Abstract
Background
Currently, no test can accurately predict the development of azotemia after treatment of hyperthyroidism. Serum cystatin C concentrations (sCysC) might be less influenced by changes in body muscle mass and so better indicate the presence of concurrent chronic kidney disease (CKD) in hyperthyroidism.
Hypotheses
sCysC will be higher in hyperthyroid cats that develop azotemia compared with hyperthyroid cats that remain nonazotemic after treatment; sCysC will be higher in nonhyperthyroid cats with azotemic CKD than healthy older cats and, sCysC will decrease after treatment of hyperthyroidism.
Animals
Ninety‐one cats treated in first opinion practice.
Methods
Case–control study. sCysC were compared between hyperthyroid cats which developed azotemia within 4 months of successful treatment of hyperthyroidism (pre‐azotemic group) and hyperthyroid cats which remained nonazotemic after treatment (nonazotemic group), and between nonhyperthyroid cats with azotemic CKD and healthy older cats. sCysC were also compared between hyperthyroid cats before treatment and at time of establishment of euthyroidism. Data are presented as median [25th, 75th percentile].
Results
Baseline sCysC were not different between the pre‐azotemic and nonazotemic groups (1.9 [1.4, 2.3] mg/L versus 1.5 [1.1, 2.2] mg/L, respectively; P = .22). sCysC in nonhyperthyroid cats with azotemic CKD and healthy older cats were not significantly different (1.5 [1.0, 1.9] mg/L versus 1.2 [0.8, 1.4] mg/L, respectively; P = .16). sCysC did not change significantly after treatment of hyperthyroidism (pretreatment 1.8 [1.2, 2.3] mg/L, after treatment 1.6 [1.1, 2.4] mg/L; P = .82).
Conclusions and Clinical Importance
sCysC do not appear to be a reliable marker of renal function in hyperthyroid cats.
Keywords: Azotemia, Clinical chemistry, Clinical pathology, Endocrinology, Renal function, Thyroid, Urinary tract, Validation
Abbreviations
- CKD
chronic kidney disease
- CV
coefficient of variation
- GFR
glomerular filtration rate
- PENIA
particle‐enhanced nephelometric immunoassay
- PETIA
particle‐enhanced turbidimetric assay
- sCysC
serum cystatin C concentrations
- TT4
total thyroxine
- UPC
urine protein:creatinine ratio
- USG
urine specific gravity
Hyperthyroidism can complicate the diagnosis of CKD, because it results in an increased glomerular filtration rate (GFR)1 and decreased body muscle mass.2 This leads to a decrease in serum creatinine concentrations, which can “mask” the presence of concurrent azotemic CKD in hyperthyroidism. As a result, many hyperthyroid cats with CKD only develop azotemia after treatment, once GFR and body muscle mass have normalized. Currently, there is no single test that can reliably predict the development of azotemia after treatment for hyperthyroidism. Identification of hyperthyroid cats with concurrent, but masked, CKD could be important, because reliable information pretreatment could influence the advice veterinarians give owners regarding the treatment options and subsequent monitoring, to avoid iatrogenic hypothyroidism,3 and would allow the institution of appropriate treatment strategies for CKD.
Cystatin C is a low molecular weight protein which is synthesized at a stable rate by most nucleated cells and is freely filtered by the glomeruli,4 therefore, serum cystatin C concentrations (sCysC) are inversely proportional to GFR. Cystatin C could better reflect GFR in hyperthyroidism because it is produced at a constant rate by all nucleated cells, and therefore should be less affected by changes in body muscle mass. However, hyperthyroidism is also associated with increased sCysC in hyperthyroid humans and cats,5, 6 which could confound its use as a marker of GFR and concurrent CKD. An automated particle enhanced turbidimetric assay (PETIA) for the measurement of cystatin C was recently validated for use in feline urine,7 however, the PETIA used in the aforementioned study has not yet been validated for the measurement of serum cystatin C in cats. A human cystatin C particle‐enhanced nephelometric immunoassay (PENIA) was recently validated for use in feline serum, and a small pilot study demonstrated that serum cystatin C concentrations were higher in cats with CKD than healthy control cats.8 However, the PENIA requires the use of a specialized immunonephelometer, whereas the PETIA can be performed using standard automated analyzers available in many commercial laboratories.
The first aim of this study was to validate a human cystatin C PETIA for use in feline serum. Using this assay, we then aimed to compare sCysC in hyperthyroid cats, with and without concurrent, but masked, azotemic CKD, both before and after treatment, to evaluate sCysC as a marker of CKD in hyperthyroidism. Finally, we also compared sCysC between healthy older cats and nonhyperthyroid cats with azotemic CKD, to establish biological validity of the PETIA.
