Abstract
The 21st century is challenging for human beings. Increased population growth, population aging, generation of new infectious agents, and natural disasters are some threatening factors for the current state of blood transfusion. However, it seems that science and technology not only could overcome these challenges but also would turn many human dreams to reality in this regard. Scientists believe that one of the future evolutionary innovations could be artificial blood substitutes that might pave the way to a new era in transfusion medicine. In this review, recent status and progresses in artificial blood substitutes, focusing on red blood cells substitutes, are summarized. In addition, steps taken toward the development of artificial blood technology and some of their promises and hurdles will be highlighted. However, it must be noted that artificial blood is still at the preliminary stages of development, and to fulfill this dream, ie, to routinely transfuse artificial blood into human vessels, we still have to strengthen our knowledge and be patient.
Keywords: artificial blood, oxygen carrier, hemoglobin, red blood cells
Introduction
Being alive is impossible without blood, the complex liquid containing millions of chemicals and cells. When William Harvey for the first time described blood circulation, scientists started to think about a proper replacement such as an artificial blood.1 However, none of the products developed since then meets all blood functions. In fact, production of a liquid that mimics all blood functions is still a dream, although it may come true in future. Some kind of blood substitutes developed over time, including simple liquids such as, urine, beer, and milk and plant resins,1 with least similarity to the blood constituents, and cells originated from stem cells with maximum similarity to blood.2 Artificial blood was highlighted after emergence of HIV in 1980 due to the risk of its transmission by blood transfusion,3 which imposes higher costs due to the necessary detection tests. In addition, low blood supplies especially in developing countries, lower number of donors due to the aging of population and consequently increased demand for blood products, short storage period, and urgent needs for blood supplies during wartime and natural disasters are other important reasons that make the development of a suitable blood substitute indispensable.4 Among the mentioned factors, the safety issue of blood products is the most important one, especially due to emerging of novel infectious agents such as Ebola and H1N1 (a kind of flu virus also called swine flu due to its similarity to those found in pigs) viruses, whose screening also imposes further costs.5 Today, there is a considerable inadequacy of blood supplies in developing countries, which are inhabited by 80% of the world’s population but collect less than 32% of total worlds’ blood supplies with low safety standards.6 Therefore, artificial blood would be of great value for developing countries. Understanding the blood behavior at the microcirculation level where blood and tissues come into contact is a key step in the development and application of blood substitutes. In other words, a blood substitute must be designed in a way that it behaves similar to natural blood in microcirculation.7 Development of an agent properly mimicking the oxygen-carrying capability of blood among its various functions has been of great interest, and many products have been established based on this property. Here, we mainly discuss and review the blood substitutes that mimic the oxygen-carrying capability of blood. In addition, potential challenges and obstacles against routine application of artificial blood in human will be discussed.
Red Blood Cell Substitutes
Red blood cells (RBCs) isolated from donated blood are an important component widely used to save patients’ lives via oxygen-carrying capacity owing to hemoglobin (Hb).8 However, there are complications associated with transfusion of RBCs to patients. These complications can be divided into noninfectious and infectious and are the most important concerns for the application of RBCs.9 Furthermore, crossmatch and blood group typing are needed before transfusion, which is challenging in case of emergencies and when rare blood group types are needed. Hence, it is essential to develop efficient RBC substitutes capable of active oxygen and carbon dioxide transfer.10,11 The most important features of an RBC substitute include its ability to transport oxygen and carbon dioxide, low cost, no need for crossmatching and blood typing, lack of contamination and infectious agents, easy access, trouble-free storage conditions, extended half-life in circulation, full excretion from body and being not accumulated in various tissues, lack of toxicity, nonimmunogenicity, nonantigenicity, and noncarcinogenicity.10
RBC substitutes or synthetic oxygen transporters studied so far are of mainly two types: perfluorocarbon and Hb-based substitutes.
Perfluorochemical-based RBC substitutes
Perfluorochemicals (PFCs) are colorless, inert, and apparently nontoxic liquids with low boiling point temperatures and are insoluble in water and alcohol.12 The capacity of PFCs in carrying oxygen was demonstrated for the first time by Clark in 1966. PFCs include straight or cyclic hydrocarbon chains13 with a general chemical formula of CnF2n+2,14 and the straight form is a better carrier for oxygen than the cyclic one.10 The level of oxygen dissolved in PFCs has a direct linear relationship with oxygen pressure, and therefore, high oxygen pressure is necessary for maximum oxygen-carrying capacity.14 Since hydrogen atoms are replaced by fluorine atoms in PFCs, these compounds are not metabolized due to the strong bond between carbon and fluorine atoms.13 PFCs are insoluble in aqueous phase, and in case of their clinical application, they are solubilized using an emulsifying agent.15 One main advantage of PFCs is for people refusing blood or proteins derived from humans or animals.16 Oxygen is dissolved in PFCs at a concentration of about 40%–50%, which is 20 times higher than the capacity of water and 2 times higher than plasma. In addition, PFCs dissolve 130–160 mL carbon dioxide, which is two to three times higher than the corresponding water capacity.17 Moreover, there are PFCs with oxygen-dissolving capacities higher than erythrocytes, including FC-80 (a liquid form of PFCs), which is 10% stronger in this regard.12 The fundamental difference in O2 transfer by Hb and PFC is that the former binds O2, while the latter dissolves it.18 PFCs are heat resistant and can withstand 300°C and higher temperatures without any change, which makes them easily amenable to heat sterilization.5 Their small sizes enable them to easily pass through the vessels occluded in some diseases, where RBCs cannot pass; hence, their application helps improving the oxygenation rate. An in vitro study showed that use of PFCs as artificial blood is considerably advantageous in occluded coronary artery to maintain myocardial function.19 Moreover, Chen et al showed the usefulness of PFCs as artificial blood substitute for oxygen carrying and expanding the plasma volume during surgeries done for the treatment of traumatic and hemorrhagic shocks even in war casualties.17
Fluosol-DA was the first accepted PFC-based RBC substitute, which is an emulsion of perfluorodecaline and perfluorotripropylamine.20 Oxygen-carrying capacity of Fluosol-DA is only 7.2% at 37°C, which is lower than RBCs.20 The use of this product entails complications such as pulmonary reactions supposedly due to complement activation by the emulsifying agent in Fluosol-DA and can be prevented by steroid injection.21 Hence, the efficacy of Fluosol-DA was not demonstrated in a prospective clinical trial,14 and its clinical application was stopped. Perflubron and perfluorodecalin have been extensively studied among PFCs.22 OxyFlour™ (Hemagen, Inc.) and Oxygent™ (Aka perflubron, Alliance Pharmaceutical, Inc.) are among the second-generation PFC-based blood substitutes, which are rejected by clinical trials due to some side effects such as complications in determining the effective dose for OxyFlour™ administration and also increased risk of stroke following administration of Oxygent™.4 However, except some changes in clotting factors, no specific interaction between blood components and administered PFCs has been reported.9 Administration of PFC-based products can result in mild thrombocytopenia (10%–15% reduction in platelet count) as well as flu-like syndrome.23
Hb-based RBC substitutes
Human Hb derived from expired RBC bags is the main source of Hb for the production of Hb-based RBC substitutes. Other sources for this purpose include cord blood RBCs and animal (bovine) and recombinant Hb.
