Abstract
Purpose of Review
Evidence-based strategies are needed to address the growing complexity of care of those ageing with HIV so that as life expectancy is extended, quality of life is also enhanced.
Recent Findings
Modifiable contributing factors to the quantity and quality of life in adults ageing with HIV include: burden of harmful health behaviours, injury from HIV infection, HIV treatment toxicity, and general burden of age-associated comorbidities. In turn, these factors contribute to geriatric syndromes including multimorbidity and polypharmacy, physiologic frailty, falls and fragility fractures, and cognitive dysfunction, which further compromise the quality of life long before they lead to mortality.
Summary
Viral suppression of human immunodeficiency virus (HIV) with combination antiviral therapy (cART) has led to increasing longevity but has not enabled a complete return to health among ageing HIV-infected individuals (HIV+). As adults age with HIV, the role of HIV itself and associated inflammation, effects of exposure to antiretroviral agents, the high prevalence of modifiable risk factors for age-associated conditions (e.g. smoking), and the effects of other viral coinfections are all influencing the health trajectory of persons ageing with HIV. We must move from the simplistic notion of HIV becoming a “chronic controllable illness” to understanding the continually evolving “treated” history of HIV infection with the burden of age-associated conditions and geriatric syndromes in the context of an altered and ageing immune system.
Keywords: HIV, ageing, multimorbidity, patient centered care, polypharmacy
INTRODUCTION
Where ever effective antiretroviral therapy (ART) is available, people are ageing with HIV. The median age of HIV-infected adults has passed 50 years in the United States, and Canada, Australia and most of Europe are close behind. Similar trends are emerging in Latin America, the Caribbean, Sub-Saharan Africa, Asia, the Middle East, and North Africa.1 Nevertheless, HIV-infected adults ≥50 years of age with suppressed HIV-1 RNA and free of AIDS-defining illnesses or comorbidities experience a shorter life expectancy than uninfected individuals.2,3 Modifiable contributing factors to the quantity and quality of life in adults ageing with HIV likely include: burden of harmful health behaviours, injury from HIV infection, HIV treatment toxicity, and general burden of age-associated comorbidities. In turn, these factors contribute to geriatric syndromes including multimorbidity and polypharmacy, physiologic frailty, falls and fragility fractures, and cognitive dysfunction, which further compromise quality of life long before they lead to mortality. Evidence-based strategies are needed to address the growing complexity of care of those ageing with HIV so that life expectancy can be extended and quality of life can be preserved.
FACTORS INFLUENCING AGEING WITH HIV
Although adults ageing with HIV are subject to the same risk factors for age-related diseases and conditions as uninfected adults, they differ in prevalence of harmful health behaviours. They also experience ongoing HIV-associated inflammation and immune activation4 and adverse effects of chronic exposure to ART likely leading to excess organ system injury.5 The extent to which this excess is expressed in cellular ageing, including cellular senescence, mitochondrial dysfunction, telomere attrition, and epigenetic alteration remains an area of active research.6,7
Weight gain after ART initiation
While HIV-infected adults tend to be less obese than uninfected adults,8 the prevalence of obesity has increased over time, and is associated with the ageing HIV-infected population, earlier ART start and increasingly widespread ART coverage.9,10 Weight gain following ART has been well documented10,11, with increase in body mass index amongst HIV-positive patients in the first year on ART surpassing that of demographically-matched uninfected comparators.10,11 This weight gain is likely due in part to decreased metabolic demand from ART-induced viral suppression coupled with ART-induced fat accumulation and potential changes to appetite.12 However, weight gain after ART should be avoided among those who are overweight or obese as it is not independently associated with improved survival.11 Further, ART-associated weight gain is associated with incident cardiovascular disease (CVD), diabetes and higher waist circumference and lower hip circumference may mediate the association between HIV and frailty in HIV-infected adults.13,14
Increased visceral adipose tissue (VAT) is particularly problematic. Even with current ART medications, VAT increases by 25–35% in the two years following ART initiation.15 Elevated VAT and peripheral lipoatrophy are associated with CVD risk and risk between VAT and CVD is higher in HIV-infected compared to uninfected adults irrespective of VAT-level.16 Further, renin-angiotensin-aldosterone system activation associated with VAT accumulation contributes to insulin resistance in HIV infection,17 which likely contributes to the excess risk of diabetes associated with weight gain after ART initiation (Herrin).
Further, although information on short-term risk of side effects is required for antiretroviral drug licensing, longer-term effects must be evaluated once a drug is in clinical use. Certain antiretroviral drugs may contribute to the risk of comorbidites via related toxicity and metabolic pathway activation. For example, abacavir and some protease inhibitors (PIs) have been associated with cardiovascular risk,18 PIs have also been associated with insulin resistance and diabetes.19,20
Harmful health behaviours
Smoking, alcohol, and substance abuse all represent modifiable risk factors for comorbidities. HIV-infected adults on ART in Europe and the United States may lose more life years through smoking than through HIV.21 Alcohol is associated with an excess risk of physiologic frailty and mortality among HIV-infected compared with uninfected adults, even at low levels.22 Further, HIV-infected adults are not exempt from the epidemic of opioid abuse which is being fueled by a growing abundance of opioid prescriptions, and is a particular problem in the United States.23 Opioid-associated mortality is higher in older compared to younger adults, irrespective of HIV status.24 The paper by Kathy Petoumenous et al in this issue outlines the association between HIV, substance abuse and survival in more detail.
