Abstract
The predictive effects of age and self-rated health (SRH) on all-cause mortality are known to differ across race and ethnic groups. African American adults have higher mortality rates than Whites at younger ages, but this mortality disparity diminishes with advancing age and may “crossover” at about 75 to 80 years of age, when African Americans may show lower mortality rates. This pattern of findings reflects a lower overall association between age and mortality for African Americans than for Whites, and health-related mechanisms are typically cited as the reason for this age-based crossover mortality effect. However, a lower association between poor SRH and mortality has also been found for African Americans than for Whites, and it is not known if the reduced age and SRH associations with mortality for African Americans reflect independent or overlapping mechanisms. This study examined these two mortality predictors simultaneously in a large epidemiological study of 12,181 African Americans and 17,436 Whites. Participants were 45 or more years of age when they enrolled in the national REasons for Geographic and Racial Differences in Stroke (REGARDS) study between 2003 and 2007. Consistent with previous studies, African Americans had poorer SRH than Whites even after adjusting for demographic and health history covariates. Survival analysis models indicated statistically significant and independent race*age, race*SRH, and age*SRH interaction effects on all-cause mortality over an average 9-year follow-up period. Advanced age and poorer SRH were both weaker mortality risk factors for African Americans than for Whites. These two effects were distinct and presumably tapped different causal mechanisms. This calls into question the health-related explanation for the age-based mortality crossover effect and suggests that other mechanisms, including behavioral, social, and cultural factors, should be considered in efforts to better understand the age-based mortality crossover effect and other longevity disparities.
Introduction
Numerous reports of all-cause mortality in the United States have documented a persistent excess mortality rate and shorter life expectancy for African Americans compared to Whites (Heron, 2011; Hovert & Xu, 2012; Ng-Mak, Dohrenwend, Abraido-Lanza & Turner, 1999). This excess mortality of African Americans is believed to be an important indicator of persistent health disparities (Williams, 2012), and its impact on the population could have far-reaching consequences including socioeconomic and political effects that might serve to perpetuate those disparities (Rodriguez et al., 2015) and a lack of sufficient aging-related services being developed for African American and other disadvantaged populations (Markides & Machalek, 1984). For all of these reasons, it is vital that we better understand the root causes of this excess mortality experienced by African Americans in comparison to Whites and design programs and policies that seek to reduce this important disparity.
Detailed statistical analyses often further indicate that the excess mortality of African Americans, while being pervasive, is not consistently observed across all stages of the lifespan. At younger ages, African Americans typically have proportionally much higher mortality rates than Whites, but this imbalance clearly diminishes with increasing age. Multiple studies have shown that the excess mortality of African Americans tends to disappear altogether for older adults, when, at approximately 75 to 80 years of age, the race-specific mortality rates often reach a point where elderly African Americans have lower mortality rates than age-matched Whites (Johnson, 2000; Manton, Poss, & Wing, 1979; Markides & Machalek, 1984; Preston & Elo, 2006; Wing et al., 1985; Yao & Robert, 2011). This phenomenon, frequently referred to as the race “crossover” mortality effect, is equivalent to a statistical interaction effect such that advancing age is a stronger predictor of mortality for Whites than it is for African Americans.
A frequent interpretation of the age-based crossover mortality effect for African Americans is that it is due to a “selective survival” effect. This hypothesis maintains that, because of the higher mortality rates of younger African Americans compared to younger Whites, those in the African American population with poorer health are more likely die young, leading to a greater survival selection process and a comparatively healthier group of African Americans who survive into old age (Manton, Poss, & Wing, 1979; Markides & Machalek, 1984; Zajacova & Burgard, 2013). This is often presented as a health-related hypothesis, although selective survival effects can also emerge for other reasons (Horiuchi & Wilmoth, 1998), including different rates of physiological aging and environmental factors (Manton, Poss, & Wing, 1979). In addition, because each organism in a population dies only once, any cause of death not directly related to health or a biological mechanism, such as an accident or an act of violence, for example, removes the opportunity for that organism to die later from another cause, including an age-related disease condition. If this occurs frequently enough within any specific subpopulation, then this phenomenon would attenuate the significance of both age- and health-related factors as predictors of mortality for that subpopulation.