Methods
Validation of the PETIA for Measurement of Cystatin C in Feline Serum
Serum cystatin C concentrations were measured by an automated analyzer (Olympus AU400, Beckman Coulter, High Wycombe, UK) using a human PETIA method.1 Precision of the human PETIA was assessed by evaluating intra‐ and interassay coefficients of variation (CV) for serum samples with low, medium, and high concentrations of cystatin C. For intra‐assay precision, three replicates of each sample were evaluated within the same run. For assessment of interassay variability, pooled feline serum samples were evaluated in triplicate on 3 consecutive working days. Specificity of the assay was assessed by serial dilution of a serum sample with a high concentration of cystatin C with a serum sample containing lower concentrations of cystatin C, to avoid changes to the sample matrix. The limit of blank was determined by measurement of cystatin C concentrations in deionized water (diH2O), which was evaluated in 5 samples on 3 consecutive working days. The limit of blank was calculated as the mean interpolated cystatin C concentration in diH2O + 2 × standard deviation of the cystatin C concentration in diH2O.9
Clinical Study
Newly diagnosed nonazotemic (plasma creatinine concentration <2.0 mg/dL) hyperthyroid cats (plasma total thyroxine (TT4)>55 nmol/L) were recruited from 2 London‐based first opinion practices (People's Dispensary for Sick Animals, Bow and the Beaumont Sainsbury Animals' Hospital, Camden) between March 2010 and June 2013. Blood and urine samples were collected from these cats as part of a geriatric screening and healthcare programme at the time of diagnosis with the consent of the owner. The Ethics and Welfare Committee of the Royal Veterinary College approved the diagnostic protocol. Jugular venous blood samples were collected and placed in heparinized and nonanticoagulated tubes, and urine samples collected by cystocentesis. Samples were kept at 4°C before processing which occurred within 6 hours of collection. Blood samples were centrifuged at 2016 × g for 10 minutes to enable separation of plasma and serum from cellular components. Heparinized plasma was submitted to a single external laboratory2 for biochemical analysis including total thyroxine concentrations (TT4). Residual serum was stored at −80°C until batch analysis of sCysC. Residual serum was also used to measure TT4 by enzyme immunoassay.10 , 3 Urine samples underwent full in‐house urinalysis including measurement of urine specific gravity (USG) by refractometry, dipstick analysis and urine sediment examination. If bacteria or pyuria (>5 white blood cells/1000× field) was identified on sediment examination the patient was excluded from the study. Hyperthyroid cats treated with glucocorticoids were also excluded.
Hyperthyroid cats were treated with anti‐thyroid medication (carbimazole or methimazole) alone, or in combination with thyroidectomy, and were monitored for a 4 months period after successful treatment of hyperthyroidism (TT4 < 40 nmol/L). Blood and urine samples were obtained at the time of establishment of euthyroidism and after this monitoring period. sCysC were measured at baseline and the time of establishment of euthyroidism only (usually 4–8 weeks after starting treatment).3 In hyperthyroid cats, renal azotemia was defined as a plasma creatinine concentration >2.0 mg/dL (the upper limit of the first commercial laboratory2 reference interval, derived in an internal unpublished study, Federico Sacchini, personal communication) in conjunction with inadequate urine concentrating ability (USG < 1.035), or persistent azotemia on two or more consecutive occasions (usually approximately 4 weeks apart) without evidence of a prerenal cause. Hyperthyroid cats which developed azotemia within the 4 months follow‐up period were defined as pre‐azotemic, and were presumed to have had concurrent, but masked, azotemic CKD at the time of diagnosis. All other hyperthyroid cats were defined as nonazotemic.
In addition, blood and urine samples were obtained from cats at 3 UK first‐opinion practices between March 2013 and April 2015, as part of a free‐of‐charge screening programme. Samples from these practices were used to establish the healthy older cat and nonhyperthyroid azotemic CKD groups. The Ethics and Welfare Committee of the Department of Veterinary Medicine at the University of Cambridge approved the diagnostic protocol (project code CR56). To be included, cats had to be at least 8 years old, and have no known major systemic diseases (eg cardiac disease, diabetes mellitus, or hyperthyroidism). Exclusion criteria included feeding of a low protein low phosphate (renal care) diet, recent or ongoing treatment with corticosteroids, diuretics or angiotensin converting enzyme inhibitors, and recent or concurrent intravenous fluid therapy at the time of sampling. Blood samples (in EDTA and nonanticoagulated tubes) were taken by jugular venepuncture and urine samples were taken by cystocentesis if possible. If cystocentesis was not possible, the owners were asked to obtain a free‐catch urine sample and submit it for analysis within 3 days of blood sampling. Blood and urine samples were submitted to a commercial laboratory3 for complete blood count, serum biochemistry including TT4 (by enzyme immunoassay)10 and urinalysis including urine protein:creatinine ratio (UPC). Urinalysis included measurement of USG by refractometry, urine dipstick, and sediment analysis. Residual serum was stored at −80°C until batch analysis of sCysC.3 Cats were excluded from further analysis if; TT4 was >40 nmol/L, there was evidence of bacteriuria, pyuria (>5 white blood cells/1000× field), or gross hematuria, there was evidence of severe systemic illness on hematology and biochemistry, or if the samples were more than 3 days old at the time of sample analysis.
Nonhyperthyroid cats were classified as having azotemic CKD if they had a serum creatinine concentration >1.7 mg/dL (the upper limit of the second commercial laboratory3 reference interval, derived in an internal unpublished study of 30 cats) with concurrent USG < 1.035. Cats that were nonazotemic, with a UPC < 0.4 and no clinical history of disease (except for dental disease or degenerative joint disease) were included in the healthy older cat group. Nonazotemic cats with a clinical history of disease were excluded from the healthy older cat group.