COMORBIDITIES: AGE- AND HIV-ASSOCIATED NON-AIDS CONDITIONS
Among those ageing with HIV, increased life expectancy has been accompanied by dramatic decreases in AIDS-related morbidity and mortality and an excess burden of comorbidities compared with uninfected demographically similar adults.25–28 The TEMPRANO and START trials have demonstrated that immediate ART initiation can reduce the incidence of comorbidities compared to deferred treatment initiation, pointing to the role of long-term immune activation and inflammation.29,30 Of note, while those with HIV are at greater risk of comorbidites across age strata and while certain cellular markers may suggest more rapid “ageing,” comorbid events do not appear to occur dramatically earlier.26,31 In fact, and perhaps even more worrisome, differences in risk may grow with age.26
Cardiovascular disease (CVD)
The risk of myocardial infarction (MI), for example, is 1.5- to 2-fold higher among HIV-infected adults without any major CVD risk factor than uninfected adults, and even higher amongst those with HIV and CVD risk factors;25,32 it is possible that the relative risk of MI is changing over time in HIV-infected adults.33 ill Detectable HIV-1 RNA has been associated with CVD in HIV-infected adults.34 Risk prediction models for CVD specific to HIV-infected adults, such as the D:A:D CVD risk model, take into account HIV-related risk factors (e.g. CD4 count and ART regimens) and have demonstrated superior risk prediction of myocardial infarction compared to the Framingham and ATP3 risk scores, both developed in the general population.35,36 The 2013 American College of Cardiology/American Heart Association atherosclerotic CVD (ASCVD) risk prediction model however, which was developed in the general population and is used to determine likelihood of benefit from statin treatment, has recently been shown to be a better model for discriminating those with high and low risk compared to the D:A:D model.36 However, the ASCVD model suffers from issues with calibration when applied to HIV-infected adults, resulting in underestimates of high CVD risk and underutilization of statins.37 A new clinical trial, REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV), will test the impact of pitavastatin on primary prevention of CVD among people with HIV at low to moderate a priori CVD risk.38
Cancer
Non-AIDS cancers are now the leading cause of death among HIV-infected individuals in care27 and a major source of morbidity for people ageing with HIV. Virally associated cancers, including hepatocellular, anal, oropharyngeal, cervical cancer, and Hodgkin’s lymphoma, represent a significant burden of disease among those ageing with HIV. Anal cancer remains high, particularly in men-who-have-sex-with-men, and is linked to infection with human papillomavirus and possibly time on PI-based regimens.39,40 The new multi-centre trial, ANCHOR, will help determine if treatment of pre-cancerous lesions among those with HIV can prevent anal cancer from developing.41 In addition, age-related cancers increasingly contribute to cancer burden among those with HIV.42 HIV-infected adults have a disproportionate burden of lung cancer, and HIV has been found to be an independent risk factor for lung cancer after adjustment for smoking and other risk factors.43
Liver Disease
Risk of liver disease is elevated among HIV-infected adults without viral hepatitis. The risk of hepatocellular cancer, a major form of cancer among those with HIV, only occurs among those with pre-existing liver fibrosis and most typically, advanced fibrosis or cirrhosis. ART medications can exacerbate liver injury through multiple mechanisms including metabolic host-mediated injury (tipranavir), hypersensitivity (abacavir and nevirapine), and mitochondrial toxicity (didanosine, stavudine, and zidovudine).44
Additionally, the odds of having hepatitis C virus (HCV) infection are six time higher in people living with HIV than in those without HIV.45 Co-infection with HCV has a synergistic effect on liver injury leading to more rapid progression of cirrhosis which is slowed, but not neutralized, after ART initiation.46 Highly effective direct-acting antivirals for HCV47 may slow progression of fibrosis but the impact of direct-acting antivirals on the long-term risk of hepatocellular carcinoma remains to be established. The paper by Klein et al in this issue outlines the association between HIV and HCV in more detail. Co-infection with hepatitis B is also relatively common, especially in Asia. Its effects in association with HIV are less well understood, but may be contributing to the risk of hepatocellular cancer.
Renal insufficiency
Renal insufficiency, a major risk factor for drug toxicity and CVD, is a contra-indication for several antiretroviral drugs, including tenofovir disoproxil fumarate. Renal insufficiency is likely multifactorial including decreasing renal function with age, greater genetic susceptibility to renal disease among those of African descent and renal toxicity related to long-term use of ART. Tenofovir alafenamide, which has demonstrated similar efficacy in suppressing HIV but an improved renal safety profile compared to tenofovir disoproxil fumarate, may help preserve renal function but will need to be evaluated in long-term studies.48 Finally, lower CD4 cell counts are associated with a greater risk of renal disease progression and ART (excluding tenofovir disoproxil fumarate) appears to ameliorate some of this risk.49,50
Pulmonary disease
Lung diseases associated with HIV after accounting for smoking include pulmonary hypertension, bacterial pneumonia, and Chronic Obstructive Pulmonory Disease (COPD).51 Infection with HIV, especially with lower CD4 cell count, is an independent risk factor for acute exacerbations of COPD,52 and is also associated with reduced diffusing capacity.53 While a relatively rare event in the general population, pulmonary arterial hypertension occurs 1,000-fold more commonly among those with HIV for reasons that are not well understood. Lung disease in HIV has a major impact on symptom burden, functional status, and frailty.54,55 Vaccination may be an important part of resilient ageing in those ageing with HIV; pneumococcal vaccination of those with HIV infection offers protection against pneumonia.
