Abstract
Centrosome integrity and microtubule network are crucial to the events around fertilization, including pronuclear development, migration and fusion, and the first mitotic division. The present review highlights the importance of bull spermatozoal centrosomes to function as a microtubule‐organizing center for successful fertilization and the subsequent embryonic development. Spermatozoal centrosomes need to be blended with ooplasmic pericentriolar materials accurately to nucleate and organize the sperm aster. Dysfunction of the spermatozoal centrosomes is associated with fertilization failure, which has been overcome with supplemental stimuli for oocyte activation following intracytoplasmic sperm injection in humans. Even though the spermatozoal centrosomes are functionally intact, abnormal sperm aster formation was frequently observed in vitrified‐warmed bovine oocytes, with delayed pronuclear development and migration. Treatment of the post‐warm oocytes with Rho‐associated coiled‐coil kinase inhibitor or α‐tocopherol inhibited the incidence of the abnormal aster formation, resulting in higher blastocyst yields following in vitro fertilization and culture. Thus, understanding of centrosomal function made it possible to improve the performance of advanced reproductive technologies.
Keywords: aster formation, fertilization, microtubule‐organizing center, oocyte vitrification, spermatozoal centrosomes
Introduction
Many reproductive biotechnologies have been applied to efficient production of domestic species, such as cattle and pigs, with high economic values. Those originally developed in cattle include artificial insemination (AI) and multiple ovulations and embryo transfer (MOET), and successful cryopreservation of spermatozoa (Polge & Rowson 1952) and embryos (Wilmut & Rowson 1973) made these AI and MOET technologies more practical and available for commercial use because estrus synchronization of recipients is no longer necessary. Discovery of ‘sperm capacitation’ phenomenon (Chang 1959) activated the research field of in vitro fertilization (IVF), and IVF‐derived calves were first obtained using in vivo‐matured oocytes (Brackett et al. 1982). The IVF technique was successfully combined with in vitro maturation (IVM) of immature oocytes retrieved from abattoir‐derived ovaries (Shioya et al. 1988) and in vitro culture (IVC) of the presumptive zygotes (Lu et al. 1988). Thus, large numbers of transferable bovine blastocysts can be prepared by the in vitro production (IVP) system.
Since Uehara and Yanagimachi (1976) reported that hamster oocytes microinjected with spermatozoa exhibited pronuclear development, the intracytoplasmic sperm injection (ICSI) technique has been used not only as a powerful research tool to study the fertilization process but also as one of the assisted reproductive technologies (ART) in various mammalian species, including rabbits (Iritani & Hosoi 1989), cattle (Goto et al. 1990), humans (Palermo et al. 1992) and small rodents (Kimura & Yanagimachi 1995; Hirabayashi et al. 2002). Despite the current routine use of the ICSI technique in humans to treat male factor infertility, bovine ICSI has not been very successful, probably due to failure of oocyte activation and compromised sperm chromatin remodeling, as well as technical difficulties (Hochi et al. 2011).
Further understanding of the mechanism responsible for fertilization and embryonic development would be helpful to efficiently increase the number of transferable bovine blastocysts produced by the IVP or ART system. Centrosome integrity and microtubule network are crucial to the events around fertilization, including the pronuclear development, migration and fusion, and the first mitotic division (Schatten et al. 1985). The present review highlights the importance of spermatozoal centrosomes and microtubule assembly for successful fertilization and subsequent embryonic development in cattle.
Centrosomes and Cytoskeletons
In the cellular cytoskeletal system, the microfilaments of actin (7 nm in diameter two‐standard helix) are involved in cell shape modifications and movements, the intermediate filaments (10 nm in diameter) relate to mechanical resistance to stress, and the microtubules (25 nm in diameter cylindrical bundle, composed from heterodimers of α‐ and β‐tubulin) form the spindle apparatus (Fuge 1974; Ferreira et al. 2009). The microtubules adhere to motor proteins such as dynein, dynactin and kinesin, which bind to molecules (such as enzyme or substrate) and to membranes of the organelles (such as mitochondria) and promote the movement along the microtubules (Steffen et al. 1997; Quintyne & Schroer 2002). The motor activity of dynein/dynactin‐driven transportation directs to the minus‐end, while that of kinesin‐driven transportation directs to the plus‐end. Thus, the microtubules are more directly involved in the process of organelle movement (Sun & Schatten 2006).
Typical centrioles are cylinder‐shaped structures composed from nine symmetrically oriented microtubular triplets (500‐nm in length, 200‐nm in diameter). Standard centrosome is composed from a pair of the centrioles surrounded by pericentriolar materials (PCMs; more than 100 different types of proteins), such as γ‐tubulin, centrin and pericentrin (Fig. 1). Their shapes and activities change dynamically among different stages of the cell cycle, and the centrosome structure is highly variable among different cell types and organisms. A spermatozoon of most mammalian species (except for rodents; referred later) has a pair of distinct centriolar structures, such as the proximal centriole located within the connecting piece under the sperm head and the distal centriole organized vertically to dispersedthe proximal counterpart and aligned with the sperm tail (Sathananthan et al. 1996). After gametogenesis, spermatozoon is left with centrioles but has dispersedlost most of the PCMs. In contrast, unfertilized oocyte has lost centrioles that can serve as a focal center for centrosomal aggregation, but has retained PCMs that are dispersed throughout the ooplasm (Schatten 1994). In pronuclear‐stage zygotes as well as cycling cells, the centrosomes duplicate before entry into mitosis, split and then form the poles of bipolar spindles during the prometaphase (Vorobjev & Nadezhdina 1987; Crozet et al. 2000). Because an interphase network of microtubules and the mitotic bipolar spindle are nucleated from the centrosome, the centrosome is considered as the microtubule‐organizing center (MTOC) (Glover et al. 1993).