Transmission of infection is an important concern in the application of Hbs with human or animal origins. In particular, the assessment of transmission risk of infectious agents causing bovine spongiform encephalitis is very important.24 However, animal-derived Hb has some advantages over human Hb, including unlimited access, higher resistance to degradation of heme, and the use of chloride ion instead of 2,3-DPG as an allosteric effector present in plasma.25 Production of genetically modified Hb in microorganisms and plants can address several problems associated with these products. Furthermore, Hb can be genetically manipulated by this approach to overcome some side effects associated with these products, for example, the affinity of Hb for NO and O2 can be changed.10 High-level production of recombinant Hb using simple Escherichia coli expression system has been reported by Hoffman et al26 in 1990.
The half-life of Hb is equal inside and outside the RBCs; however, outside the RBCs, the natural tetramer molecule of Hb rapidly converts to dimer and monomer Hb species, which cause severe complications such as kidney damage. Furthermore, outside the RBCs, Hb and 2,3-DPG are separated, resulting in higher affinity for oxygen.4,16 On the other hand, it has been shown that Hb scavenges the existing NO molecules by its heme groups. NO is also involved in relaxation of smooth muscles of blood vessels, and this property is responsible for the vasoactivity of Hb-based products.27 Overall, this type of Hb must be modified before its application as an oxygen carrier.4,16 The Hb-based oxygen carriers (HBOCs) are divided into the following two groups: acellular and cellular HBOCs.
Acellular HBOCs
Acellular HBOCs have been developed to increase Hb performance and decrease its side effects (Table 1). These are now in various phases of clinical trials and belong to three categories including cross-linked HBOC, polymerized HBOC, and conjugated HBOC (Fig. 1). However, among different modifications of Hb, only nanotechnology-based polyhemoglobin (PolyHb) and conjugated Hb are effective.28 However, due to their short blood half-lives and side effects, a majority of these products did not achieve required criteria in clinical trials.4
Table 1.
Summary of acellular Hb-based oxygen carriers.
| TYPE OF HBOC | PRODUCT | BIOGENESIS | ACTION | PROPERTIES |
|---|---|---|---|---|
| Cross-linked HBOC | Diaspirin cross-linked Hb (DCLHb) or HemAssist | Human hemoglobin | Carrier of oxygen | In phase lll clinical trial, it seems to increase mortality rates (6), lacking the ability to outregulate the oxidative state of iron in their heme group (4) |
| Polymerized HBOC | Hemopure | Glutaraldehyde bovine Hb | Carrier of oxygen | Lacking the ability to outoregulate the oxidative state of iron in their heme group (4), contains higher amount of free α2β2, increases the risk of cardiovascular problems, risk of transmission of diseases due to the use of bovine hemoglobin (6) |
| PolyHeme | Glutaraldehyde, pyridoxal human Hb | Carrier of oxygen | Increasing the risk of cardiovascular problems (6), trauma victins (6) | |
| Oxyglobin | Bovine hemoglobin | Carrier of oxygen | Lacking the ability to outoregulate the oxidative state of iron in their heme group (4), risk of transmission of diseases due to the use of bovine hemoglobin (6) | |
| PolyHb-SOD-CAT-CA | Bovine hemoglobin | Carrier of oxygen, removal of oxygen radical, transportation of CO2 | Risk of transmission of diseases due to the use of bovine hemoglobin (6) | |
| PolyHb-Fibrinogen | Carrier of oxygen and coagulation | Lacking the ability to outoregulate the oxidative state of iron in their heme group (4) | ||
| Conjugated HBOC | Hemospan | Maleimide PEG-human Hb | Carrier of oxygen | Lacking the ability to outoregulate the oxidative state of iron in their heme group (4) |
| MP4 | Malemide PEG-hemoglobin | Carrier of oxygen |
Figure 1.
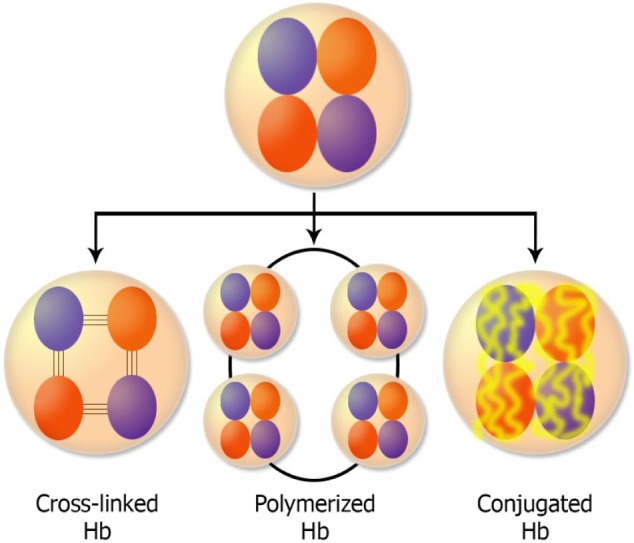
Three major classes of cellular HBOCs are polymerized, cross-linked, and conjugated Hbs. Spontaneous separation of Hb chains is prevented by various modifications. For example, in the cross-linked type, Hb chains are bound by intermolecular covalent bonds, in the polymerized type, they are bound by intermolecular covalent bond, and in the conjugated type, a polymer is bound to the surface of Hb.