Depression
An estimated 13% of HIV-infected adults experience major depression.56 Depression has been linked to reduced retention in care57 and reduced cognitive performance.58 Traditionally, depression has been associated with stigma in HIV-infected populations.59 In the geriatric population additional triggers for depression may include social isolation and additional stresses, such as ill health and stress caused by the loss of a loved one. A recent study showed significant discrepancy in physician perception and self-reported depressive symptoms and called for mandatory depression screening in all HIV-infected adults.60 Measuring depression is challenging, with many options for screening, diagnosis, and rating symptoms and the need to account for cultural considerations. Bipolar disorders and schizophrenia may interact with depression. Depressive symptoms in people with HIV with bipolar disorder have been associated with poor psychotropic medication adherence.61 Both bipolar disorder and schizophrenia have also been linked to poor adherence to ART in HIV-infected adults.62 Some ART medications may also contribute; there remains controversy concerning efavirenz and suicide with a recent analysis suggesting a two-fold increased risk.63
GERIATRIC SYNDROMES
The increased burden of comorbidities in adults ageing with HIV requires a shift in the HIV care paradigm from targeted disease-specific management to a holistic, geriatrics-based, approach (Figure 1). The goal in geriatric care is not exclusively one of extending survival. It is instead focused on also maintaining quality of life for as long as possible. Geriatric syndromes are characterized by multifaceted aetiologies and recursive associations; for example, multimorbidity leads to polypharmacy. Polypharmacy can lead to declines in neurocognitive performance, which can contribute to falls and fragility fractures causing pain, additional psychoactive medication, and increased polypharmacy.
Figure 1.
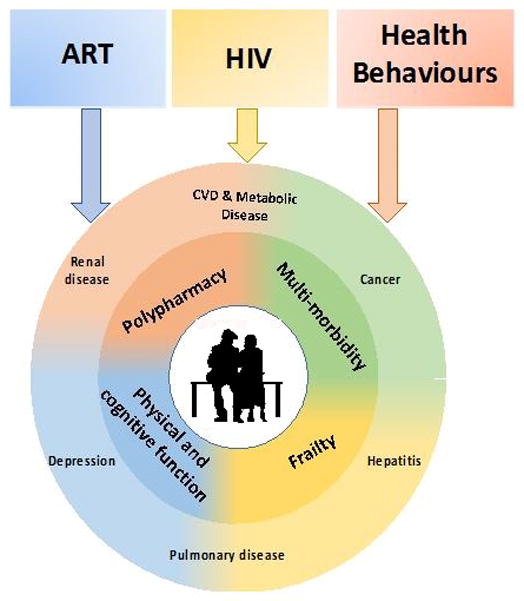
Moving from standard care of ageing HIV-positive patients to care that incorporates key geriatric principles. The boxes illustrate elements amongst HIV-positive patients that may further contribute to disease and condition in ageing. Within the circle are the levels of clinical/medical elements that patients may suffer from as they age with HIV.
Depiction of the complexities of aging with HIV and caring for this population.
Multimorbidity and polypharmacy
Multimorbidity and polypharmacy go hand-in-hand. Multimorbidity, defined as “multiple, potentially interacting, medical and psychiatric conditions,” is a geriatrics-rooted concept with applicability to ageing with HIV.64 Multimorbidity is typically measured as a count of the number of comorbid conditions, which makes multimorbidity a function of the number of conditions considered.64 Multimorbidity is both a result of risk factors for individual HIV and age-related conditions, as well as the propensity for one condition to increase the risk of others. As the number of conditions increases, so does the proportion of individuals taking multiple medications (polypharmacy). Patients on 5 or more medications experience a significant number of medication-related adverse effects.65 Among HIV-infected individuals 50 years or older with access to care, polypharmacy is becoming the norm in North America.66–68 Approximately a third of long-term HIV-infected adults in Canada are taking 5 or more medications.66 In contrast, in the Netherlands only 5% of all patients in care are taking 3 or more medications in addition to ART and these proportions increase with age64 and will likely increase with time.67 Opioid and benzodiazepine medications are particularly problematic and confer an independent association with mortality after adjustment for severity of illness and for number of chronic medications.69
A recent modelling study forecasts that multi-morbidity amongst HIV-infected adults in Europe will increase to 84% by 2030, with 54% using multiple medications, and CVD contributing the largest burden.67 Harms of multimorbidity and polypharmacy include: 1) complicated drug interactions (with other drugs and with alcohol and substance use), 2) a potential decrease in ART adherence due to confusion of medication dosing and timing and/or medication fatigue, 3) cumulative toxicity, 4) mortality and 5) expense. Successful management of multimorbidity and polypharmacy will be vital for future HIV care. A proposed framework for managing polypharmacy in people ageing with HIV includes 1) an annual medication reconciliation, 2) assessment of tobacco, alcohol, and substance use, 3) a risk and benefit assessment and ranking of medications, and 4) prioritization with patient input.68 As a result of multimorbidity and polypharmacy, ageing HIV-infected adults are likely to suffer from the geriatric syndromes discussed below.