Figure 1.
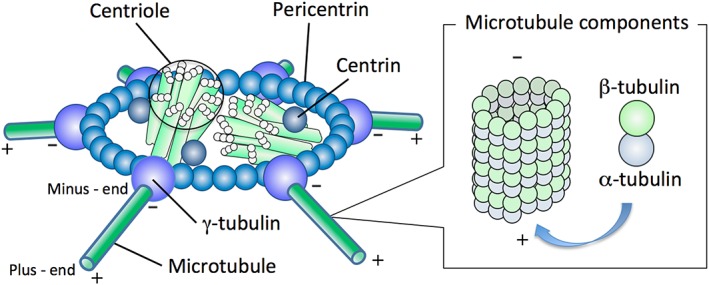
Centrosome structure. The standard centrosome is composed from a pair of centrioles, surrounded by pericentriolar materials such as pericentrin (ring structure), centrin and γ‐tubulin in either fibrous or amorphous form. The molecules of γ‐tubulin, access points for microtubules (25‐nm in diameter cylindrical bundle, composed from heterodimers of α‐ and β‐tubulin) are organized for aster formation by the conformational change through reduction of disulfide bonds in sperm centrosome and the recruitment of γ‐tubulin molecules present in the ooplasm. During aster formation, microtubules anchored with their minus‐ends are polymerized with recruiting α‐ and β‐tubulin molecules toward the distal plus‐end.
The microtubule nucleating activity of MTOCs requires the presence of γ‐tubulin. The γ‐tubulin ring complexes, composed from γ‐tubulin and other PCMs, are permanently associated with the centrosome core structure and nucleate microtubules throughout the cell cycle. Unfertilized oocytes contain γ‐tubulin that is recruited to the sperm centrosome to nucleate increasing numbers of microtubules as the sperm aster grows. Accurate recruitment of γ‐tubulin is important, as recruitment of insufficient amounts of γ‐tubulin will result in aster formation abnormalities and decreased developmental potential. Sperm aster formation and size has been analyzed during bovine fertilization and correlated to in vitro embryonic development to the blastocyst stage (Navara et al. 1996). A bull‐dependent variation was also shown in the degree of sperm‐derived centrosome and aster organization (Navara et al. 1996) that affects male fertility and early development in humans (Rawe et al. 2002).
Microtubule Assembly During Fertilization
Once oocytes arrested at prometaphase‐I resume the first meiotic division with stimulation by gonadotrophins, the nuclear envelope (germinal vesicle: GV) is disintegrated, allowing the nuclear materials to mix dispersedinto the ooplasm. Some alterations also occur in organelles such as mitochondria, cytoskeleton and cortical granules, along with disruption of oocyte‐cumulus gap junctions. The GV‐breakdown oocytes reach through metaphase‐I, anaphase‐I and telophase‐I to metaphase‐II (M‐II) stage when ovulation takes place in vivo. Then, the oocytes become arrested again at the M‐II stage till they are fertilized. During the oocyte maturation, microtubule asters appear close to the condensed chromatin in bovine oocytes (Albertini 1992; Li et al. 2005). The microtubules are nucleated at both poles anchoring the chromosomes (Schatten & Sun 2009). The lack of centrioles in the M‐II spindle poles has been documented in many mammalian species, including mice, humans and cattle (Hertig & Adams 1967; Szollosi et al. 1972; Manandhar et al. 2005). The perinuclear MTOC stability at the meiotic poles is crucial for maintaining spindle integrity to ensure accurate microtubule‐chromosome attachment and chromosome segregation. Dysfunction of the meiotic acentriolar MTOC is associated with inaccurate chromosomal segregation that may relate to female factor infertility and developmental abnormalities.
During fertilization, the centrosome brought into the M‐II stage oocyte by spermatozoon plays a crucial role in assembly of the microtubule network (sperm aster, Fig. 2A) that brings both male and female pronuclei to the center of the newly formed zygote. Motor proteins, dynactin and dynein, are located on the surface of female pronucleus and male pronucleus in bovine zygotes, respectively (Payne et al. 2003). This phenomenon has been reported in many mammalian species, including humans (Simerly et al. 1995), rhesus monkeys (Hewitson et al. 1996), rabbits (Pinto‐Correia et al. 1994) and cattle (Navara et al. 1994). Following centrosomal duplication, the microtubules are nucleated from paternal centrosomes at both poles anchoring the chromosomes during the first mitotic cleavage (Chen et al. 2003; Schatten & Sun 2009). For successful fertilization, sperm and oocyte centrosomal components need to be blended accurately to form a functional centrosome that can nucleate and organize sperm aster. Abnormalities of the spindle / MTOC function / sperm aster have been shown to directly correlate with the loss of developmental competence after IVF or ICSI, because they are crucial for migration and fusion of the pronuclei and formation of the first mitotic spindles (Schatten et al. 1985). The centrosome further serves as a unique signaling platform to recruit and distribute regulatory components and enzymes for cell cycle‐specific and adaptive regulations. Impaired MTOC function as a possible cause of male factor infertility will be referred later.
Figure 2.