Statistical Analysis
Using the Mann‐Whitney U‐test, serum cystatin C concentrations were compared between; hyperthyroid cats which developed renal azotemia in the follow up period (pre‐azotemic) and those which remained nonazotemic throughout the follow up period (nonazotemic), hyperthyroid cats and healthy older cats, and nonhyperthyroid cats with azotemic CKD and healthy older cats. Serum cystatin C concentrations in hyperthyroid cats before treatment and at the time of establishment of euthyroidism were compared using the Wilcoxon signed‐rank test. Correlations between baseline serum concentrations of TT4 and cystatin C (in all cats), and between serum concentrations of creatinine and cystatin C (in untreated hyperthyroid cats only), were assessed by Spearman's correlation coefficient. Data are presented as median [25th, 75th percentile] and statistical significance was defined as P < .05.
Results
The PETIA for serum cystatin C demonstrated excellent precision and reproducibility (CV <10%) at all levels tested (Table 1). The assay was linear in the range 0.5–3.5 mg/L (r 2 = 0.99) and the limit of blank was determined to be <0.01 mg/L.
Table 1.
Intra‐ and interassay coefficients of variation (CV) at low, medium, and high serum concentrations of cystatin C calculated using a human particle enhanced turbidimetric assay
| Cystatin C Concentration | Intra‐assay Variability (n = 3) | Interassay Variability (n = 3) | ||
|---|---|---|---|---|
| Mean Cystatin C Concentration (mg/L) | CV (%) | Mean Cystatin C Concentration (mg/L) | CV (%) | |
| Low | 0.79 | 1.4 | 0.80 | 2.9 |
| Medium | 1.60 | 0.8 | 1.66 | 8.2 |
| High | 2.97 | 0.8 | 3.03 | 2.7 |
Fifty‐five hyperthyroid cats were included in the study, 21 of which developed azotemia within 4 months of successful treatment of hyperthyroidism (pre‐azotemic group). Therefore, there were 34 hyperthyroid cats included in the nonazotemic group. In addition, 24 healthy older cats (including 1 cat diagnosed with mild entropion) and 12 cats with azotemic CKD were recruited to the study. Baseline clinicopathological data for these 4 groups are shown in Table 2. Pre‐azotemic and nonazotemic hyperthyroid cats were age matched (Table 2, P = .68), however, the hyperthyroid group was significantly older than the healthy older cat group (Table 2, P < .001). There was no significant difference in the age of the nonhyperthyroid azotemic CKD group and the healthy older cat group (Table 2, P = .25).
Table 2.
Selected baseline clinicopathological data in initially nonazotemic hyperthyroid cats that developed azotemia within 4 months of successful treatment of hyperthyroidism (pre‐azotemic), hyperthyroid cats that remained nonazotemic throughout the study period (nonazotemic), nonhyperthyroid cats with azotemic CKD, and healthy older cats
| Variable | Hyperthyroid | Nonhyperthyroid Azotemic CKD | Healthy Older Cat | |
|---|---|---|---|---|
| Pre‐azotemic | Nonazotemic | |||
| Age (years) | 15.1 [12.3, 16.2] | 14.8 [12.9, 16.2] | 12.5 [11.3, 16.1] | 12.0 [10.5, 14.0] |
| Serum total thyroxine concentrations (nmol/L) | 82.1 [62.4, 117.9] | 108.8 [73.0, 177.8] | 23.0 [18.6, 27.5] | 21.2 [17.6, 24.8] |
| Serum/plasma blood urea nitrogen concentrations (mg/dL) | 34.7 [29.7, 46.2] | 25.2 [23.0, 31.6] | 42.3 [29.7, 58.5] | 28.3 [24.9, 34.4] |
| Serum/plasma creatinine concentrations (mg/dL) | 1.4 [1.1, 1.6] | 1.1 [0.9, 1.3] | 2.2 [1.9, 2.8] | 1.4 [1.2, 1.6] |
| Urine specific gravity | 1.027 [1.020, 1.039] | 1.031 [1.019, 1.041] | 1.022 [1.017, 1.028] | 1.044 [1.034, >1.050] |
Normal reference interval for serum total thyroxine concentrations is 7–45 nmol/L.10 Data are presented as median [25th, 75th percentiles].
In hyperthyroid cats, sCysC were not different between the pre‐azotemic and azotemic groups at baseline (1.9 [1.4, 2.3] mg/L versus 1.5 [1.1, 2.2] mg/L respectively; P = .22, Fig 1), whereas plasma creatinine concentrations were significantly higher in pre‐azotemic cats compared with nonazotemic hyperthyroid cats (Table 2, P < .001). Urine specific gravity data were available in 17 pre‐azotemic and 24 nonazotemic hyperthyroid cats, and in 23 healthy older cats and 12 azotemic CKD cats (Table 2).
Figure 1.