Functional decline
Decline in physical performance can be reflected in slow gait speed and weak grip strength, both of which appear to be predictors of disability, morbidity and mortality in the general population. Faster rate of decline and an increased risk for slow gait speed have been observed among HIV-infected compared to uninfected men.70 Functional impairment, including gait speed and grip strength, has been associated with low muscle mass and loss of bone density in those ageing with HIV.71 Increased physical activity are recommended for reducing functional impairment among those ageing with HIV.72
Cognitive dysfunction
Cognitive dysfunction in HIV is multifaceted in nature and dysfunction is estimated to effect as many as half of HIV-infected adults.73 Contributing factors associated with cognitive dysfunction may include HIV itself including serious immune-deficiency, on-going substance use, neurocognitively active medications, depression, and multimorbidity.74,75 Changes in brain structure have been correlated with cognitive impairment in HIV-infected adults,73 with a recent study in suppressed HIV-infected and uninfected adults showing that microstructural abnormalities were more common in HIV-infected adults.76 Most recently, a novel high-resolution subcortical shape analysis technique was found to be more sensitive to associations between brain volume and CD4 counts as well as neurocognitive scores over traditional whole volume subcortical analyses.77 As people age with HIV beyond 65 years of age we will likely see substantially more cognitive dysfunction with consequence for HIV care, including lower adherence to ART78 and reduced retention.57,58 Alzheimer’s disease and vascular dementia will likely play an increasing role.
Frailty
Frailty, another geriatric syndrome, is commonly defined as a loss of physiologic reserve and increased vulnerability to negative health outcomes. Many recent studies have shown an increased prevalence of frailty in HIV-infected compared with uninfected adults.14,79 Frailty has commonly been measured using the Fried criteria (≥3 of the following criteria: weakness, slowness, unintentional weight loss, exhaustion, and low physical activity), which were developed and validated in a general population sample age 80 years or older,80 and there is controversy regarding how to operationalize this definition among people ageing with HIV. 79
Alternative measures of frailty include the Rockwood index and the Veterans Aging Cohort Study (VACS) Index (http:vacs.med.yale.edu). While the former is based on accumulation of deficits, and has not been widely applied amongst ageing adults with HIV81 the VACS Index was developed specifically for HIV-infected adults and is based on an accumulation of physiologic deficits. The VACS Index incorporates age and biomarkers specific for HIV and for liver, renal, and bone marrow disease. Further, the VACS Index has been associated with physiologic frailty,82–84 cognitive performance,74 functional status,85 hospitalizations,86 and inflammatory markers 87,88 and reproducibly estimates the probability of all-cause mortality among HIV infected individuals.89,90 The VACS Index is also a good predictor of cardiovascular mortality and can improve clinical assessment of mortality risk even when all the component measures are available.91,92 It is increasingly used in HIV clinical settings to guide decision-making as to the frequency of medical follow-up, clinical risk assessment and end of life planning.
Falls and fractures
Falls and fractures are important health outcomes that have been linked to multimorbidity, polypharmacy, functional impairment, CVD, diabetes, HCV co-infection, and tenofovir disoproxil fumarate use.82,93–96 Markers of HIV-infection (CD4 count, viral load), however, have not been associated with falls.7272,70 Interestingly, the perception of balance has been associated with falls in HIV-infected men.97 Alcohol and other substance use likely contribute substantially to risk of falls and fractures. Proton pump inhibitors, a commonly used medication to reduce gastric acid production, have negative effects on bone health (and have also been linked to an increased risk of chronic kidney disease).98 A single infusion of zoledronic acid, a long-acting bisphosphonate for the treatment of osteoporosis, at ART initiation was shown to prevent ART-induced bone loss.99 However, the long-term effects of bisphosphonates, the first-line therapy for low bone mineral density, as well as the impact of calcium and vitamin D supplementation to prevent bone disease, are unknown in HIV-infected adults.
CONCLUSION
As disease patterns become more complex among those ageing with HIV, HIV care will need to carefully consider how to appropriately prioritize prevention, screening and treatment (see Text Box and Figure 1) and clinical research will need to embrace the complexity of ageing with HIV (see Text Box and Figure 1) to maintain a high quality of life while continuing to extend survival.
Table 1.
Integrated Care for those Aging with HIV
To ensure improved survival and quality of life for those ageing with HIV, we recommend integrating the following principles into HIV care:
|
Table 2.
Research Recommendations
If we are to optimize care for those aging with HIV infection, we need substantially more information from studies that span the continuum from basic science to observational research to operations research to clinical trials. Questions that need to be addressed include:
|
Table 3.
Online Resources
The following are resources for additional information relevant to aging well with HIV and caring for those aging with HIV.
|
Key Points.
Ageing with HIV is characterized by multimorbidity and polypharmacy, which is strongly influenced by chronic infection with HIV.
Many factors influence quality and quantity of life in this group, HIV-1 RNA suppression is the first therapeutic goal, but many other management issues follow.
Attention to mental health, geriatric syndromes, maintaining physical activity, and avoiding excess weight gain after ART are advised.
Acknowledgments
We would like to thank Drs. Perry Wilson, Kristina Crothers, and Leslie Park for critical reading and suggestions on this manuscript.