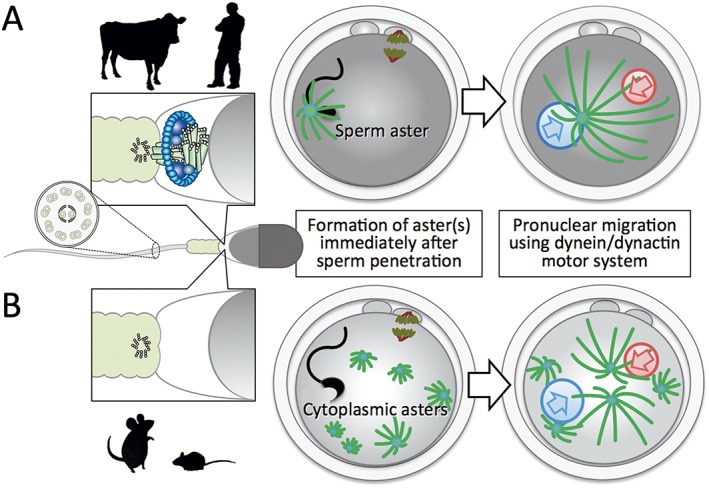
Species‐specific organization of microtubule‐organizing center (MTOC). (A) In many mammalian species, including primates and large domestic animals, spermatozoal centrosomes are brought into oocytes during fertilization and function as MTOC to form a monopolar array of microtubules, sperm aster. The well‐developed microtubule network is essential for pronuclear migration to mix the male and female genomes using the dynein/dynactin motor system. (B) In rodents, paternal centrosomes degenerate during spermiogenesis while basal body (‘9 triplets + 0’ arrangement) for axoneme (‘9 doublets + 2 singlets’ arrangement) remains intact. Microtubules assemble with pericentriolar materials scattered in ooplasm during the pronuclear stage, and acentriolar cytoplasmic multiple asters can function as MTOCs.
On the other hand, the paternal inheritance of MTOC does not occur in rodents such as the mouse (Schatten et al. 1985) and the rat (Woolley & Fawcett 1973) and the microtubule network developed from multiple cytoplasmic asters, instead of a single sperm aster, is involved in the migration and fusion of pronuclei (Fig. 2B). The rodent spermatozoa lack centrioles and majority of PCMs after the spermiogenesis (Woolley & Fawcett 1973; Manandhar et al. 1998). Thus, the pattern of centrosome inheritance during fertilization is dependent on the animal species.
Chemical Treatment in Bovine ICSI
In addition to studying the fundamental aspects around fertilization or to overcome male factor infertility, the homologous ICSI technique is used for offspring production with spermatozoa freeze‐dried and rehydrated (Wakayama & Yanagimachi 1998), retrieved from testicular tissues (Silber et al. 1995) or exposed to foreign DNA (Perry et al. 1998; Hirabayashi & Hochi 2010). However, the ICSI procedure may disturb some post‐fertilization events essential for early development, including sperm‐induced oocyte activation, epigenetic remodeling of paternal genomes, and microtubule organization for pronuclear fusion (Yoshizawa et al. 2010; Hara et al. 2011). In cattle, the ICSI technique is applicable to achieve the best utilization of genetically superior bulls with low sperm concentration and/or low motility in their ejaculate, or suboptimal reaction with the in vitro capacitation process required for IVF protocol (Ushijima & Nakane 2006).
Pretreatment of bull spermatozoa with a reducing agent dithiothreitol (DTT) induced a partial decondensation of the sperm nucleus in the ICSI oocytes (Rho et al. 1998), probably due to the stabilized and condensed structure of sperm nucleus by disulfide bonds of protamine, a specific nuclear protein in the sperm (Calvin & Bedford 1971) which has been modified. The DTT treatment also improved the proportion of oocytes with a sperm aster‐derived microtubule network on bovine ICSI (our unpublished results). The DTT not only destabilizes nuclear packaging in the sperm head, but also organizes γ‐tubulin in the sperm centrosome, from which microtubules are nucleated. The conformational change induced by reducing the disulfide bonds would facilitate the γ‐tubulin to access the microtubule components present in the ooplasm.
Unlike the IVF‐derived bovine oocytes, the majority of bovine ICSI oocytes did not show normal pattern of calcium oscillations, resulting in the failure of oocyte activation (Malcuit et al. 2006), even though sperm‐borne oocyte activating factor (SOAF), possibly phospholipase C‐ζ (Fissore et al. 1995; Wu et al. 2001), was mechanically incorporated into the oocytes. To overcome this problem, bovine ICSI oocytes have been treated to induce intracellular calcium spike with a direct current pulse(s) (Hwang et al. 2000), calcium ionophore (Keskintepe & Brackett 2000), ionomycin (Rho et al. 1998; Galli et al. 2003; Oikawa et al. 2005; Abdalla et al. 2009) or ethanol (Horiuchi et al. 2002; Oikawa et al. 2005; Abdalla et al. 2009). Because these stimuli do not induce long‐lasting oscillations, they have been combined with other chemicals, such as cycloheximide (Galli et al. 2003) or 6‐dimethylaminopurine (Rho et al. 1998; Oikawa et al. 2005) that interfere with either the re‐synthesis or the activation of the metaphase‐promoting factor (MPF), respectively. In our laboratory, the best blastocyst yield from bovine ICSI oocytes (30% vs. 40% in IVF control group) is achieved by a supplemental activation treatment composed from 5 µmol/L ionomycin for 5 min (immediately after ICSI) plus 7% ethanol for 10 min (4 h after the ICSI), without additional chemicals for MPF inactivation (Abdalla et al. 2009).