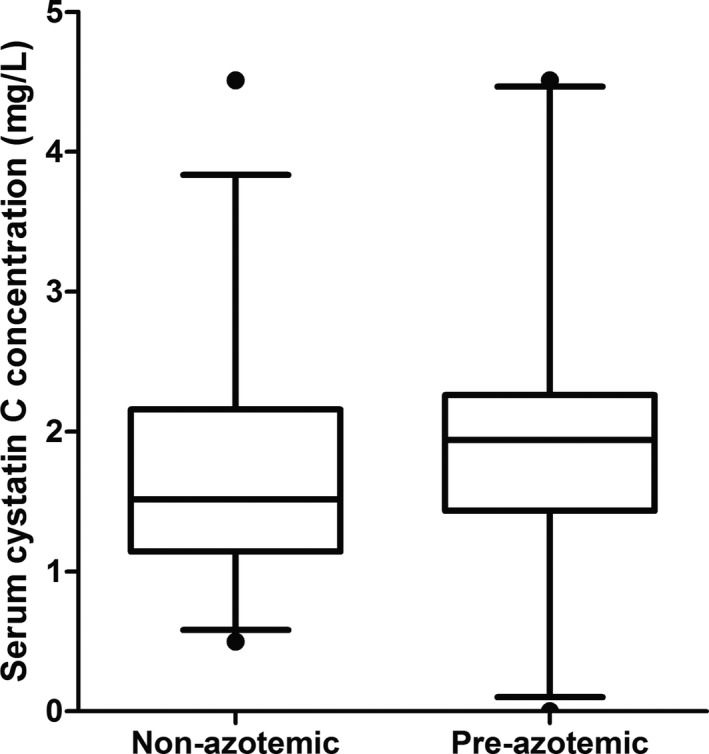
Box and whisker plots showing baseline serum cystatin C concentrations in a group of hyperthyroid cats which remained nonazotemic after treatment (n = 34) and initially nonazotemic hyperthyroid cats which developed azotemia within 4 months of successful treatment of hyperthyroidism (n = 21). Whiskers represent the 5th and 95th percentiles and circles represent outliers. Serum cystatin C concentrations were not significantly different between the 2 groups (P = .22).
Two cats were treated with 2.5 mg methimazole daily, 22 cats received 5 mg methimazole daily, 25 cats received 10 mg methimazole daily and 3 cats received 15 mg methimazole daily (mostly divided into 2 doses). In addition, 1 cat was treated with 15 mg carbimazole daily (divided into 3 doses) and 2 cats were treated by thyroidectomy. At the time of establishment of euthyroidism, serum TT4 concentrations in the pre‐azotemic and nonazotemic hyperthyroid groups were 13.0, [5.0, 24.9] nmol/L and 10.7, [3.7, 17.9] nmol/L, respectively. Six (of 21) pre‐azotemic and 12 (out of 35) nonazotemic hyperthyroid cats had a serum TT4 concentration below the lower limit of the reference interval (7 nmol/L) at the time of establishment of euthyroidism, however, evaluation of serum TSH concentrations was not performed to confirm or rule out iatrogenic hypothyroidism. sCysC did not change significantly between baseline and the time of establishment of euthyroidism (pretreatment 1.8 [1.2, 2.3] mg/L, after treatment 1.6 [1.1, 2.4] mg/L; P = .82, Fig 2), whereas plasma creatinine concentrations increased significantly over this time (1.2 [1.0, 1.4] mg/dL versus 1.7 [1.3, 2.0] mg/dL; P < .001). If the nonazotemic group was analyzed separately, there was still no significant change in sCysC after treatment (pretreatment 1.5 [1.1, 2.2] mg/L, after treatment 1.5 [1.0, 2.4] mg/L; P = .86). Similarly, there was no significant change in sCysC if only the pre‐azotemic group were analyzed separately (P = .65).
Figure 2.
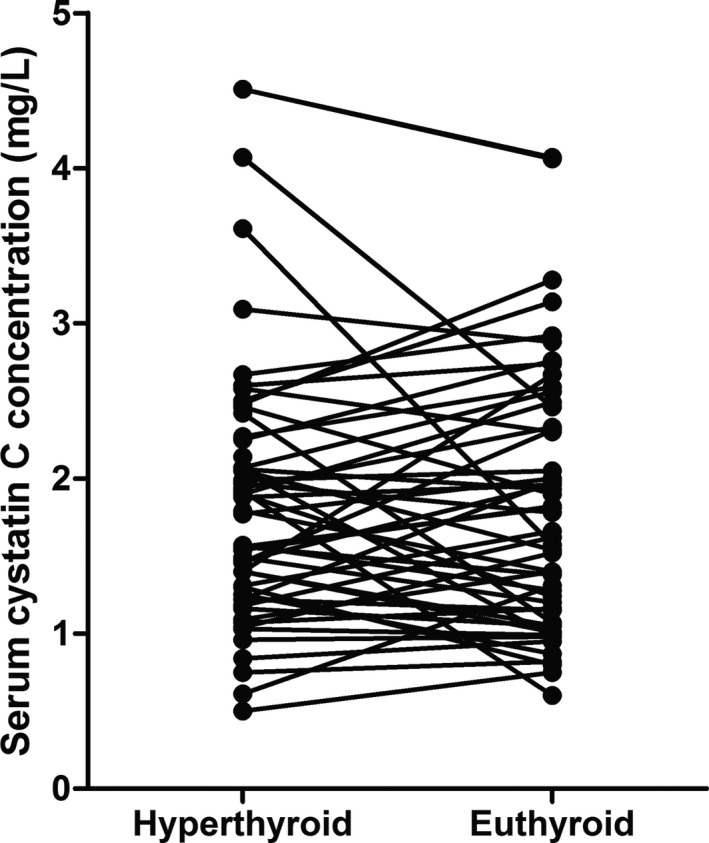
Line chart showing serum cystatin C concentrations in hyperthyroid cats before treatment (hyperthyroid) and at time of establishment of euthyroidism (euthyroid). Serum cystatin C concentrations were not significantly different between the hyperthyroid and euthyroid time points (P = .82).