Financial Support
Dr. Justice’s research is funded through the National Institutes of Health in the United States including National Institute of Alcohol Abuse and Alcoholism (U01 AA020790; U24 AA020794; U24 AA022001) and National Heart, Lung, and Blood Institute (R01 HL125032-01). Dr. Keri Althoff is by the National Institute of Allergy and Infectious Diseases (K01 AI093197). Dr Mikaela Smit’s research in funded by the Stichting HIV Monitoring Foundation, The Netherlands.
Footnotes
Conflict of Interest
Dr. Justice reports no conflict of interest. Dr. Reiss through his institution has received independent scientific grant support from Gilead Sciences, Inc., Janssen Pharmaceuticals Inc, Merck & Co, Bristol-Myers Squibb and ViiV Healthcare; he has served on scientific advisory board for Gilead Sciences, Inc.; he serves on data safety monitoring committee for Janssen Pharmaceuticals Inc; chaired a scientific symposium by ViiV Healthcare, for which his institution has received remuneration. Dr. Keri Althoff has served on scientific advisory boards for Gilead Sciences, Inc. Dr Mikaela Smit received consultancy fees from Gilead Science to present at the Advisory Board and HIV team meeting, and is receiving through her institution scientific grant support from Gilead Science.
Bibliography
- 1.UNAIDS. The Gap report 2014 - People aged 50 years and older. 2014 [Google Scholar]
- **2.Legarth RA, et al. Long-Term Mortality in HIV-Infected Individuals 50 Years or Older: A Nationwide, Population-Based Cohort Study. J Acquir Immune Defic Syndr. 2016;71:213–218. doi: 10.1097/QAI.0000000000000825. This Danish study shows that although life expectancy amongst HIV-infected individuals over 50 years old has increased by more than 10 years from 1996–1999 to 2006–2014, it remains lower than the general population, even in those well treated and without comorbidity. [DOI] [PubMed] [Google Scholar]
- 3.Marcus J, Chao C, Leyden WA, et al. Narrowing the Gap in Life Expectancy for HIV+ Compared With HIV− Individuals. CROI; Boston Massachusetts. 2016. Abstract #54. [Google Scholar]
- 4.Wada NI, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horvath S, Levine AJ. HIV-1 Infection Accelerates Age According to the Epigenetic Clock. J Infect Dis. 2015;212:1563–1573. doi: 10.1093/infdis/jiv277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS. 2016;30:273–291. doi: 10.1097/QAD.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koethe JR, et al. Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32:50–58. doi: 10.1089/aid.2015.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasse B, et al. Obesity Trends and Body Mass Index Changes After Starting Antiretroviral Treatment: The Swiss HIV Cohort Study. Open Forum Infect Dis. 2014;1:ofu040. doi: 10.1093/ofid/ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuh B, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015;60:1852–1859. doi: 10.1093/cid/civ192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batterham MJ. Investigating heterogeneity in studies of resting energy expenditure in persons with HIV/AIDS: a meta-analysis. Am J Clin Nutr. 2005;81:702–713. doi: 10.1093/ajcn/81.3.702. [DOI] [PubMed] [Google Scholar]
- *13.Achhra AC, et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med. 2015 doi: 10.1111/hiv.12294. This multi-centre cohort study of HIV-infected individuals in Europe, USA and Australia found that short-term BMI gain following ART initiation may increase longer-term cardiovascular disease risk amongst people with normal pre-ART BMI, and diabetes irrespective of pre-ART BMI. [DOI] [PubMed] [Google Scholar]
- *14.Kooij KW, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. AIDS. 2016;30:241–250. doi: 10.1097/QAD.0000000000000910. This study of HIV-infected adults over 45 years of age and uninfected controls in the Netherlands found that pre-frailty and frailty was significantly higher amongst older HIV-infected compared to age-matched controls. This may be mediated by higher waist-and lower hip-circumference in HIV-infected individuals, potentially caused by lipodystrophy and weight loss associated with advanced HIV disease. [DOI] [PubMed] [Google Scholar]
- 15.McComsey GA. Visceral Adiposity in the Modern HIV Treatment Era. CROI; Boston Massachusetts. 2016. Abstract #123. [Google Scholar]
- 16.Lake JE, et al. Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study. AIDS Care. 2011;23:929–938. doi: 10.1080/09540121.2010.543885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasa S, et al. RAAS Activation Is Associated With Visceral Adiposity and Insulin Resistance Among HIV-infected Patients. J Clin Endocrinol Metab. 2015;100:2873–2882. doi: 10.1210/jc.2015-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friis-Møller N, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2015 doi: 10.1177/2047487315579291. [DOI] [PubMed] [Google Scholar]
- 19.De Wit S, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31:1224–1229. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samaras K. The burden of diabetes and hyperlipidemia in treated HIV infection and approaches for cardiometabolic care. Curr HIV/AIDS Rep. 2012;9:206–217. doi: 10.1007/s11904-012-0124-x. [DOI] [PubMed] [Google Scholar]