Assessment of Centrosomal Dysfunction
Interspecies assays with rodent oocytes have been used to evaluate the capacitation status via IVF (Hanada & Chang 1972) and the SOAF activity via ICSI (Rybouchkin et al. 1995). However, such rodent oocytes are not valid for assessing the functional integrity of non‐rodent spermatozoal centrosomes, because the cytoplasmic asters play a crucial role in pronuclear development, migration and fusion in the rodent zygote (Schatten et al. 1985). In fact, normal human spermatozoa did not organize the sperm aster in hamster oocytes (Hewitson et al. 1997). Therefore, heterologous ICSI using oocytes from rabbits (Terada et al. 2002, 2004) or cattle (Nakamura et al. 2001; Terada et al. 2002) has been proposed to assess the dysfunction of human spermatozoal centrosomes in relation to male infertility. Bovine assay system is more convenient to achieve, because preparation of IVM oocytes is relatively easy as the abattoir‐derived ovaries are available, and is used especially for clinical application in humans.
The proportion of bovine oocytes with human sperm aster formation is independent from semen characteristics and pronuclear formation rate on clinical IVF, but correlates with embryonic cleavage and clinical pregnancy (Yoshimoto‐Kakoi et al. 2008). Spermatozoa from globozoospermia and dysplasia of the fibrous sheath (DFS) patients have centrosomal dysfunction, as shown by the decreased aster formation rates in bovine oocytes (Nakamura et al. 2002; Rawe et al. 2002). The low aster formation rate in globozoospermia (16% vs. 68% in normal sperm) doubled to 32% when supplemental oocyte activation treatment was given with ethanol to the post‐ICSI bovine oocytes. An immunofluorescent study showed that expression of centrin is observed in only 2% of DFS patient sperm mid‐piece (Nakamura et al. 2005). The intrinsic function of spermatozoal centrosomes can be restored by a combined treatment of human dead sperm with DTT and of heterologous ICSI oocytes with paclitaxel (TaxolTM), which can act as a cytoskeletal stabilizer by enhancing microtubule polymerization; the expected effect is not found in DFS patients (Nakamura et al. 2005). Pre‐ICSI treatment of human oocytes with calcium ionophore resulted in a successful pregnancy for a couple with sperm centrosomal dysfunction (Terada et al. 2009), and such a supplemental treatment for oocyte activation is used to increase the clinical pregnancy rate. A novel challenging approach may be to replace dysfunctional centrosomes with functional donor sperm centrosomes (Mitchison & Kirschner 1984).
Hyper‐Rescue of Cryopreserved Oocytes
Successful cryopreservation of spermatozoa and embryos made bovine AI and MOET technologies more practical for commercial use. Thereafter, the bovine IVP system became a more or less routine technique with frozen semen and vitrified blastocysts. In contrast, cryopreservation of bovine oocytes, which can be combined with ART technologies such as ICSI, somatic cell nuclear transplantation or germinal vesicle transplantation, is less successful (Hwang & Hochi 2014). Low fertilization rate of cryopreserved oocytes were reported to be associated with chilling and freezing injuries, including zona hardening due to premature release of cortical granules (Carroll et al. 1990; Fuku et al. 1995) and spindle disorganization and loss or clumping of microtubules (Aman & Parks 1994). In addition, we propose a third hypothesis for cryodamage of bovine oocytes, in which multiple aster formation frequently observed in vitrified‐warmed oocytes following IVF is related to loss of ooplasmic function responsible for normal microtubule assembly (Hara et al. 2012). These multiple asters differ from cytoplasmic asters as seen in rodents, and pronuclear development and migration in such post‐warm bovine oocytes are significantly delayed.
Vitrification protocol, characterized by an extremely high cooling rate, has been well adapted to oocytes with various types of cryodevices such as open‐pulled straws (Vajta et al. 1998) or Cryotop (Kuwayama et al. 2005). Oocytes from domestic species are rich in cytoplasmic lipid droplets that can serve as energy sources but are increased to chilling sensitivity during cryopreservation. Supplementation of L‐carnitine to IVM medium of bovine oocytes has been reported to reduce the amount of cytoplasmic lipid droplets and improve the cryotolerance of the oocytes at the blastocyst yield of 34% (Chankitisakul et al. 2013), but it is still controversial whether the positive effect of L‐carnitine is reproducible (Phongnimitr et al. 2013). Supplementation of L‐carnitine to IVC medium reduced the lipid content in IVF‐derived bovine embryos and increased cryotolerance and developmental competence (Takahashi et al. 2013). Stabilizing cytoskeletal systems during vitrification may be beneficial to improve oocyte cryotolerance. Treatment of bovine IVM oocytes with paclitaxel (Morató et al. 2008) prior to vitrification improved their revivability, but the positive effect of paclitaxel on blastocyst yield is very limited.
The incidence of multiple aster formation, a possible cause for low developmental potential of vitrified‐warmed bovine oocytes as described above, can be inhibited by a short‐term culture of the post‐warm oocytes in the presence of Rho‐associated coiled‐coil kinase (ROCK) inhibitor (Y‐27632), with an improved blastocyst yield of 21% after vitrification (Hwang et al. 2013). ROCK can regulate cellular growth, adhesion, migration, metabolism and apoptosis through controlling the actin‐cytoskeletal assembly and cell contraction (Riento & Ridley 2003). Because the ROCK regulates microtubule acetylation via phosphorylation of the tubulin polymerization‐promoting protein 1, inhibition of ROCK activity resulted in increased cellular microtubule acetylation (Schofield et al. 2012). Use of an antioxidant α‐tocopherol (vitamin E) during the recovery culture also rescued the post‐warm bovine oocytes to give the maximum blastocyst yield at 37% (Yashiro et al. 2015). Interestingly, another antioxidant ascorbic acid (vitamin C) was not effective in post‐warm oocyte rescue. α‐tocopherol also inhibited the formation of multiple asters in the post‐warm oocytes, although the action mechanism is unknown. Thus, chemical treatment of bovine oocytes before or after vitrification protocol enables increases in their revivability to 20–40% when evaluated with blastocyst yield.