One of the main problems limiting the application of these products is their inability to convert Fe3+ to Fe2+, which is an important function of RBCs. As a result, Met-Hb with low oxygen-carrying capacity was produced, showing that such complications can be avoided by attaching reducing agents to Hb surface in this product series.4
First, acellular HBOCs were cross-linked HBOC in which intramolecular covalent bonds were formed between globin chains in order to prevent their detachment. Diaspirin cross-linked Hb or HemAssist in which human Hb molecule has been intramolecularly cross-linked between alpha chains has been subjected to extensive investigation. This product has been used for various purposes, such as reduction of neurological damage in a mouse model,29 intraoperative anemia in sheep,30 cardiopulmonary resuscitation in pigs,31 and postcardiac bypass surgery to avoid allogeneic blood transfusion.32 However, the efficiency of diaspirin cross-linked Hb in the above-mentioned studies has been challenging.
Polymerized HBOCs are another acellular HBOC in which Hb molecules are cross-linked intermolecularly to increase the molecular size. Each PolyHb molecule is composed of 4–5 Hb molecules, and various kinds of PolyHb molecules have been produced so far. For example, Hemopure is a polymerized bovine Hb developed by OPK Biotech, which is currently in phase III clinical trial.5 PolyHeme is another product of this family consisted of pyridoxylated human Hb. However, its administration has been rejected by the United States Food and Drug Administration (US FDA) due to its adverse effects5 including hypertension, safety and toxicity issues attributed to vasoactivity, and oxidative stress.5
Oxyglobin is another product developed by OPK biotech in which bovine Hb has been used. This product contains 2% α2β2 Hb, which comparing to Hemopure that contains 31% α2β2 Hb, fewer hypertensive complications associated with free α2β2 is observed following its administration.5,33 In fact, it is the first product approved by the US FDA and the European commission for veterinary use.
In a study conducted by Bian and Chang,34 superoxide dismutase (SOD), catalase (CAT), and carbonic anhydrase (CA) were cross-linked to Hb and soluble nanobiotechnology-based product of PolyHb–SOD–CAT–CA RBC was created, whose efficiency was shown in a mouse model. This product could be stored at room temperature for 320 days and its lyophilized form can be sterilized.
PolyHb–fibrinogen is a product showing both oxygen-carrying and blood coagulation properties, which can be used in massive bleedings where the oxygen-carrying capacity is not sufficient by itself.35
Winslow and other researchers have shown that the modified Hbs with low P50 (partial pressure of oxygen required to achieve 50% saturation of Hb) and macromolecular diffusivities have an oxygen delivery profile similar to RBCs.25 Therefore, they used oxidized mono-, di-, tri-, and polysaccharides as cross-linking agents. Their study showed that application of larger polysaccharides led to higher Hb cross-linking and polymerization, and O-methylglucopyranoside is the best polysaccharide to achieve this goal.25
Conjugated HBOCs are other acellular HBOCs in which inert polymers are attached to the surface of Hb molecules. Due to unique characteristics, low toxicity, and lack of immunogenicity or antigenicity in body, PEG can be the best polymer for conjugation.36
Hemospan is a PEG-conjugated Hb, which is under clinical trial as an oxygen carrier. This modification has been shown to increase the circulation half-life of the product.37
MP4 is another PEG–Hb conjugate designed as an oxygen carrier. This product did not cause vasoconstriction in animal models, and its efficacy to deliver oxygen to hypoxic tissues was demonstrated; MP4 is now under human clinical trials.38
In addition to PEG, other polymers have been used to conjugate Hb, including benzene tetracarboxylate dextran,39 hydroxyethyl starch (called HRC 101),40 and albumin.41
Recombinant Hb
The highest Hb activity is seen when it is within RBCs. Therefore, when Hb is going to be used in a cell-free format, it must be subjected to various modifications for increasing its half-life in circulation and preventing related complications in the body. In fact, recombinant production of Hb makes it easier to be modified specially through site-directed mutagenesis.42
In 1980s, human Hb was produced in large quantities in transgenic organisms. Since then, investigations for higher quality and more efficient production of recombinant Hb were started. It should be noted that most studies have focused on the production of Hb by E. coli expression system.43 However, it has been also expressed in other bacterial systems and also by transgenic mouse and pig.43 Nevertheless, the most important problems on the way of recombinant production of Hb are low expression yields and expensive production processes,42 in addition to difficulties in obtaining desired purity.43
There have been several attempts to increase the production yield of recombinant Hb. For example, in a study, E. coli expression system was used to produce high quantities of human α-globin and bovine β-globin for therapeutic purposes. Then, the produced Hbs (Hb minotaur) were polymerized using intermolecular disulfide bonds and designated as Hb Polytaur. Animal studies revealed some advantages for this product over the other products of this type.44
It has been stated that mutating the Hb chains (βGly16 to Ala and αGly15 to Ala) might increase its production yield by preventing degradation and aggregation of the expressed chains. In addition, to increase the production yield of heme for the generation of functional Hb, the strategy of simultaneous heme transporter generation in bacterial membrane can be used, which might increase heme uptake by the bacterial host and subsequently increase the production yield.45
Targeted mutations of Hb might also cause several improvements in its functions outside RBCs. For example, targeted mutations could result in increased oxygen affinity, reduced capacity of Hb to scavenge NO, reduced autooxidation, decreased rate of heme loss, preventing the detachment of subunits, and decreased irreversible denaturation of subunits.43
In a study, in order to improve Hb vasoactivity, and also to prevent its tetramer to dimer conversions and associated complications, rHbβG83C was generated using targeted mutation in which according to Hb Ta-Li, the Cys amino acid substituted for Gly in position 83 of β-chain (β83 Gly → Cys). This polymerizes using intertetramer disulfide bonds, and the molecular size of this product was shown to be stable in fresh frozen plasma.46
In another study, rHb (βN108Q) was produced by an E. coli expression system. In this Hb version, N → Q mutation occurred in position 108 of β-chain, which is the α1β1 subunit interface and also the central cavity of Hb. This mutation causes reduced oxygen affinity, increased cooperativity, and decreased autooxidation of the product.47
These are only a few cases of successful efforts for the production of recombinant HBOCs being able to be used as a favorable blood substitute. However, none of the products have received therapeutic licensure in the USA.43
Cellular HBOCs
Other types of Hb-based products as artificial blood are cellular HBOCs, in which Hb is encapsulated in a cell-like structure. In this way, some products with highest similarity to RBCs were produced, which do not cause vasoactivation due to scavenging of NO. A summary of products corresponding to the cellular-based Hb is shown in Table 2.