- **21.Helleberg M, et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS. 2015;29:221–229. doi: 10.1097/QAD.0000000000000540. Analysis of data from the multicentre ART Cohort Collaboration (ART-CC)\of European and North American HIV-positive individuals found that successfully treated HIV-infected individuals may loose more life-years through smoking than HIV, and this excess mortality may increase with age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **22.Justice AC, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016 doi: 10.1016/j.drugalcdep.2016.01.017. This analyses of the large US-based Veterans Aging Cohort Study found that ongoing alcohol use is common among HIV-infected individuals, that HIV-infected individuals experience greater mortality and physiological injury with lower levels of alcohol consumption compared to uninfected controls, this work suggests that drinking limits for those with HIV infection should be no more than one drink per day (half the current recommended limit for men in the general population) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker WC, et al. Trends in Any and High-Dose Opioid Analgesic Receipt Among Aging Patients With and Without HIV. AIDS Behav. 2016;20:679–686. doi: 10.1007/s10461-015-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West NA, Severtson SG, Green JL, Dart RC. Trends in abuse and misuse of prescription opioids among older adults. Drug and Alcohol Dependence. 2015;149:117–121. doi: 10.1016/j.drugalcdep.2015.01.027. [DOI] [PubMed] [Google Scholar]
- *25.Schouten J, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV Cohort Study. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu701. This study of HIV-infected adults over 45 years of age and uninfected controls in the Netherlands found that peripheral arterial, cardiovascular and impaired renal function were significantly more prevalent amongst HIV-positive individuals compared to uninfected controls, with risk factors including cardiovascular risk factors, HIV infection, and longer duration of immunodeficiency. [DOI] [PubMed] [Google Scholar]
- *26.Althoff KN, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis. 2015;60:627–638. doi: 10.1093/cid/ciu869. This study of HIV-infected, 97% of which are male, and demographically matched controls from the US-based Veteran Aging Cohort Study found that while HIV-infected adults had a higher risk of age-associated non-communicable events they occurred at similar ages than those without HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Smith CJ, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. This multi-centre cohort study of HIV-infected individuals in Europe, USA and Australia found that reductions in AIDS-related deaths over time may be linked to improvements in CD4 count, while the reduction in liver disease and cardiovascular disease deaths over time could be explained by non-HIV-specific preventive interventions. The leading cause of non-AIDS mortality are non-AIDS cancers. [DOI] [PubMed] [Google Scholar]
- 28.Cox JA, et al. Temporal trends in death causes in adults attending an urban HIV clinic in Uganda: a retrospective chart review. BMJ Open. 2016;6:e008718. doi: 10.1136/bmjopen-2015-008718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. New England Journal of Medicine. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.TEMPRANO ANRS 12136 Study Group et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen LD, et al. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV. 2015;2:e288–298. doi: 10.1016/S2352-3018(15)00077-6. [DOI] [PubMed] [Google Scholar]
- 32.Paisible AL, et al. Human immunodeficiency virus infection, cardiovascular risk factor profile and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68:209–216. doi: 10.1097/QAI.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein DB, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis. 2015;60:1278–1280. doi: 10.1093/cid/civ014. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, et al. Episodes of HIV viremia and the risk of non-AIDS diseases in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60:265–272. doi: 10.1097/QAI.0b013e318258c651. [DOI] [PubMed] [Google Scholar]
- 35.Pirš M, et al. Cardiovascular risk assessment in HIV-infected male patients: a comparison of Framingham, SCORE, PROCAM and DAD risk equations. Acta Dermatovenerol Alp Pannonica Adriat. 2014;23:43–47. doi: 10.15570/actaapa.2014.11. [DOI] [PubMed] [Google Scholar]
- 36.Crain HM, et al. Comparing Cardiovascular Disease Risk Scores for Use in HIV-Infected Individuals. CROI; Boston Massachusetts. 2016. Abstract #42. [Google Scholar]
- 37.Regan S, et al. Evaluation of the ACC/AHA CVD Risk Prediction Algorithm Among HIV-Infected Patients. CROI; Seattle, Washington. 2015. Abstract #751. [Google Scholar]
- 38.Gilbert JM, Fitch KV, Grinspoon SK. HIV-Related Cardiovascular Disease, Statins, and the REPRIEVE Trial. Top Antivir Med. 2015;23:146–149. [PMC free article] [PubMed] [Google Scholar]
- 39.Mbang PA, et al. Association between Time on Protease Inhibitors and the Incidence of Squamous Cell Carcinoma of the Anus among U.S. Male Veterans. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruyand M, et al. Cancer Risk and Use of Protease Inhibitor or Nonnucleoside Reverse Transcriptase Inhibitor–Based Combination Antiretroviral Therapy: The D. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015;68:568–577. doi: 10.1097/QAI.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 41.The Anchor Study. [Accessed: 7th March 2016]; Available at: https://anchorstudy.org/
- **42.Silverberg MJ, et al. Cumulative Incidence of Cancer Among Persons With HIV in North America: A Cohort Study. Ann Intern Med. 2015;163:507–518. doi: 10.7326/M14-2768. This large North American cohort collaboration found that Kaposi’s sarcoma, non-Hodgkin’s lymphoma and lung cancer contributed greatly to cumulative cancer incidence by age 75 amongst HIV-infected individuals after accounting for the competing risk of death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigel K, et al. HIV as an independent risk factor for incident lung cancer. AIDS. 2012;26:1017–1025. doi: 10.1097/QAD.0b013e328352d1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed: 19th April 2016];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.