sCysC in healthy older cats ranged from 0.5 mg/L to 2.7 mg/L, and were greater in hyperthyroid cats than healthy older cats (1.8 [1.2, 2.2] mg/L versus 1.2 [0.8, 1.4] mg/L; P = .001, Fig 3). When considering only nonazotemic hyperthyroid cats, the sCysC were still higher than those of healthy older cats (1.5 [1.1, 2.2] mg/L versus 1.2 [0.8, 1.4] mg/L; P = .017). However, sCysC were not significantly different between nonhyperthyroid cats with azotemic CKD and healthy older cats (nonhyperthyroid azotemic CKD group 1.5 [1.0, 1.9] mg/L, healthy older group 1.2 [0.8, 1.4] mg/L; P = .16, Fig 3). Conversely, serum creatinine concentrations were significantly higher in the nonhyperthyroid azotemic CKD group than the healthy older cat group (P < .001, Table 2). No nonhyperthyroid azotemic CKD cats had a sCysC above the highest value recorded in a healthy older cat (2.7 mg/L). At baseline, 2/21 pre‐azotemic and 3/35 nonazotemic hyperthyroid cats had a sCysC above the highest value observed in healthy older cats (2.7 mg/L). sCysC were also weakly positively correlated with plasma creatinine concentrations (rs = 0.320, n = 55; P = .018) and serum TT4 (rs = 0.307, n = 91; P = .005).
Figure 3.
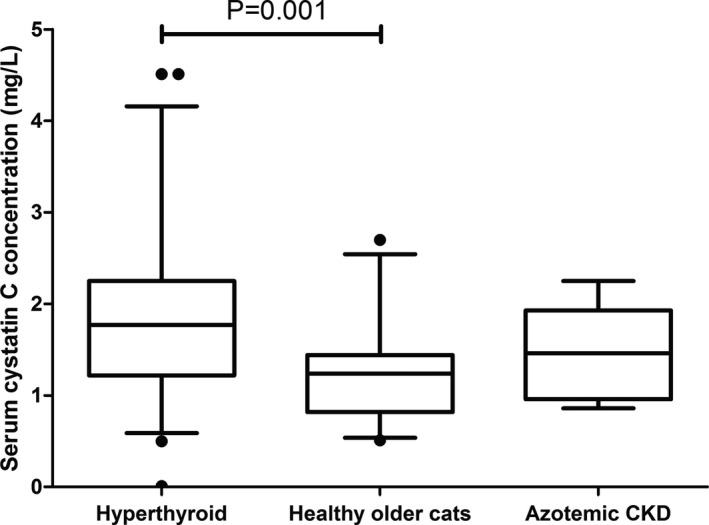
Box and whisker plots showing serum cystatin C concentrations in a group of initially nonazotemic (serum creatinine concentration <2.0 mg/dL) hyperthyroid cats (n = 55), healthy nonazotemic older cats (n = 24, serum creatinine concentration <1.7 mg/dL) and nonhyperthyroid cats diagnosed with azotemic CKD (n = 12). Whiskers represent the 5th and 95th percentiles and circles represent outliers. Serum cystatin C concentrations were significantly higher in hyperthyroid cats than healthy nonazotemic older cats (P = .001). Serum cystatin C concentrations were not significantly different between the healthy older cat and nonhyperthyroid azotemic CKD groups (P = .16).
Discussion
The human PETIA for serum cystatin C demonstrated excellent precision and reproducibility and appeared linear. Binding of the monoclonal anti‐cystatin C antibody to feline cystatin C was not definitively demonstrated by western blotting in this study, although biological validity was suggested, based on the expected, albeit weak, positive correlation between sCysC and serum creatinine concentrations and TT4. This suggests that the avian anti‐human cystatin C antibody does cross‐react with feline cystatin C, although we could not determine how much of the total feline cystatin C was detected by the human PETIA. Recently, an alternative PETIA, which utilizes rabbit anti‐human antibodies against cystatin C, was also validated for use in feline serum in a preliminary study.11 Further studies to compare the sCysC between these 2 assays would be of interest and could help to validate the PETIA used in this study.