Table 2.
Summary of cellular Hb-based oxygen carriers.
| PRODUCT | BIOGENESIS | ACTION | PROPERTIES |
|---|---|---|---|
| Neo red cell | Hemoglobin | Carrier of oxygen | High oxygen transport efficiency, has a strong capsule membrane, readily circulates due to its low viscosity (48) |
| Hemoglobin vesicle (HbV) | Carbonyl human hemoglobin | Carrier of oxygen | Transient decrease in phagocytic activity one day after infusion (49), cause splenomegaly (49), higher encapsulation efficiency (50). The advantages of HbV over the conventional Hb vesicles are also the surface modification of HbV with poly(ethylene glycol) that allows better hemodynamics, reduced complement activation and longer circulation time and a moderate rate of entrapment and metabolism (49) |
| Liposome encapsulated actin-hemoglobin (LEAcHb) | Bovin hemoglobin | Carrier of oxygen | High circulation half-life, disk like shape (35) |
| Hemoglobin-loaded polymeric nanocapsule (PNP) | Hemoglobin | Carrier of oxygen | Rapid clearance by phagocytosis in blood stream, high encapsulation efficiency, biocompatible in a large concentration range (51) |
| Cationizad HbPNP | Bovin hemoglobin | Carrier of oxygen | High half-life in circulation in comparison to PNP due to low uptake by macrophages, no significant aggregation and sedimentation even after 5 days, biocompatibility and biofunctionality, high encapsulation efficiency, controlled particle size, biocompatible in a large concentration range, lack of cytotoxicity (51) |
| Fe(ll) porphyrin loaded dendrimer | Porphyrin | Carrier of oxygen, efficient oxidation catalyst | The shape and size of this product is similar to RBCs, production of this product is time consuming and costly (53) |
| Nanocapsule bearing a membrane made of ultrathin PEG-PLA, containing polymerized Hb and all RBC enzymes | Hemoglobin | Carrier of oxygen, all other action of RBC | Containg all RBC enzymes specially reductase (56), high half-life due to reduced phagocytosis (57) |
| Nanoscale hydrogel particles (NHP) | Bovine hemoglobin | Carrier of oxygen | Releases hemoglobin to blood stream, good oxygen uptake and release characteristics (58) |
| Lipogel | Bovine hemoglobin | Carrier of oxygen | High hemoglobin loading capacity, low recognition by immune cells, good oxygen uptake and release (58) |
| Polymersome-encapsulated hemoglobin (PEH) | Human and bovine hemoglobin | Carrier of oxygen, drug delivery in cancer (polymersome encapsulated drug) | High Hb loading capacity even higher than lipogel and NHP (59), can be prepared in large quantities, affinity to oxygen, comparable to human RBC, size distribution, Hb encapsulation efficiency, oxygen affinity (P50), cooperativity coefficient, and methemoglobin (metHb) level of these novel PEH dispersions were consistent with values required for efficient oxygen delivery in the systemic circulation (60) |
| Single protein nanocapsule (SNP) | Human hemoglobin | Carrier of oxygen, use of polymer for drug delivery | Mechanical, heat and PH resistant, polymer layer can essentially stabilize different type of proteins, the quaternary hemoglobin structure does not change during preparation of SNP (61) |
| Hemoglobin conjugated biodegradable polymer micelles | Bovin hemoglobin | Carrier of oxygen |
Encapsulation of Hb by a phospholipid layer (liposome-encapsulated Hb [LEH]) prolonged its half-life and shelf-life comparing to acellular products. LEH particles are much smaller than RBCs (1:30). This small size enables their entry into areas of body that are not accessible for RBCs. Hence, they can pass through clots and blockages causing more oxygenation during stroke.3 However, this product has a short circulation half-life, which can be solved by a number of approaches for example by PEGylation of the particles’ surface.3
In a study, liposome-encapsulated Hbs known as neo red cells were developed, and their efficiency as artificial RBCs was demonstrated in total cardiopulmonary bypass in an animal study, which showed even higher oxygen delivery capacity than RBC.48
Modifying the surface of these liposomes, including PEGylation, can result in products with higher half-life, stability, and solubility, as well as lower antigenicity and immunogenicity. Hb vesicle is a PEGylated product with increased serum half-life and decreased recognition by the immune system.49,50
In addition to the removal by reticuloendothelial system, another reason for low serum half-life of LEHs is shear-induced liposome destruction in bloodstream. Hence, to address this issue, an actin matrix was introduced into the aqueous core of the submicron liposomes to increase their mechanical strength. This strategy caused increased half-life of the product known as LEAcHb.35
Another series of products used as RBC imitators are biodegradable Hb-loaded polymeric nanoparticle (HbPNP). However, the most important problem with their application is rapid clearance by phagocytes either directly or through opsonins. To address this problem, several studies have been conducted. In one study, changing the surface charge by cationized cetyltrimethylammonium bromide increased half-life of the PEGylated HbPNP. It was demonstrated that surface charge of products used as artificial blood substitutes has a profound effect on their circulation time, where anionized HbPNPs are rapidly cleared from bloodstream and cationized HbPNPs have high circulation half-life.51
Other cellular-based biocompatible Hb products with repetitively branched molecular structures are dendrimers. Poly(propylene), poly(amide amine), and polyether are among synthetic dendrimer products.52 Fe(II) porphyrin-loaded dendrimers were prepared in some studies to simulate Hb, playing its role. The shape and size of these products are similar to Hb, and they are able to bind and release oxygen. However, their production is time consuming and costly. Therefore, a kind of dendrimer known as hyperbranched polymer has been developed, with reduced problems, which can be used as oxygen carrier by some adaptations.53,54 Dendrimers are also used for encapsulation in drug delivery. Therefore, it has been suggested that dendrimers could be used as artificial oxygen carriers by encapsulating Hb.55
As an Hb-based RBC substitute, nanocapsules bearing a membrane made of ultrathin polyethylene glycol–polylactic acid (PEG–PLA), containing polymerized Hb and all RBC enzymes, were also studied.56 The advantage of this method compared to the previous product in which only PLA membranes have been used was a significant increase in the half-life of the product due to reduction of phagocytosis shown in mice.56,57 High circulation half-life and presence of enzymatic systems, including reductase, are the most important advantages of this system. The presence of enzyme system, especially the reductase, results in prevention of the accumulation of methemoglobin.56