- 45.Platt L, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. The Lancet Infectious Diseases. 2016 doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- *46.Anderson JP, et al. Antiretroviral therapy reduces the rate of hepatic decompensation among HIV− and hepatitis C virus-coinfected veterans. Clin Infect Dis. 2014;58:719–727. doi: 10.1093/cid/cit779. This paper reported that treatment of HIV infection slows the rate of liver disease among those who are co-infected with HCV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam BP, Jeffers T, Younoszai Z, Fazel Y, Younossi ZM. The changing landscape of hepatitis C virus therapy: focus on interferon-free treatment. Therap Adv Gastroenterol. 2015;8:298–312. doi: 10.1177/1756283X15587481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sax PE, et al. Tenofovir Alafenamide Vs. Tenofovir Disoproxil Fumarate in Single Tablet Regimens for Initial HIV-1 Therapy: A Randomized Phase 2 Study. J Acquir Immune Defic Syndr. 2014;67:52–58. doi: 10.1097/QAI.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 49.Ryom L, et al. Predictors of advanced chronic kidney disease and end-stage renal disease in HIV-positive persons. AIDS. 2014;28:187–199. doi: 10.1097/QAD.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 50.Kalayjian RC, et al. Risk factors for chronic kidney disease in a large cohort of HIV-1 infected individuals initiating antiretroviral therapy in routine care. AIDS. 2012;26:1907–1915. doi: 10.1097/QAD.0b013e328357f5ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drummond MB, Kirk GD. HIV-associated obstructive lung diseases: insights and implications for the clinician. Lancet Respir Med. 2014;2:583–592. doi: 10.1016/S2213-2600(14)70017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Depp TB, et al. Risk factors associated with acute exacerbation of chronic obstructive pulmonary disease in HIV-infected and uninfected patients. AIDS. 2016;30:455–463. doi: 10.1097/QAD.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *53.Crothers K, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr. 2013;64:271–278. doi: 10.1097/QAI.0b013e3182a9215a. This provactive paper suggests that those with HIV infection may experience different lung disease pathology and invites further translational work in this area. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campo M, et al. Association of chronic cough and pulmonary function with 6-minute walk test performance in HIV infection. J Acquir Immune Defic Syndr. 2014;65:557–563. doi: 10.1097/QAI.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akgün KM, et al. Association of chronic obstructive pulmonary disease (COPD) with frailty measurements in HIV-infected and uninfected Veterans. AIDS. 2016 doi: 10.1097/QAD.0000000000001162. [DOI] [PubMed] [Google Scholar]
- *56.Do AN, et al. Excess burden of depression among HIV-infected persons receiving medical care in the United States: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS ONE. 2014;9:e92842. doi: 10.1371/journal.pone.0092842. This study showed a 3-fold increase in the prevalence of current depression in HIV-infected adults receiving care compared to the general population in the United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuniga JA, Yoo-Jeong M, Dai T, Guo Y, Waldrop-Valverde D. The Role of Depression in Retention in Care for Persons Living with HIV. AIDS Patient Care STDS. 2016;30:34–38. doi: 10.1089/apc.2015.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schouten J, et al. Determinants of reduced cognitive performance in HIV-1-infected middle-aged men on combination antiretroviral therapy. AIDS. 2016 doi: 10.1097/QAD.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 59.Shacham E, Rosenburg N, Önen NF, Donovan MF, Overton ET. Persistent HIV-related stigma among an outpatient US clinic population. Int J STD AIDS. 2015;26:243–250. doi: 10.1177/0956462414533318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marando F, et al. Discrepancies between physician’s perception of depression in HIV patients and self-reported CES-D-20 assessment: the DHIVA study. AIDS Care. 2016;28:147–159. doi: 10.1080/09540121.2015.1080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Casaletto KB, et al. Predictors of psychotropic medication adherence among HIV+ individuals living with bipolar disorder. Int J Psychiatry Med. 2016;51:69–83. doi: 10.1177/0091217415621267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore DJ, et al. HIV-infected individuals with co-occurring bipolar disorder evidence poor antiretroviral and psychiatric medication adherence. AIDS Behav. 2012;16:2257–2266. doi: 10.1007/s10461-011-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mollan KR, et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med. 2014;161:1–10. doi: 10.7326/M14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyd CM, Fortin M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design. Public Health Rev. 2010;32:451–474. [Google Scholar]
- 65.Gnjidic D, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–995. doi: 10.1016/j.jclinepi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- *66.Krentz HB, Gill MJ. The Impact of Non-Antiretroviral Polypharmacy on the Continuity of Antiretroviral Therapy (ART) Among HIV Patients. AIDS Patient Care STDS. 2016;30:11–17. doi: 10.1089/apc.2015.0199. This study of HIV-infected adults in Canada found that polypharmacy was associated with lower CD4 count, AIDS, older age and increased risk of non-continuous ART use. [DOI] [PubMed] [Google Scholar]