The primary aim of this study was to assess if serum cystatin C concentrations might be a marker of concurrent, but masked, azotemic CKD in hyperthyroidism. Although a recent study has demonstrated that the development of azotemia after treatment of hyperthyroidism is not associated with a reduced survival time in cats that are euthyroid after treatment, there is an association between the development of azotemia and reduced survival time in cats with iatrogenic hypothyroidism.3 Therefore, the identification of hyperthyroid cats which have concurrent CKD would be helpful because the development of iatrogenic hypothyroidism in these cats could lead to a reduced survival time, and therefore the treatment plan might need to be altered to avoid hypothyroidism (perhaps by starting on a lower dose of anti‐thyroid medication or giving a lower dose of radioiodine). Various studies have attempted to identify reliable markers of CKD in hyperthyroidism,12, 13, 14, 15, 16 however, no single reliable test has been identified. Early studies suggested that measurement of GFR might be useful in predicting the development of azotemia after treatment of hyperthyroidism,17 however, other studies have suggested that measurement of GFR alone is not a reliable predictor of this.18, 19 Serum creatinine concentrations are likely to be poor markers of GFR, and hence the presence of concurrent CKD, partly due to the changes in body muscle mass that occur in hyperthyroidism. The use of sCysC as a marker of GFR might be advantageous in hyperthyroidism because cystatin C is produced by all nucleated cells rather than just myocytes. One previous study identified an association between hyperthyroidism and increased sCysC in cats,6 which could confound the use of sCysC as a marker of renal function. However, the presence of concurrent, but masked, azotemic CKD, which in turn might have increased sCysC, was not fully ruled out in the aforementioned study. In this study, sCysC were not significantly different between hyperthyroid cats with and without concurrent, but masked, azotemic CKD, therefore sCysC might not be a useful marker of GFR and renal function in hyperthyroidism. In addition, the increased sCysC identified in nonazotemic hyperthyroid cats suggests that sCysC are increased in hyperthyroid cats independent of the presence of concurrent, but masked, azotemic CKD.
It is likely that the increased sCysC associated with hyperthyroidism are secondary to increased cellular production of cystatin C associated with the hypermetabolic state. The weak positive correlation that existed between sCysC and TT4 in this study would support this conclusion. Increased cellular production of cystatin C must also outweigh the increased renal clearance of cystatin C that would be expected in hyperthyroidism secondary to elevated GFR.1 Alternatively, hyperthyroidism could also be associated with increased renal reabsorption of cystatin C. Cystatin C is usually freely filtered by the glomeruli and mostly reabsorbed and catabolized by the proximal tubules of the kidney, therefore, tubular damage (in CKD) could lead to decreased tubular reabsorption of cystatin C.4 Other work from our laboratory has indicated that overall urinary cystatin C excretion (accounting for renal clearance and tubular reabsorption) is decreased in early CKD (IRIS stage 2 and 3),7 although another study reported that cats with CKD had increased overall urinary cystatin C excretion.8 Further studies of renal handling of cystatin C in hyperthyroid cats and nonhyperthyroid cats with azotemic CKD are warranted to ascertain if altered renal reabsorption of cystatin C occurs in these conditions which could contribute to altered sCysC in these patients.
Based on the results of this study, sCysC also do not appear to be reliable markers of renal function in nonhyperthyroid cats with azotemic CKD. The reason why sCysC do not appear to be a reliable marker of renal function in nonhyperthyroid cats is, however, unclear at this time. Although one previous small study indicated that sCysC are increased in cats with azotemic CKD,8 a subsequent study calculated a reference interval for sCysC from healthy older cats which encompassed serum cystatin C concentrations previously observed in many cats with azotemic CKD.8, 20 This could suggest that the sensitivity of sCysC for the detection of CKD is poor. Furthermore, 2 more recent preliminary studies have indicated that there is a poor correlation between sCysC and GFR11 and the presence of CKD.21 This could be secondary to altered cellular production, metabolism, renal reabsorption and elimination, or elimination of cystatin C from the body via other mechanisms (for example, through the gastrointestinal tract) in CKD. Given the results of our previous study,7 altered renal reabsorption and elimination of cystatin C could be contributory to altered sCysC in CKD and the poor performance of sCysC as a test for azotemic CKD in cats.
Serum cystatin C concentrations were also assessed before treatment and at the time of establishment of euthyroidism, and despite sCysC being increased in hyperthyroidism in this study, the sCysC did not decrease after successful treatment. This could reflect a combination of the opposing effects of treatment of hyperthyroidism on GFR, which decreases after treatment and thus would cause an increase in sCysC, and cellular production of cystatin C, which would also decrease and thus would decrease sCysC. The overall effect might therefore be that sCysC remain unchanged after treatment, however, this does not explain why sCysC are increased in hyperthyroidism overall. As stated earlier, increased cellular production of cystatin C must outweigh the increased renal clearance of cystatin C that would be expected in hyperthyroidism secondary to elevated GFR, therefore leading to an elevated sCysC in hyperthyroid cats overall, when compared with healthy older cats. Increased cellular production of cystatin C might occur for a prolonged period in hyperthyroid cats after restoration of euthyroidism, which could account for the lack of a decrease in sCysC up to the time of establishment of euthyroidism. Further longitudinal studies evaluating the change in sCysC over a longer time period after treatment of hyperthyroidism are warranted to investigate this.
An additional confounding factor in the evaluation of sCysC after treatment of hyperthyroidism could be the development of iatrogenic hypothyroidism, because hypothyroidism is associated with decreased sCysC in human patients.5 Although some cats had low serum TT4 concentrations after treatment in this study, unfortunately, assessment of thyroid stimulating hormone concentrations, to rule out iatrogenic hypothyroidism, was not possible, due to financial constraints. The change in sCysC after treatment of hyperthyroidism remained nonsignificant if cats with subnormal TT4 concentrations after treatment were excluded from the analysis (data not shown), therefore the effect of iatrogenic hypothyroidism on sCysC after treatment is unlikely to be a significant confounding factor.