A new class of materials for encapsulation of Hb is lipogel and NHPs (nanoscale hydrogel particles). In case of NHPs, nonbiodegradable but biocompatible hydrophilic polymers are used to enclose Hb within solid and spherical nanoscale hydrogel particles. Various complications caused by Hb release subsequent to the degradation of biodegradable polymers resulted in higher interests for application of nonbiodegradable polymers. Lipogels are NHPs enclosed within a lipid bilayer that is actually a hydrogel–liposome. They are made by photopolymerization of poly(N-isopropylacrylamide) and polyacrylamide monomers. This approach in turn enhances the mechanical strength of liposomal system. In fact, this product has the advantages of hydrogel and liposome together. These products have high Hb-loading capacity and low susceptibility that can be recognized by the reticuloendothelial system.58
An increased Hb-loading capacity even higher than that of lipogel and NHP is observed in polymersome-encapsulated Hb (PEH), which is a product made up of biodegradable, biocompatible, amphiphilic diblock copolymers and is used to enclose human or animal Hb. PEHs can be easily produced in large quantities, and their oxygen affinity is comparable to human erythrocytes. The mechanical resistance and possibility of changing the size and thickness is higher in PEH than in liposomes, and they can withstand temperatures up to 60°C. These products are stable in saline solution and blood for several months and at least five days, respectively. According to accumulating evidence, these products could herald the emergence of an ideal artificial oxygen.59,60
In single-protein nanocapsules, a protein molecule is encapsulated with a thin polymer layer and the resulting product has mechanical, heat, and pH resistance. This method has been used for Hb (Fig. 2).61
Figure 2.

Structure of a typical cellular Hb-based oxygen carrier in a single-protein nanocapsule. In this product, Hb is covered by a thin layer of acrylamide and bisacrylamide monomers. This thin layer increases the thermal and pH stability of Hb and also protects Hb against protease degradation in blood circulation.
An oxygen carrier has been developed by a research group, in which Hb is conjugated to biodegradable polymer micelles.17 The micelle is a PEG–PMPC–PLA triblock copolymer in which PEG acts as shell, PMPC (PLA-b-poly (2-methacryloyloxyethyl phosphorylcholine)) bearing propargyl groups as the middle layer and PLA as core layer. Following the production of these micelles in an aqueous solution, Hb groups bind micelles via propargyl groups in which Hb is surrounded and protected by PEG chains (Fig. 3).17
Figure 3.

Structure of a micelle formed from triblock copolymers. In this product, Hb is conjugated to biodegradable polymer micelles. It contains a triblock copolymer made up of PEG (as external layer), PMPC consisting of propargyl groups (as middle layer), and PLA (as internal layer).
RBCs Differentiated From Stem Cells
Perhaps, the real RBCs are RBCs differentiated from stem cells. These cells can be used as an ideal product for injection to patients requiring chronic blood transfusion as well as patients with rare blood groups or autoantibodies. Stem cells derived from various sources including bone morrow, cord blood, embryonic stem cells, and induced pluripotent stem cells (iPSCs) have been used for this purpose.62 RBCs were derived from iPSCs from fetal and adult human fibroblasts using a two-step protocol for the first time by Lapillonne et al.13 This suggests that iPSCs could represent an unlimited supply for the production of RBCs for clinical application. Mass production of RBCs in laboratory for their application in transfusion have been performed by adjusting various production conditions such as providing optimal culture conditions for cord blood-derived hematopoietic stem cells and subsequent coculture of erythroid progenitors with human fetal liver stromal cells. This resulted in improved terminal erythroid maturation process and plenty of cells were obtained, which were comparable with natural RBCs in many aspects.63 Immortalized erythrocyte progenitor cells are obtained through the introduction of C-MYC and BCL-XL into multipotent hematopoietic progenitor cells derived from pluripotent stem cells. Overexpression of these proteins led to erythroblasts with consistently high self-replication capacity. Elimination of overexpression of these genes induced the differentiation of these erythroblasts to mature erythrocytes.64 Exposing CD34+ cells to a short pulse of cytokines favorable for erythroid differentiation before their expansion and their subsequent expansion is another approach in this respect.65
Mass production and high production costs for clinical applications are the most important problems in this field. It would be expected to overcome these obstacles in near future to make an unlimited source with maximum similarity and minimum complications to replace RBCs derived from donated blood.62
Conclusion
Due to the increased demand for blood transfusion and concerns about blood-borne pathogens, development of artificial blood substitutes, especially HBOCs, is under intensive focus. However, although many important steps have been taken to date, no oxygen-carrying blood substitutes are approved for use by the US FDA. Side effects and short half-life are the two main reasons that they did not met criteria for being approved. The fact of having no approved product in this field shows that there is an important challenge against formulation and application of promising and effective blood substitutes. In addition, it indicates the immense potential that exists in this field. However, being optimistic, it seems that science and technology would facilitate developing real blood substitutes, at least oxygen-carrying blood substitutes, whose production will substantially alleviate the worldwide shortage of blood needed for transfusion. It seems that future studies on artificial blood substitutes would focus on real blood substitutes, ie, RBCs obtained through differentiation of stem cells. Hence, by considering the first steps that have been taken successfully toward this ideal product, it would be expected in the near future to have a source of required RBCs with minimum complications and maximum similarity to replace RBCs derived from donated blood.