Epidemiological research examining the predictors of mortality have identified other factors besides age that may also have differential impacts on mortality across minority subgroups. One such predictor is the relatively simple rating of one’s overall health as excellent, very good, good, fair, or poor. This simple self-rated health (SRH) measure is a surprisingly strong and robust predictor of mortality even after controlling for many medical, behavioral, and demographic risk factors (Benyamini, Blumstein, Lusky, & Modan, 2003; Benyamini & Idler, 1999; DeSalvo et al., 2006; Lima-Costa, Cesar, et al., 2012; McGee, Liao, Cao, & Cooper, 1999). It is sometimes considered to be a remarkably sensitive overall summary indicator of one’s health-related risk for subsequent mortality (Idler & Benyamini, 1997; Jylha, 2009). Interestingly, many studies have found minority groups to report poorer SRH in comparison to Whites even after adjusting for relevant sociodemographic, health, and physical performance covariates (Boardman, 2004; Borrell & Crawford, 2006; Ferraro, 1993; Ren & Amick, 1996; Skarupski et al., 2007; Spencer et al., 2009).
Similar to research on the age-related crossover mortality effect, some investigators have sought to determine whether the SRH – mortality association is stronger or weaker in certain demographic subgroups compared to a referent group. Data from the national Health and Retirement Study, for example, have shown that the SRH – mortality association is much weaker for African Americans than it is for Whites (Lee et al., 2007). In that analysis, poor SRH, in comparison to excellent SRH, was much more strongly linked with subsequent mortality for Whites (odds ratio (OR) = 10.4) than for African Americans (OR = 2.9). Similar findings of attenuated SRH – mortality associations have been found for Hispanics compared to Whites (McGee et al., 1999) and for those with less education or income compared to their respective reference groups (Dowd & Zajacova, 2007; Lima-Costa, Steptoe, et al., 2012). Socioeconomic differences, however, do not appear to explain the attenuated SRH – mortality associations that have been found for African Americans (Ferraro & Kelley-Moore, 2001; Lee et al., 2007).
It is interesting that the diminished SRH – mortality association for African Americans compared to Whites has a pattern that is quite similar to the age-based “crossover” mortality effect for African Americans. In both instances, two straightforward and replicable risk factors for mortality – poorer SRH and advancing age – show weaker associations with subsequent mortality for African Americans than for Whites. Surprisingly, in spite of the numerous studies on both age- or SRH-based mortality crossover effects for African Americans, no previous study has, to our knowledge, examined both of these possible crossover effects simultaneously. Furthermore, if the age-based mortality crossover effect is due to a health-related selective survival effect, then SRH effects in an age-based model should reduce some of the diminished age-related mortality differences between African Americans and Whites. That is, it is possible that the age-based race crossover mortality effect for African Americans is confounded, or overlapping, with a SRH-related race crossover mortality effect, and such a finding would support the health-related selective survival effect as an explanation for the age-based crossover effect. A simultaneous analysis would, therefore, determine whether these effects are overlapping or if they reflect mostly distinct phenomena due to different and independent mechanisms.
To advance understanding of these effects and inform future investigation into the root causes of race disparities in mortality, we sought to examine both age-based and SRH-related race crossover mortality effects in the national REasons for Geographic and Racial Differences in Stroke (REGARDS) study. This study enrolled a large and well-characterized national sample of African Americans and Whites who were 45 years of age or older at the time of enrollment (Howard et al., 2005) and it provides a unique opportunity to further examine African American vs. White differences in the predictors of all-cause mortality. Using rigorously collected mortality data from the REGARDS study, we conducted an independent examination of potential SRH- and age-crossover mortality effects for the two race groups enrolled in this national cohort study. Two hypotheses were advanced based on the proposition that the age-based crossover mortality effect for African Americans is due to a health-related selective survival effect. First, this selective survival effect should result in a finding that race-based differences in SRH are diminished for older participants compared to younger participants. Second, adding health history covariates, SRH, and SRH*age interaction effects to survival models should diminish the significance of the race*age interaction effect that reflects the age-based crossover mortality effect for African Americans.