A limitation of this study was the lack of GFR measurements in our healthy older cats. Serum creatinine concentrations remain within the laboratory reference intervals in experimental models when 75–83% of renal function has been lost,22 therefore, it is possible that some of the cats included in the healthy older cat group and the nonazotemic hyperthyroid group might have had subclinical, nonazotemic CKD. This could have confounded the comparison in sCysC between healthy older cats and nonhyperthyroid cats with azotemic CKD, and between pre‐azotemic and nonazotemic hyperthyroid cats. It has been speculated that serum symmetrical dimethylarginine (SDMA) concentrations are a more sensitive indicator of reduced GFR,23 and therefore determination of SDMA concentrations might have allowed us to detect early nonazotemic CKD in some of the healthy older cats and nonazotemic hyperthyroid cats in this study. However, the effect of thyroid function on SDMA concentrations is currently unknown, therefore, SDMA could be subject to the same limitations as creatinine for the determination of renal function in hyperthyroid cats. Unfortunately, measurement of GFR was not possible within the first opinion clinics where these samples were obtained, and determination of serum SDMA concentrations was not available commercially at the time of the study.
The samples were obtained over a different time period for the hyperthyroid cats (2010–2013) and the healthy older cats and nonhyperthyroid azotemic CKD cats (2013–2015), therefore the storage time of the samples (at −80°C) differed between these groups. This would only have influenced the comparison between hyperthyroid cats and healthy older cats, however, given that the findings in this study concur with those of a previous study,6 it seems unlikely that this was a significant limiting factor in this study.
A further limitation of this study was that hyperthyroid cats were significantly older than healthy older cats, which could have confounded the results of this analysis because older cats would have been more likely to have concurrent nonazotemic CKD, which might have increased sCysC. However, no significant correlation between age and sCysC was identified in this study (data not shown) or a previous study.20
In this study, the serum creatinine concentrations were measured at 2 different commercial laboratories which had different upper limits of the reference interval for serum creatinine concentration. As a result, the inclusion criteria for the hyperthyroid (serum creatinine concentration <2.0 mg/dL) and the healthy older cat (serum creatinine concentration <1.7 mg/dL) groups were different, which could have confounded the comparison of sCysC between these groups. However, patient results should be compared against reference intervals derived in the same laboratory, since there can be significant differences in results obtained between laboratories,24 therefore, it is appropriate to utilize the laboratory specific upper limit of the reference interval rather than classify the cats utilizing an arbitrary cutoff value. Furthermore, higher sCysC have been reported in hyperthyroid cats compared with healthy cats previously, which concurs with the findings of our study.6
Finally, samples from healthy older cats and nonhyperthyroid cats with azotemic CKD in this study were up to 3 days old at the time of submission. One previous study has identified a small, likely clinically insignificant (1.9%), increase in sCysC after storage of samples at room temperature for a 24 hours period,25 however, it is currently not known if longer storage would result in a more clinically relevant increase in sCysC. A previous study from our laboratory reported that cystatin C is stable in urine for up to 3 days,7 however stability of sCysC was not assessed as part of this study. In this study, submission of samples within a 24 hours period was not possible from cats in the healthy older cat and nonhyperthyroid azotemic CKD groups, since these samples were submitted from first opinion clinics off site. In addition, the age of the samples in the healthy older cat group and the nonhyperthyroid azotemic CKD group at the time of submission was not significantly different between the groups, therefore, it is unlikely that this would have confounded the comparison of sCysC between these groups. It is possible that the inclusion of more older samples in the healthy older cat group might have confounded the comparison of sCysC with the hyperthyroid group, however, our findings concur with those of a previous study.6 Comparison of sCysC between pre‐azotemic and nonazotemic hyperthyroid cats was not confounded in this study since these samples were all frozen to −80°C within 6 hours of sampling. Further studies to examine the effect on sCysC of storage of samples at room temperature for longer than a 24 hours period are warranted.
In conclusion, the human PETIA for cystatin C was successfully validated for use in feline serum, however, the findings of this study indicate that serum cystatin C concentrations are increased in hyperthyroidism, independent of the presence of masked, azotemic CKD, which might limit the utility of serum cystatin C concentrations as a marker of CKD in hyperthyroid cats. The reason why serum cystatin C concentrations are a poor marker of GFR in hyperthyroid and nonhyperthyroid cats remains unknown.
Acknowledgments
The authors acknowledge the clients and staff at the Beaumont Sainsbury Animals Hospital and PDSA in Bow for participating in the study. We also acknowledge Miranda Wright, Alexa Selwyn, Vanessa Nichols, Rachel Watson, Martha Cannon and colleagues (from the Oxford Cat Clinic) who submitted samples for this study, and Nicola Lötter for providing follow‐up data on the hyperthyroid cats.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Some results of this study were presented at the Society for Comparative Endocrinology bi‐annual meeting, French Lick, Indiana, 2015.