Acknowledgments
We are grateful to thank Dr Ahmad Mehdipour for his help in drawing figures.
Footnotes
ACADEMIC EDITOR: Prabitha Natarajan, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 663 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Provenance: the authors were invited to submit this paper.
Author Contributions
Analyzed the data: MHR. Wrote the first draft of the manuscript: SM. Contributed to the writing of the manuscript: AJ-N. Agree with manuscript results and conclusions: MHR, SM, AJ-N. Jointly developed the structure and arguments for the paper: AJ-N. Made critical revisions and approved final version: MHR. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Sarkar S. Artificial blood. Indian J Crit Care Med. 2008;12(3):140. doi: 10.4103/0972-5229.43685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giarratana M-C, Kobari L, Lapillonne H, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23(1):69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 3.Sharma A, Arora S, Grewal P, Dhillon V, Kumar V. Recent innovations in delivery of artificial blood substitute: a review. Int J Appl Pharm. 2011;3(2):1–5. [Google Scholar]
- 4.Tao Z, Ghoroghchian PP. Microparticle, nanoparticle, and stem cell-based oxygen carriers as advanced blood substitutes. Trends Biotechnol. 2014;32(9):466–473. doi: 10.1016/j.tibtech.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Cabrales P, Intaglietta M. Blood substitutes: evolution from non-carrying to oxygen and gas carrying fluids. ASAIO J. 2013;59(4):337. doi: 10.1097/MAT.0b013e318291fbaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shekhar C. Blood relatives: artificial oxygen carriers between promise and concern. Chem Biol. 2007;14(10):1091–1092. doi: 10.1016/j.chembiol.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Intaglietta M. Whitaker lecture 1996: microcirculation, biomedical engineering, and artificial blood. Ann Biomed Eng. 1997;25(4):593–603. doi: 10.1007/BF02684838. [DOI] [PubMed] [Google Scholar]
- 8.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370(9585):415–426. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications and complications. Am Fam Physician. 2011;83(6):719. [PubMed] [Google Scholar]
- 10.Lowe K. Perfluorinated blood substitutes and artificial oxygen carriers. Blood Rev. 1999;13(3):171–184. doi: 10.1054/blre.1999.0113. [DOI] [PubMed] [Google Scholar]
- 11.Vercellotti GM, Hammerschmidt DE, Craddock PR, Jacob HS. Activation of plasma complement by perfluorocarbon artificial blood: probable mechanism of adverse pulmonary reactions in treated patients and rationale for corticosteroids prophylaxis. Blood. 1982;59(6):1299–1304. [PubMed] [Google Scholar]
- 12.Agarwal VK. Organo-fluorine compounds as artificial blood substitute. Def Sci J. 2014;30(1):51–54. [Google Scholar]
- 13.Lapillonne H, Kobari L, Mazurier C, et al. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2010;95(10):1651–1659. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane TA. Perfluorochemical-based artificial oxygen carrying red cell substitutes. Transfus Sci. 1995;16(1):19–31. doi: 10.1016/0955-3886(94)00067-t. [DOI] [PubMed] [Google Scholar]
- 15.Biro GP, Blais P. Perfluorocarbon blood substitutes. Crit Rev Oncol Hematol. 1987;6(4):311–374. doi: 10.1016/s1040-8428(87)80018-5. [DOI] [PubMed] [Google Scholar]
- 16.Squires JE. Artificial blood. Science. 2002;295(5557):1002–1005. doi: 10.1126/science.1068443. [DOI] [PubMed] [Google Scholar]
- 17.Shi Q, Huang Y, Chen X, Wu M, Sun J, Jing X. Hemoglobin conjugated micelles based on triblock biodegradable polymers as artificial oxygen carriers. Biomaterials. 2009;30(28):5077–5085. doi: 10.1016/j.biomaterials.2009.05.082. [DOI] [PubMed] [Google Scholar]
- 18.Cohn CS, Cushing MM. Oxygen therapeutics: perfluorocarbons and blood substitute safety. Crit Care Clin. 2009;25(2):399–414. doi: 10.1016/j.ccc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Mushlin PS, Boucek RJ, Parrish MD, Graham TP, Olson RD. Beneficial effects of perfluorochemical artificial blood on cardiac function following coronary occlusion. Life Sci. 1985;36(22):2093–2102. doi: 10.1016/0024-3205(85)90305-4. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura N, Nitsuno T, Naito R. Clinical studies of a perfluorochemical, whole blood substitute. Critical Care Med. 1981;9(3):168. [Google Scholar]
- 21.Bowman RJ. Red blood cell substitutes and artificial blood. Hum Pathol. 1983;14(3):218–220. doi: 10.1016/s0046-8177(83)80020-3. [DOI] [PubMed] [Google Scholar]
- 22.Lowe KC. Blood substitutes: from chemistry to clinic. J Mater Chem. 2006;16(43):4189–4196. [Google Scholar]
- 23.Kresie L, editor. Artificial blood: an update on current red cell and platelet substitutes; Baylor University Medical Center Proceedings; Dallas, Texas, USA: Baylor University Medical Center; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowe K, Farrell K, Ferguson E, James V. Current perceived risks of transfusion in the UK and relevance to the future acceptance of blood substitutes. Artif Cells Blood Substit Immobil Biotechnol. 2001;29(3):179–189. doi: 10.1081/bio-100103042. [DOI] [PubMed] [Google Scholar]