- **67.Smit M, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. The Lancet Infectious Diseases. 2015 doi: 10.1016/S1473-3099(15)00056-0. This modelling study quantifies how the HIV-infected adults in the Netherlands will be aging over the coming 20 years, resulting in a large increase in the burden of comorbidity, polypharmacy and potential complications in the sense of ART contra-indications and drug-interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edelman EJ, et al. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging. 2013;30:613–628. doi: 10.1007/s40266-013-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **69.Weisberg DF, et al. Long-term Prescription of Opioids and/or Benzodiazepines and Mortality Among HIV-Infected and Uninfected Patients. J Acquir Immune Defic Syndr. 2015;69:223–233. doi: 10.1097/QAI.0000000000000591. This important paper reported increased risk for mortality among those taking prescriptions opioids and benzodiazepines beyond that from polypharmacy alone, with excess risk among HIV infected compared with uninfected individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *70.Schrack JA, et al. Accelerated Longitudinal Gait Speed Decline in HIV-Infected Older Men. J Acquir Immune Defic Syndr. 2015;70:370–376. doi: 10.1097/QAI.0000000000000731. This study in the US-based Multicenter AIDS Cohort Study (MACS) showed HIV-infected men had a greater decrease in gait speed (an important measure of physical function) as compared to similar uninfected men. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Erlandson KM, et al. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr. 2013;63:209–215. doi: 10.1097/QAI.0b013e318289bb7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erlandson KM, et al. Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials. 2012;13:324–334. doi: 10.1310/hct1306-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen RA, Seider TR, Navia B. HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther. 2015;7:37. doi: 10.1186/s13195-015-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marquine MJ, et al. The veterans aging cohort study index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr. 2014;65:190–197. doi: 10.1097/QAI.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schouten J, et al. Determinants of reduced cognitive performance in HIV-1-infected middle-aged men on combination antiretroviral therapy. AIDS. 2016;1 doi: 10.1097/QAD.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 76.Underwood J, et al. Brain MRI Changes Associated With Poorer Cognitive Function in Treated HIV Infection. CROI; 2016; Boston Massachusetts. 2016. Abstract #148. [Google Scholar]
- 77.Ching CR, et al. NeuroHIV: A Novel High-Resolution Subcortical Shape Analysis. CROI; Boston Massachusetts. 2016. Abstract #386. [Google Scholar]
- 78.Campbell NL, et al. Self-Reported Medication Adherence Barriers Among Ambulatory Older Adults with Mild Cognitive Impairment. Pharmacotherapy. 2016;36:196–202. doi: 10.1002/phar.1702. [DOI] [PubMed] [Google Scholar]
- 79.Althoff KN, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci. 2014;69:189–198. doi: 10.1093/gerona/glt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fried LP, et al. Frailty in Older Adults Evidence for a Phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 81.Rockwood K, Bergman H. FRAILTY: A Report from the 3(rd) Joint Workshop of IAGG/WHO/SFGG, Athens, January 2012. Can Geriatr J. 2012;15:31–36. doi: 10.5770/cgj.15.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Womack JA, et al. Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis. 2013;56:1498–1504. doi: 10.1093/cid/cit056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Akgün KM, et al. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr. 2014;67:397–404. doi: 10.1097/QAI.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Escota GV, et al. Short communication: The Veterans Aging Cohort Study Index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS Res Hum Retroviruses. 2015;31:313–317. doi: 10.1089/AID.2014.0225. [DOI] [PubMed] [Google Scholar]
- 85.Oursler KK, et al. Association of the veterans aging cohort study index with exercise capacity in HIV-infected adults. AIDS Res Hum Retroviruses. 2013;29:1218–1223. doi: 10.1089/aid.2012.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akgün KM, et al. Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV-infected Veterans. J Acquir Immune Defic Syndr. 2013;62:52–59. doi: 10.1097/QAI.0b013e318278f3fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Justice AC, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mooney S, Tracy R, Osler T, Grace C. Elevated Biomarkers of Inflammation and Coagulation in Patients with HIV Are Associated with Higher Framingham and VACS Risk Index Scores. PLoS ONE. 2015;10:e0144312. doi: 10.1371/journal.pone.0144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Justice AC, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62:149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tate JP, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27:563–572. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Justice AC, et al. Can the Veterans Aging Cohrt Study Index Improve Clinical Judgement?. CROI; Atlanta, GA. 2013. Abstract #573. [Google Scholar]
- 92.Justice AC, Tate JP, Freiberg MS, Rodriguez-Barradas MC, Tracy R. Reply to Chow et al. Clin Infect Dis. 2012;55:751–752. [Google Scholar]
- 93.Sharma A, et al. Increased Fracture Incidence in Middle-Aged HIV-Infected and HIV-Uninfected Women: Updated Results From the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2015;70:54–61. doi: 10.1097/QAI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peters BS, et al. A cross-sectional randomised study of fracture risk in people with HIV infection in the probono 1 study. PLoS ONE. 2013;8:e78048. doi: 10.1371/journal.pone.0078048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Neill TJ, Rivera L, Struchkov V, Zaheen A, Thein HH. The effect of HIV-hepatitis C co-infection on bone mineral density and fracture: a meta-analysis. PLoS ONE. 2014;9:e101493. doi: 10.1371/journal.pone.0101493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McComsey GA, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown T, Li X, Jacobson LP, et al. Balance confidence predicts falls better than physical function testing in HIV+ men. CROI; Seattle, Washington. 2015. Abstract #786. [Google Scholar]
- 98.Lazarus B, et al. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern Med. 2016;176:238–246. doi: 10.1001/jamainternmed.2015.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ofotokun I, et al. A Single Dose Zoledronic Acid Prevents Antiretroviral-Induced Bone Loss. CROI; 2016; Boston Massachusetts. 2016. Abstract #47. [Google Scholar]