Conclusions
Sperm centrosomal integrity and microtubule network are crucial to post‐fertilization events, including pronuclear development, migration and fusion, and the first mitotic division. Spermatozoal centrosomes need to be blended with ooplasmic PCMs accurately to act as MTOC and organize the sperm aster. Dysfunction of human spermatozoal centrosomes can be assessed by interspecies ICSI using bovine oocytes, and supplemental stimuli for oocyte activation (e.g. ethanol and ionomycin) and/or for microtubule stabilization (e.g. DTT and paclitaxel) may improve fertilization outcome and the subsequent embryonic development. Abnormal incidence of sperm aster formation in vitrified‐warmed bovine oocytes can be inhibited by treatment either with ROCK inhibitor or α‐tocopherol, resulting in higher blastocyst yields. Thus, understanding of centrosomal function contributed to improve ART performance.
Acknowledgments
The author thanks Dr. Masumi Hirabayashi (National Institute for Physiological Sciences, Aichi, Japan) for his careful reading and comments and Mr. Kazuya Tashima (Shinshu University, Nagano, Japan) for his excellent contribution in preparing illustrations.
Hochi, S. (2016) Microtubule assembly crucial to bovine embryonic development in assisted reproductive technologies. Anim Sci J, 87: 1076–1083. doi: 10.1111/asj.12621.
References
- Abdalla H, Shimoda M, Hirabayashi M, Hochi S. 2009. A combined treatment of ionomycin with ethanol improves blastocyst development of bovine oocytes harvested from stored ovaries and microinjected with spermatozoa. Theriogenology 72, 453–460. [DOI] [PubMed] [Google Scholar]
- Albertini DF. 1992. Cytoplasmic microtubular dynamics and chromatin organization during mammalian oogenesis and oocyte maturation. Mutation Research 296, 57–68. [DOI] [PubMed] [Google Scholar]
- Aman RR, Parks JE. 1994. Effects of cooling and rewarming on the meiotic spindle and chromosomes of in vitro‐matured bovine oocytes. Biology of Reproduction 50, 103–110. [DOI] [PubMed] [Google Scholar]
- Brackett BG, Bousquet D, Boice ML, Donawick WJ, Evans JF, Dressel MA. 1982. Normal development following in vitro fertilization in the cow. Biology of Reproduction 27, 147–158. [DOI] [PubMed] [Google Scholar]
- Calvin HI, Bedford JM. 1971. Formation of disulfide bonds in the nucleus and accessory structures of mammalian spermatozoa during maturation in the epididymis. Journal of Reproduction and Fertility. Supplement 13, 65–75. [PubMed] [Google Scholar]
- Carroll J, Depypere H, Matthews CD. 1990. Freeze‐thaw‐induced changes of the zona pellucida explains decreased rates of fertilization in frozen‐thawed mouse oocytes. Journal of Reproduction and Fertility 90, 547–553. [DOI] [PubMed] [Google Scholar]
- Chang MC. 1959. Fertilization of rabbit ova in vitro . Nature 184, 466–467. [DOI] [PubMed] [Google Scholar]
- Chankitisakul V, Somfai T, Inaba Y, Techakumphu M, Nagai T. 2013. Supplementation of maturation medium with L‐carnitine improves cryo‐tolerance of bovine in vitro matured oocytes. Theriogenology 79, 590–598. [DOI] [PubMed] [Google Scholar]
- Chen SU, Lien YR, Chao KH, Ho HN, Yang YS, Lee TY. 2003. Effects of cryopreservation on meiotic spindles of oocytes and its dynamics after thawing: clinical implications in oocyte freezing ‐ a review article. Molecular and Cellular Endocrinology 202, 101–107. [DOI] [PubMed] [Google Scholar]
- Crozet N, Dahirel M, Chesne P. 2000. Centrosome inheritance in sheep zygotes: centrioles are contributed by the sperm. Microscopy Research and Technique 49, 445–450. [DOI] [PubMed] [Google Scholar]
- Ferreira EM, Vireque AA, Adona PR, Meirelles FV, Ferriani RA, Navarro PAAS. 2009. Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology 71, 836–848. [DOI] [PubMed] [Google Scholar]
- Fissore RA, Pinto‐Correia C, Robl JM. 1995. Inositol triphosphate‐induced calcium release in the generation of calcium oscillation in bovine eggs. Biology of Reproduction 53, 766–774. [DOI] [PubMed] [Google Scholar]
- Fuge H. 1974. Ultrastructure and function of the spindle apparatus microtubules and chromosomes during nuclear division. Protoplasma 82, 289–320. [DOI] [PubMed] [Google Scholar]
- Fuku E, Xia L, Downey BR. 1995. Ultrastructural changes in bovine oocytes cryopreserved by vitrification. Cryobiology 32, 139–156. [DOI] [PubMed] [Google Scholar]
- Galli C, Vassiliev I, Lagutina I, Galli A, Lazzari G. 2003. Bovine embryo development following ICSI: effect of activation, sperm capacitation and pre‐treatment with dithiothreitol. Theriogenology 60, 1467–1480. [DOI] [PubMed] [Google Scholar]
- Glover DM, Gonzalez C, Raff JW. 1993. The centrosome. Scientific American 268, 62–68. [DOI] [PubMed] [Google Scholar]
- Goto K, Kinoshita A, Tamura Y, Ogawa K. 1990. Fertilization of bovine oocytes by the injection of immobilized, killed spermatozoa. Veterinary Record 127, 517–520. [PubMed] [Google Scholar]
- Hanada A, Chang MC. 1972. Penetration of zona‐free eggs by spermatozoa of different species. Biology of Reproduction 6, 300–309. [DOI] [PubMed] [Google Scholar]