- 25.Eike JH, Palmer AF. Oxidized Mono-, di-, tri-, and polysaccharides as potential hemoglobin cross-linking reagents for the synthesis of high oxygen affinity artificial blood substitutes. Biotechnol Prog. 2004;20(3):953–962. doi: 10.1021/bp0499754. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman SJ, Looker DL, Roehrich JM, et al. Expression of fully functional tetrameric human hemoglobin in Escherichia coli. Proc Natl Acad Sci U S A. 1990;87(21):8521–8525. doi: 10.1073/pnas.87.21.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lui FE, Kluger R. Reviving artificial blood: meeting the challenge of dealing with NO scavenging by hemoglobin. Chembiochem. 2010;11(13):1816–1824. doi: 10.1002/cbic.201000291. [DOI] [PubMed] [Google Scholar]
- 28.Chang TMS. Blood substitutes based on nanobiotechnology. Trends Biotechnol. 2006;24(8):372–377. doi: 10.1016/j.tibtech.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Bowes MP, Burhop KE, Zivin JA. Diaspirin cross-linked hemoglobin improves neurological outcome following reversible but not irreversible CNS ischemia in rabbits. Stroke. 1994;25(11):2253–2257. doi: 10.1161/01.str.25.11.2253. [DOI] [PubMed] [Google Scholar]
- 30.Vane LA, Funston JS, Kirschner R, et al. Comparison of transfusion with DCLHb or pRBCs for treatment of intraoperative anemia in sheep. J Appl Physiol. 2002;92(1):343–353. doi: 10.1152/jappl.2002.92.1.343. [DOI] [PubMed] [Google Scholar]
- 31.Chow MS, Fan C, Tran H, Zhao H, Zhou L. Effects of diaspirin cross-linked hemoglobin (DCLHb) during and post-CPR in swine. J Pharmcol Exp Ther. 2001;297(1):224–229. [PubMed] [Google Scholar]
- 32.Lamy ML, Daily EK, Brichant J-F, et al. Randomized trial of diaspirin cross-linked hemoglobin solution as an alternative to blood transfusion after cardiac surgery. Anesthesiology. 2000;92(3):646–656. doi: 10.1097/00000542-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Staub A, Rudaz S, Saugy M, Veuthey JL, Schappler J. Analysis of hemoglobin-based oxygen carriers by CE-UV/Vis and CE-ESI-TOF/MS. Electrophoresis. 2010;31(7):1241–1247. doi: 10.1002/elps.200900513. [DOI] [PubMed] [Google Scholar]
- 34.Bian Y, Chang TMS. A novel nanobiotherapeutic poly-[hemoglobin-superoxide dismutase-catalase-carbonic anhydrase] with no cardiac toxicity for the resuscitation of a rat model with 90 minutes of sustained severe hemorrhagic shock with loss of 2/3 blood volume. Artif Cells Nanomed Biotechnol. 2015;43(1):1–9. doi: 10.3109/21691401.2014.964554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Nickels J, Palmer AF. Liposome-encapsulated actin–hemoglobin (LEAcHb) artificial blood substitutes. Biomaterials. 2005;26(17):3759–3769. doi: 10.1016/j.biomaterials.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Pasut G. Polymers for protein conjugation. Polymers. 2014;6(1):160–178. [Google Scholar]
- 37.Svergun DI, Ekström F, Vandegriff KD, et al. Solution structure of poly (ethylene) glycol-conjugated hemoglobin revealed by small-angle X-ray scattering: implications for a new oxygen therapeutic. Biophys J. 2008;94(1):173–181. doi: 10.1529/biophysj.107.114314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winslow RM. MP4, a new nonvasoactive polyethylene glycol–hemoglobin conjugate. Artif Organs. 2004;28(9):800–806. doi: 10.1111/j.1525-1594.2004.07392.x. [DOI] [PubMed] [Google Scholar]
- 39.Jia Y, Wood F, Menu P, Faivre B, Caron A, Alayash AI. Oxygen binding and oxidation reactions of human hemoglobin conjugated to carboxylate dextran. Biochim Biophys Acta. 2004;1672(3):164–173. doi: 10.1016/j.bbagen.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Crawford MW, Shichor T, Engelhardt T, et al. The novel hemoglobin-based oxygen carrier HRC 101 improves survival in murine sickle cell disease. Anesthesiology. 2007;107(2):281–287. doi: 10.1097/01.anes.0000271872.14311.b4. [DOI] [PubMed] [Google Scholar]
- 41.Hu T, Su Z. Bovine serum albumin-bovine hemoglobin conjugate as a candidate blood substitute. Biotechnol Lett. 2002;24(4):275–278. [Google Scholar]
- 42.Fronticelli C, Koehler RC. Design of recombinant hemoglobins for use in transfusion fluids. Crit Care Clin. 2009;25(2):357–371. doi: 10.1016/j.ccc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Varnado CL, Mollan TL, Birukou I, Smith BJ, Henderson DP, Olson JS. Development of recombinant hemoglobin-based oxygen carriers. Antioxid Redox Signal. 2013;18(17):2314–2328. doi: 10.1089/ars.2012.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bobofchak KM, Mito T, Texel SJ, et al. A recombinant polymeric hemoglobin with conformational, functional, and physiological characteristics of an in vivo O2 transporter. Am J Physiol Heart Circ Physiol. 2003;285(2):H549–H561. doi: 10.1152/ajpheart.00037.2003. [DOI] [PubMed] [Google Scholar]