Methods
Participants
Participants in the REGARDS study were randomly sampled from a commercially available nationwide list purchased through Genesys, Incorporated (Howard et al., 2005). Exclusion criteria included age less than 45, race other than African American or White, previous diagnosis of cancer requiring chemotherapy, or residence in or on a waiting list for a nursing home. The goals of the REGARDS study are to examine the reasons why African Americans and residents of southern states of the United States have higher rates of stroke mortality than their respective comparison groups. For this reason, African Americans and residents from the southern "stroke belt" and “stroke buckle” regions of the United States were oversampled by design based on a stratified random sampling design. The stroke belt is a collection of Southern states characterized by high stroke mortality (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana) and the stroke buckle (coastal plains of North Carolina, South Carolina, and Georgia) is a subregion of the stroke belt that has even higher rates of mortality due to stroke (Howard et al., 1997).
Demographic, socioeconomic factors, medical history, and verbal informed consent were obtained during a baseline computer-assisted telephone interview (CATI) conducted by trained interviewers employed in the Survey Research Unit of the School of Public Health at the University of Alabama at Birmingham (UAB). During a subsequent in-home examination, written informed consent and physical measurements were obtained. A total of 30,239 participants were enrolled in the REGARDS study from January 2003 through October 2007. Data were missing on mortality status or at least of the mortality predictors analyzed (see below) for 622 (2.1%) participants, leaving 29,617 participants with complete data for the analyses reported here. All interview and research procedures were reviewed and approved by the UAB Institutional Review Board. The design, enrollment, and interviewing procedures for the REGARDS study have been previously described in more detail elsewhere (G. Howard et al., 2011; V. Howard et al., 2005; Roth et al., 2013).
Procedure and Measures
Trained interviewers contacted potential participants, established eligibility, obtained verbal informed consent, and administered the baseline CATI at enrollment. The following variables were obtained during the baseline CATI and are used in the present analyses:
Demographic Variables
Race (African American vs. White) and gender (male vs. female) were dichotomous variables based on self-report. Age was calculated based on the number of days between the participant’s reported date of birth and the date of the baseline interview. For analytic purposes, age was categorized into 5 groups based on 10-year intervals (45-54, 55-64, 65-74, 75-84 and 85 or greater). Region was analyzed based on the stratified sampling categories that were used (Stroke belt, Stroke buckle, Nonbelt). Marital status, education, and annual household income were obtained by self-report and coded into categorical variables as indicated in Table 1.
Table 1.
Descriptive Information by Race.
| Variables | Total (N = 29,617) |
African-American (N = 12,181) |
White (N = 17,436) |
|---|---|---|---|
| Gender, N (%) | |||
| Female | 16,309 (55.1) | 7,571 (62.2) | 8,738 (50.1) |
| Male | 13,308 (44.9) | 4,610 (37.8) | 8,698 (49.9) |
| Age Group, N (%) | |||
| 45-54 | 3,681 (12.4) | 1,702 (14.0) | 1,939 (11.4) |
| 55-64 | 11,295 (38.1) | 4,869 (40.0) | 6,246 (36.9) |
| 65-74 | 9,553 (32.3) | 3,816 (31.3) | 5,737 (32.9) |
| 75-84 | 4,508 (15.2) | 1,596 (13.1) | 2,912 (16.7) |
| 85+ | 580 (2.0) | 198 (1.6) | 382 (2.2) |
| Region, N (%) | |||
| Stroke Belt | 10,256 (34.6) | 4,050 (33.3) | 6,206 (35.6) |
| Stroke Buckle | 6,202 (20.9) | 2,188 (18.0) | 4,014 (23.0) |
| Non-Belt | 13,159 (44.4) | 5,943 (48.8) | 7,216 (41.4) |
| Marital status, N (%) | |||
| Married | 17,467 (59.0) | 5,514 (45.3) | 11,953 (68.6) |
| Widowed | 5,598 (18.9) | 2,796 (23.0) | 2,802 (16.1) |