- Hara H, Abdalla H, Morita H, Kuwayama M, Hirabayashi M, Hochi S. 2011. Procedure for bovine ICSI, not sperm freeze‐drying, impairs the function of microtubule‐organizing center. Journal of Reproduction and Development 57, 428–432. [DOI] [PubMed] [Google Scholar]
- Hara H, Hwang IS, Kagawa N, Kuwayama M, Hirabayashi M, Hochi S. 2012. High incidence of multiple aster formation in vitrified‐warmed bovine oocytes after in vitro fertilization. Theriogenology 77, 908–915. [DOI] [PubMed] [Google Scholar]
- Hertig AT, Adams E. 1967. Studies on the human oocyte and its follicle: ultrastructural and cytological observation on the primary follicle. Journal of Cell Biology 34, 647–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson LC, Simerly CR, Tengowski MW, Sutovsky P, Navara CS, Haavisto AJ, et al. 1996. Microtubule and chromatin configurations during rhesus intracytoplasmic sperm injection: successes and failures. Biology of Reproduction 55, 271–280. [DOI] [PubMed] [Google Scholar]
- Hewitson LC, Haavisto A, Simerly C, Jones J, Schatten G. 1997. Microtubule organization and chromatin configurations in hamster oocytes during fertilization and parthenogenetic activation. Biology of Reproduction 57, 967–975. [DOI] [PubMed] [Google Scholar]
- Hirabayashi M, Hochi S. 2010. Generation of transgenic rats by ooplasmic injection of sperm cells exposed to exogenous DNA. Methods in Molecular Biology 597, 127–136. [DOI] [PubMed] [Google Scholar]
- Hirabayashi M, Kato M, Aoto T, Sekimoto A, Ueda M, Miyoshi I, et al. 2002. Offspring derived from intracytoplasmic injection of transgenic rat sperm. Transgenic Research 11, 221–228. [DOI] [PubMed] [Google Scholar]
- Hochi S, Abdalla H, Hirabayashi M. 2011. Intracytoplasmic sperm injection in cattle, In: Steiger SP. (ed.), In Vitro Fertilization, pp. 35–63. Nova Biomedical, New York. [Google Scholar]
- Horiuchi T, Emuta C, Yamauchi Y, Oikawa T, Numabe T, Yanagimachi R. 2002. Birth of normal calves after intracytoplasmic sperm injection of bovine oocytes: a methodological approach. Theriogenology 57, 1013–1024. [DOI] [PubMed] [Google Scholar]
- Hwang IS, Hara H, Chung HJ, Hirabayashi M, Hochi S. 2013. Rescue of vitrified‐warmed bovine oocytes with Rho‐associated coiled‐coil kinase inhibitor. Biology of Reproduction 89, 26. [DOI] [PubMed] [Google Scholar]
- Hwang IS, Hochi S. 2014. Recent progress in cryopreservation of bovine oocytes. BioMed Research International 2014, 570647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Lee E, Yoon J, Yoon BK, Lee JH, Choi D. 2000. Effects of electric stimulation on bovine oocyte activation and embryo development in intracytoplasmic sperm injection procedure. Journal of Assisted Reproduction and Genetics 17, 310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani A, Hosoi Y. 1989. Microfertilization by various methods in mammalian species. Progress in Clinical and Biological Research 294, 145–149. [PubMed] [Google Scholar]
- Keskintepe L, Brackett BG. 2000. Cryopreservation of bovine blastocysts obtained by intracytoplasmic sperm injection. Theriogenology 53, 1041–1052. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Yanagimachi R. 1995. Intracytoplasmic sperm injection in the mouse. Biology of Reproduction 52, 709–720. [DOI] [PubMed] [Google Scholar]
- Kuwayama M, Vajta G, Kato O, Leibo SP. 2005. Highly efficient vitrification method for cryopreservation of human oocytes. Reproductive Biomedicine Online 11, 300–308. [DOI] [PubMed] [Google Scholar]
- Li GP, Liu Y, Bunch TD, White KL, Aston KI. 2005. Asymmetric division of spindle microtubules and microfilaments during bovine meiosis from metaphase I to metaphase III. Molecular Reproduction and Development 71, 220–226. [DOI] [PubMed] [Google Scholar]
- Lu KH, Gordon I, Chen HB, Gallagner M, McGovern H. 1988. Birth of twins after transfer of cattle embryos produced by in vitro techniques. Veterinary Record 122, 539–540. [DOI] [PubMed] [Google Scholar]
- Malcuit C, Maserati M, Takahashi Y, Page R, Fissore RA. 2006. Intracytoplasmic sperm injection in the bovine induces abnormal [Ca2+]i responses and oocyte activation. Reproduction, Fertility and Development 18, 39–51. [DOI] [PubMed] [Google Scholar]
- Manandhar G, Schatten H, Sutovsky P. 2005. Centrosome reduction during gametogenesis and its significance. Biology of Reproduction 72, 2–13. [DOI] [PubMed] [Google Scholar]
- Manandhar G, Sutovsky P, Joshi HC, Stearns T, Schatten G. 1998. Centrosome reduction during mouse spermiogenesis. Developmental Biology 203, 424–434. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. 1984. Microtubule assembly nucleated by isolated centrosome. Nature 312, 232–237. [DOI] [PubMed] [Google Scholar]