- 45.Graves PE, Henderson DP, Horstman MJ, Solomon BJ, Olson JS. Enhancing stability and expression of recombinant human hemoglobin in E coli: progress in the development of a recombinant HBOC source. Biochim Biophys Acta. 2008;1784(10):1471–1479. doi: 10.1016/j.bbapap.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Fablet C, Marden MC, Green BN, Ho C, Pagnier J, Baudin-Creuza V. Stable octameric structure of recombinant hemoglobin α2β283 Gly → Cys. Protein Sci. 2003;12(4):690–695. doi: 10.1110/ps.0234403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai C-H, Fang T-Y, Ho NT, Ho C. Novel recombinant hemoglobin, rHb (βN108Q), with low oxygen affinity, high cooperativity, and stability against autoxidation. Biochemistry. 2000;39(45):13719–13729. doi: 10.1021/bi001116a. [DOI] [PubMed] [Google Scholar]
- 48.Usuba A, Motoki R, Ogata Y, Suzuki K, Kamitani T. Effect and safety of liposome-encapsulated hemoglobin neo red cells (NRCs) as a perfusate for total cardiopulmonary bypass. Artif Cells Blood Substit Immobil Biotechnol. 1995;23(3):337–346. doi: 10.3109/10731199509117950. [DOI] [PubMed] [Google Scholar]
- 49.Sakai H, Horinouchi H, Tomiyama K, et al. Hemoglobin-vesicles as oxygen carriers: influence on phagocytic activity and histopathological changes in reticuloendothelial system. Am J Pathol. 2001;159(3):1079–1088. doi: 10.1016/S0002-9440(10)61783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sou K, Klipper R, Goins B, Tsuchida E, Phillips WT. Circulation kinetics and organ distribution of Hb-vesicles developed as a red blood cell substitute. J Pharmacol Exp Ther. 2005;312(2):702–709. doi: 10.1124/jpet.104.074534. [DOI] [PubMed] [Google Scholar]
- 51.Xu F, Yuan Y, Shan X, et al. Long-circulation of hemoglobin-loaded polymeric nanoparticles as oxygen carriers with modulated surface charges. Int J Pharm. 2009;377(1):199–206. doi: 10.1016/j.ijpharm.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Scott RW, Wilson OM, Crooks RM. Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J Phys Chem B. 2005;109(2):692–704. doi: 10.1021/jp0469665. [DOI] [PubMed] [Google Scholar]
- 53.Twyman LJ, Ge Y. Porphyrin cored hyperbranched polymers as heme protein models. Chem Commun. 2006;(15):1658–1660. doi: 10.1039/b600831n. [DOI] [PubMed] [Google Scholar]
- 54.Twyman LJ, Ellis A, Gittins PJ. Pyridine encapsulated hyperbranched polymers as mimetic models of haeme containing proteins, that also provide interesting and unusual porphyrin-ligand geometries. Chem Commun. 2011;48(1):154–156. doi: 10.1039/c1cc14396d. [DOI] [PubMed] [Google Scholar]
- 55.Astruc D, Boisselier E, Ornelas C. Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev. 2010;110(4):1857–1959. doi: 10.1021/cr900327d. [DOI] [PubMed] [Google Scholar]
- 56.Chang TM, Powanda D, Yu W. Analysis of polyethylene-glycol-polylactide nano-dimension artificial red blood cells in maintaining systemic hemoglobin levels and prevention of methemoglobin formation. Artif Cells Blood Substit Immuobil Biotechnol. 2003;31(3):231–247. doi: 10.1081/bio-120023155. [DOI] [PubMed] [Google Scholar]
- 57.Sheng Y, Yuan Y, Liu C, Tao X, Shan X, Xu F. In vitro macrophage uptake and in vivo biodistribution of PLA–PEG nanoparticles loaded with hemoglobin as blood substitutes: effect of PEG content. J Mater Sci Mater Med. 2009;20(9):1881–1891. doi: 10.1007/s10856-009-3746-9. [DOI] [PubMed] [Google Scholar]
- 58.Patton JN, Palmer AF. Photopolymerization of bovine hemoglobin entrapped nanoscale hydrogel particles within liposomal reactors for use as an artificial blood substitute. Biomacromolecules. 2005;6(1):414–424. doi: 10.1021/bm049432i. [DOI] [PubMed] [Google Scholar]
- 59.Rameez S, Alosta H, Palmer AF. Biocompatible and biodegradable polymersome encapsulated hemoglobin: a potential oxygen carrier. Bioconjug Chem. 2008;19(5):1025–1032. doi: 10.1021/bc700465v. [DOI] [PubMed] [Google Scholar]
- 60.Arifin DR, Palmer AF. Polymersome encapsulated hemoglobin: a novel type of oxygen carrier. Biomacromolecules. 2005;6(4):2172–2181. doi: 10.1021/bm0501454. [DOI] [PubMed] [Google Scholar]
- 61.Hegedüs I, Dojcsak ÉK-T, Szalai AJ, et al. Single haemoglobin nanocapsules as test materials for artificial blood. Period Polytech Chem Eng. 2014;58(suppl):11. [Google Scholar]
- 62.Kim HO. In-vitro stem cell derived red blood cells for transfusion: are we there yet? Yonsei Med J. 2014;55(2):304–309. doi: 10.3349/ymj.2014.55.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xi J, Li Y, Wang R, et al. In vitro large scale production of human mature red blood cells from hematopoietic stem cells by coculturing with human fetal liver stromal cells. Biomed Res Int. 2013;2013:807863. doi: 10.1155/2013/807863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirose S-I, Takayama N, Nakamura S, et al. Immortalization of erythroblasts by c-MYC and BCL-XL enables large-scale erythrocyte production from human pluripotent stem cells. Stem Cell Reports. 2013;1(6):499–508. doi: 10.1016/j.stemcr.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olivier E, Qiu C, Bouhassira EE. Novel, high-yield red blood cell production methods from CD34-positive cells derived from human embryonic stem, yolk sac, fetal liver, cord blood, and peripheral blood. Stem Cells Transl Med. 2012;1(8):604–614. doi: 10.5966/sctm.2012-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]


