functional connectivity in Parkinson's disease‐related apathy. Mov Disord 2015; 30: 671–679. [DOI] [PubMed] [Google Scholar]
- 65. Carriere N, Besson P, Dujardin K, et al Apathy in Parkinson's disease is associated with nucleus accumbens atrophy: a magnetic resonance imaging shape analysis. Mov Disord 2014; 29: 897–903. [DOI] [PubMed] [Google Scholar]
- 66. Aarsland D, Brønnick K, Alves G, et al The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson's disease. J Neurol Neurosurg Psychiatry 2009; 80: 928–930. [DOI] [PubMed] [Google Scholar]
- 67. Mayberg H. Modulating dysfunctional limbic‐cortical circuits in depression: towards development of brain‐based algorithms for diagnosis and optimised treatment. Br Med Bull 2003; 65: 193–207. [DOI] [PubMed] [Google Scholar]
- 68. Rutten S, Ghielen I, Vriend C, et al Anxiety in Parkinson's disease: symptom dimensions and overlap with depression and autonomic failure. Parkinsonism Relat Disord 2015; 21: 189–1993. [DOI] [PubMed] [Google Scholar]
- 69. Pagonabarraga J, Kulisevsky J, Strafella A, Krack P. Apathy in Parkinson's disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol 2015; 14: 518–531. [DOI] [PubMed] [Google Scholar]
- 70. Wen M‐C, Steffens D, Chen M, Zainal N. Diffusion tensor imaging studies in late‐life depression: systematic review and meta‐analysis. Int J Geriatr Psychiatry 2014; 29: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 71. de Visser L, Baars A, Lavrijsen M, van der Weerd C, van den Bos R. Decision‐making performance is related to levels of anxiety and differential recruitment of frontostriatal areas in male rats. Neuroscience 2011; 184: 97–106. [DOI] [PubMed] [Google Scholar]
- 72. Lavretsky H, Ballmaier M, Pham D, Toga A, Kumar A. Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. Am J Geriatr Psychiatry 2007; 15: 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical characteristics of neuroimaging studies in depression in PD.
Table S2. Clinical characteristics of neuroimaging studies in anxiety in PD.
Table S3. Clinical characteristics of neuroimaging studies in apathy in PD.