Footnotes
Gentian, Moss, Norway
IDEXX Laboratories, Wetherby, UK
Central Diagnostic Services, University of Cambridge, UK
References
- 1. Adams WH, Daniel GB, Legendre AM. Investigation of the effects of hyperthyroidism on renal function in the cat. Can J Vet Res 1997;61:53–56. [PMC free article] [PubMed] [Google Scholar]
- 2. Shiel RE, Mooney CT. Testing for hyperthyroidism in cats. Vet Clin North Am Small Anim Pract 2007;37:671–691, vi. [DOI] [PubMed] [Google Scholar]
- 3. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med 2010;24:1086–1092. [DOI] [PubMed] [Google Scholar]
- 4. Uchida K, Gotoh A. Measurement of cystatin‐C and creatinine in urine. Clin Chim Acta 2002;323:121–128. [DOI] [PubMed] [Google Scholar]
- 5. den Hollander JG, Wulkan RW, Mantel MJ, Berghout A. Is cystatin C a marker of glomerular filtration rate in thyroid dysfunction? Clin Chem 2003;49:1558–1559. [DOI] [PubMed] [Google Scholar]
- 6. Ghys LF, Paepe D, Taffin ER, et al. Serum and urinary cystatin C in cats with feline immunodeficiency virus infection and cats with hyperthyroidism. J Feline Med Surg 2016; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams TL, Archer J. Evaluation of urinary biomarkers for azotaemic chronic kidney disease in cats. J Small Anim Pract 2016;57:122–129. [DOI] [PubMed] [Google Scholar]
- 8. Ghys LF, Meyer E, Paepe D, et al. Analytical validation of a human particle‐enhanced nephelometric assay for cystatin C measurement in feline serum and urine. Vet Clin Pathol 2014;43:226–234. [DOI] [PubMed] [Google Scholar]
- 9. Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev 2008;29(Suppl 1):S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 10. Williams T, Archer J. Validation of an automated enzyme immunoassay for the measurement of serum total thyroxine in cats. Vet Clin Pathol 2016;45:148–153. [DOI] [PubMed] [Google Scholar]
- 11. Ghys LFE, Paepe D, Lefebvre HP, et al. Evaluation of cystatin C for the detection of chronic kidney disease in cats. J Vet Intern Med 2016;30:381 (abstract). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lapointe C, Belanger MC, Dunn M, et al. N‐acetyl‐beta‐D‐glucosaminidase index as an early biomarker for chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med 2008;22:1103–1110. [DOI] [PubMed] [Google Scholar]
- 13. Williams TL, Peak KJ, Brodbelt D, et al. Survival and the development of azotemia in hyperthyroid cats. J Vet Intern Med 2010;24:863–869. [DOI] [PubMed] [Google Scholar]
- 14. van Hoek I, Lefebvre HP, Peremans K, et al. Short‐ and long‐term follow‐up of glomerular and tubular renal markers of kidney function in hyperthyroid cats after treatment with radioiodine. Domest Anim Endocrinol 2009;36:45–56. [DOI] [PubMed] [Google Scholar]
- 15. van Hoek I, Meyer E, Duchateau L, et al. Retinol‐binding protein in serum and urine of hyperthyroid cats before and after treatment with radioiodine. J Vet Intern Med 2009;23:1031–1037. [DOI] [PubMed] [Google Scholar]
- 16. Riensche MR, Graves TK, Schaeffer DJ. An investigation of predictors of renal insufficiency following treatment of hyperthyroidism in cats. J Feline Med Surg 2008;10:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adams WH, Daniel GB, Legendre AM, et al. Changes in renal function in cats following treatment of hyperthyroidism using 131I. Vet Radiol Ultrasound 1997;38:231–238. [DOI] [PubMed] [Google Scholar]
- 18. Graves TK, Olivier NB, Nachreiner RF, et al. Changes in renal function associated with treatment of hyperthyroidism in cats. Am J Vet Res 1994;55:1745–1749. [PubMed] [Google Scholar]
- 19. Boag AK, Neiger R, Slater L, et al. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec 2007;161:711–715. [DOI] [PubMed] [Google Scholar]
- 20. Ghys LF, Paepe D, Duchateau L, et al. Biological validation of feline serum cystatin C: the effect of breed, age and sex and establishment of a reference interval. Vet J 2015;204:168–173. [DOI] [PubMed] [Google Scholar]
- 21. Farace G, Patch D, Yerramilli M. Feline serum cystatin C does not differentiate healthy cats from those with chronic kidney disease. J Vet Intern Med 2015;29:1215 (abstract). [Google Scholar]
- 22. Ross LA, Finco DR. Relationship of selected clinical renal‐function tests to glomerular‐filtration rate and renal blood‐flow in cats. Am J Vet Res 1981;42:1704–1710. [PubMed] [Google Scholar]
- 23. Hall JA, Yerramilli M, Obare E, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med 2014;28:1676–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology, 2nd ed Oxford: Wiley‐Blackwell; 2008. [Google Scholar]
- 25. Ghys LF, Paepe D, Lefebvre HP, et al. The effect of feeding, storage and anticoagulant on feline serum cystatin C. Vet J 2015;206:91–96. [DOI] [PubMed] [Google Scholar]