- Morató R, Izquierdo D, Albaarracín JL, Angulta B, Palomo MJ, Jimenez‐Macedo AR, et al. 2008. Effects of pre‐treating in vitro‐matured bovine oocytes with the cytoskeleton stabilizing agent taxol prior to vitrification. Molecular Reproduction and Development 75, 191–201. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Terada Y, Horiuchi T, Emuta C, Murakami T, Yaegashi N, et al. 2001. Human sperm aster formation and pronuclear decondensation in bovine eggs following intracytoplasmic sperm injection using a Piezo‐driven pipette: a novel assay for human sperm centrosomal function. Biology of Reproduction 65, 1359–1363. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Terada Y, Horiuchi T, Emuta C, Murakami T, Yaegashi N, et al. 2002. Analysis of the human centrosomal function and the oocyte activation ability in a case of globozoospermia, by ICSI into bovine oocytes. Human Reproduction 17, 2930–2934. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Terada Y, Rawe Y, Uehara S, Morito Y, Yoshimoto T, et al. 2005. A trial to restore defective human sperm centrosomal function. Human Reproduction 20, 1933–1937. [DOI] [PubMed] [Google Scholar]
- Navara CS, First NL, Schatten G. 1994. Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis, and nuclear transfer: the role of the sperm aster. Developmental Biology 162, 29–40. [DOI] [PubMed] [Google Scholar]
- Navara CS, First NL, Schatten G. 1996. Phenotypic variations among paternal centrosomes expressed within the zygote as disparate microtubule lengths and sperm aster organization: correlations between centrosome activity and developmental success. Proceedings of the National Academy of Sciences of the United States of America 93, 5384–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T, Takada N, Kikuchi T, Numabe T, Takenaka M, Horiuchi T. 2005. Evaluation of activation treatments for blastocyst production and birth of viable calves following intracytoplasmic sperm injection. Animal Reproduction Science 86, 187–194. [DOI] [PubMed] [Google Scholar]
- Palermo GD, Joris H, Devroey P, Van Steirteghem AC. 1992. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340, 17–18. [DOI] [PubMed] [Google Scholar]
- Payne C, Rawe V, Ramalho‐Santos J, Simerly C, Schatten G. 2003. Preferentially localized dynein and perinuclear dynactin associate with nuclear pore complex proteins to mediate genomic union during mammalian fertilization. Journal of Cell Science 116, 4727–4738. [DOI] [PubMed] [Google Scholar]
- Perry AC, Wakayama T, Kishikawa H, Kasai T, Okabe M, Toyoda Y, et al. 1998. Mammalian transgenesis by intracytoplasmic sperm injection. Science 284, 1180–1183. [DOI] [PubMed] [Google Scholar]
- Phongnimitr T, Liang Y, Srirattana K, Panyawai K, Sripunya N, Treetampinich C, et al. 2013. Effect of L‐carnitine on maturation, cryo‐tolerance and embryo developmental competence of bovine oocytes. Animal Science Journal 84, 719–725. [DOI] [PubMed] [Google Scholar]
- Pinto‐Correia C, Poccia DL, Chang T, Robl JM. 1994. Dephosphorylation of sperm midpiece antigens initiates aster formation in rabbit oocytes. Proceedings of the National Academy of Sciences of the United States of America 91, 7894–7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polge C, Rowson LE. 1952. Fertilizing capacity of bull spermatozoa after freezing at –79 °C. Nature 169, 626–627. [DOI] [PubMed] [Google Scholar]
- Quintyne NJ, Schroer TA. 2002. Distinct cell cycle‐dependent roles for dynactin and dynein at centrosomes. Journal of Cell Biology 159, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawe VY, Terada Y, Nakamura S, Chillik CF, Olmedo SB, Chemes HE. 2002. A pathology of the sperm centriole response for defective sperm aster formation, syngamy and cleavage. Human Reproduction 17, 2344–2349. [DOI] [PubMed] [Google Scholar]
- Rho GJ, Kawarsky S, Johnson WH, Kochhar K, Betteridge KJ. 1998. Sperm and oocyte treatments to improve the formation of male and female pronuclei and subsequent development following intracytoplasmic sperm injection into bovine oocytes. Biology of Reproduction 59, 918–924. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. 2003. ROCKs: multifunctional kinases in cell behavior. Nature Reviews Molecular Cell Biology 4, 446–456. [DOI] [PubMed] [Google Scholar]
- Rybouchkin A, Dozortsev D, De Sutter P, Quin C, Dhont M. 1995. Intracytoplasmic injection of human spermatozoa into mouse oocytes: a useful model to investigate the oocyte‐activating capacity and the karyotype of human spermatozoa. Human Reproduction 10, 1130–1135. [DOI] [PubMed] [Google Scholar]
- Sathananthan AH, Ratnam SS, Ng SC, Tarin JJ, Gianaroli L, Trounson A. 1996. The sperm centriole: its inheritance, replication and perpetuation in early human embryos. Human Reproduction 11, 345–356. [DOI] [PubMed] [Google Scholar]
- Schatten G, Simerly C, Schatten H. 1985. Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule‐mediated motility during mammalian fertilization. Proceedings of the National Academy of Sciences of the United States of America 82, 4152–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten G. 1994. The centrosome and its mode of inheritance: The reduction of the centrosome during gametogenesis and its restoration during fertilization. Developmental Biology 165, 299–335. [DOI] [PubMed] [Google Scholar]
- Schatten H, Sun QY. 2009. The role of centrosomes in mammalian fertilization and its significance for ICSI. Molecular Human Reproduction 15, 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield AV, Steel R, Bermard O. 2012. Rho‐associated coiled‐coil kinase (ROCK) protein controls microtubule dynamics in a novel signaling pathway that regulate cell migration. Journal of Biological Chemistry 287, 43620–43629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber SJ, Van Steirteghem AC, Kiu J, Nagy Z, Tournaye H, Devroey P. 1995. Human Reproduction 10, 148–152. [DOI] [PubMed] [Google Scholar]
- Simerly C, Wu GJ, Zoran S, Ord T, Rawlins R, Jones J, et al. 1995. The paternal inheritance of the centrosome, the cell's microtubule‐organizing center, in humans, and the implications for infertility. Nature Medicine 1, 47–52. [DOI] [PubMed] [Google Scholar]
- Shioya Y, Kuwayama M, Fukushima M, Iwasaki S, Hanada A. 1988. In vitro fertilization and cleavage capability of bovine follicular oocytes classified by cumulus cells and matured in vitro . Theriogenology 30, 489–496. [DOI] [PubMed] [Google Scholar]
- Steffen W, Karki S, Vaughan KT, Vallee RB, Holzbaur EL, Weiss DG, et al. 1997. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Molecular Biology of the Cell 8, 2077–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QY, Schatten H. 2006. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 131, 193–205. [DOI] [PubMed] [Google Scholar]
- Szollosi D, Calarco P, Donahue RP. 1972. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. Journal of Cell Biology 11, 521–541. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Inaba Y, Somfai T, Kaneda M, Geshi M, Nagai T, et al. 2013. Supplementation of culture medium with L‐carnitine improves development and cryotolerance of bovine embryos produced in vitro . Reproduction, Fertility and Development 25, 589–599. [DOI] [PubMed] [Google Scholar]
- Terada Y, Hasegawa H, Takahashi A, Ugajin T, Yaegashi N, Okamura K. 2009. Successful pregnancy after oocyte activation by a calcium ionophore for a patient with recurrent intracytoplasmic sperm injection failure, with an assessment of oocyte activation and sperm centrosomal function using bovine eggs. Fertility and Sterility 91, 935. [DOI] [PubMed] [Google Scholar]
- Terada Y, Nakamura S, Hewitson L, Simerly C, Horiuchi T, Murakami T, et al. 2002. Human sperm aster formation after intracytoplasmic sperm injection with rabbit and bovine eggs. Fertility and Sterility 77, 1283–1284. [DOI] [PubMed] [Google Scholar]
- Terada Y, Nakamura S, Simerly C, Hewitson L, Murakami T, Yaegashi N, et al. 2004. Centrosomal function assessment in human sperm using heterologous ICSI with rabbit eggs: a new male factor infertility assay. Molecular Reproduction and Development 67, 360–365. [DOI] [PubMed] [Google Scholar]
- Uehara T, Yanagimachi R. 1976. Microsurgical injection of spermatozoa into hamster eggs with subsequent transformation of sperm nuclei into male pronuclei. Biology of Reproduction 15, 467–470. [DOI] [PubMed] [Google Scholar]
- Ushijima H, Nakane T. 2006. Present status and prospects for bovine intracytoplasmic sperm injection with in vitro‐matured oocytes and frozen semen. Journal of Mammalian Ova Research 23, 107–113. [Google Scholar]
- Vajta G, Holm P, Kuwayama M, Booth PJ, Jacobsen H, Greve T, et al. 1998. Open pulled straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Molecular Reproduction and Development 51, 53–58. [DOI] [PubMed] [Google Scholar]
- Vorobjev IA, Nadezhdina ES. 1987. The centrosome and its role in the organization of microtubules. International Review of Cytology 106, 227–293. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Yanagimachi R. 1998. Development of normal mice from oocytes injected with freeze‐dried spermatozoa. Nature Biotechnology 16, 639–641. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Rowson LE. 1973. The successful low‐temperature preservation of mouse and cow embryos. Journal of Reproduction and Fertility 33, 352–353. [DOI] [PubMed] [Google Scholar]
- Woolley DM, Fawcett DW. 1973. The degeneration and disappearance of the centrioles during the development of the rat spermatozoon. Anatomical Record 177, 289–301. [DOI] [PubMed] [Google Scholar]
- Wu H, Smith J, Luzzi V, Fukami K, Takenawa T, Black SL, et al. 2001. Sperm factor induce intracellular free calcium oscillations by stimulating the phosphoinositide pathway. Biology of Reproduction 64, 1338–1349. [DOI] [PubMed] [Google Scholar]
- Yashiro I, Tagiri M, Ogawa H, Tashima K, Takashima S, Hara H, et al. 2015. High revivability of vitrified‐warmed bovine mature oocytes after recovery culture with α‐tocopherol. Reproduction 149, 347–355. [DOI] [PubMed] [Google Scholar]
- Yoshimoto‐Kakoi T, Terada Y, Tachibana M, Murakami T, Yaegashi N, Okamura K. 2008. Assessing centrosomal function of infertile males using heterologous ICSI. Systems Biology in Reproductive Medicine 54, 135–142. [DOI] [PubMed] [Google Scholar]
- Yoshizawa Y, Kato M, Hirabayashi M, Hochi S. 2010. Impaired active demethylation of paternal genome in pronuclear‐stage rat zygotes produced by in vitro fertilization or intracytoplasmic sperm injection. Molecular Reproduction and Development 77, 69–75. [DOI] [PubMed] [Google Scholar]