| Divorced | 4,302 (14.5) | 2,387 (19.6) | 1,915 (11.0) |
| Single | 1,558 (5.3) | 933 (7.7) | 625 (3.6) |
| Other | 692 (2.3) | 551 (4.5) | 141 (0.8) |
| Education, N (%) | |||
| College graduate and above | 10,322 (34.9) | 3,131 (25.7) | 7,191 (41.2) |
| Some college | 7,944 (26.8) | 3,236 (26.6) | 4,708 (27.0) |
| High school graduate | 7,657 (25.9) | 3,394 (27.9) | 4,263 (24.5) |
| Less than high school | 3,694 (12.5) | 2,420 (19.9) | 1,274 (7.3) |
| Income, N (%) | |||
| < $20,000 | 5,327 (18.0) | 3,247 (26.7) | 2,080 (11.9) |
| $20,000 - $34,000 | 7,161 (24.2) | 3,218 (26.4) | 3,963 (22.6) |
| $35,000 - $74,000 | 8,798 (29.7) | 3,098 (25.4) | 5,700 (32.7) |
| $75,000 or more | 4,695 (15.9) | 1,088 (8.9) | 3,607 (20.7) |
| Refused | 3,636 (12.3) | 1,530 (12.6) | 2,106 (12.1) |
| Self-Rated Health, N (%) | |||
| Excellent | 4,743 (16.0) | 1,189 (9.8) | 3,554 (20.4) |
| Very Good | 9,046 (30.5) | 2,865 (23.5) | 6,181 (35.5) |
| Good | 10,372 (35.0) | 4,935 (40.5) | 5,437 (31.2) |
| Fair | 4,417 (14.9) | 2,653 (21.8) | 1,764 (10.1) |
| Poor | 1,039 (3.5) | 539 (4.4) | 500 (2.9) |
| Deceased, N (%) | 4,881 (16.5) | 2,118 (17.4) | 2,763 (15.9) |
Disease History
Participants were asked several health history questions during the CATI and the following were health history categories were coded based on responses to these questions. Participants were asked if they had ever been told by a doctor or health professional that they had a stroke, and those who answered “yes” were coded as having a history of stroke. Participants were coded as having kidney disease if they reported being told so by a doctor or health professional. A history of diabetes was recorded if participants reported being told by a doctor or health professional that they had diabetes, high blood sugar, or if they were taking medications specifically for diabetes. Hypertension was coded as present if participants reported being told by a doctor or health professional they had hypertension, high blood pressure, or that they were taking medications for high blood pressure. A history of heart disease was coded for any participants who reported a history of myocardial infarction, heart attack, coronary bypass surgery, repair of aortic aneurism, a pacemaker implanted, or coronary angioplasty/stenting.
Self-Rated Health
Participants were asked, “in general, would you say that your health is excellent, very good, good, fair, or poor.”
Participants have also been interviewed by telephone every 6 months after enrollment to ascertain incident stroke events and other medical outcomes. This includes proxy reports of mortality. The semi-annual follow-up interviews continue at the present time.
All-Cause Mortality
Preliminary dates of death were typically obtained from proxy reports when participants could not be reached for their semi-annual follow-up telephone interviews. Dates of death were later verified using the Social Security index, death certificates, or other administrative data files such as the National Death Index (Doody, Hayes, & Bilgrad, 2001; Halanych et al., 2011).
Statistical Analysis
Unadjusted race differences in SRH, on the covariates, and on mortality observed over a 9-year period were analyzed with simple chi-square tests of association. In both the cumulative logistic regression models and the Cox proportional hazards survival models (see below), race was analyzed as a dichotomous variable, with White race as the referent group. Odds ratios (ORs) or hazard ratios (HRs) greater than 1.0, therefore, reflected poorer SRH or elevated mortality, respectively, for African Americans compared to Whites. Covariates used in the cumulative logistic regression models and the proportional hazards survival models included age group (45-54 referent); 5 other demographic variables: gender (male referent), region (non-belt referent), marital status (married referent), education (college graduate and above referent), and income category ($75,000 and above referent); and 5 dichotomous indicators for self-reported disease history (stroke, kidney disease, diabetes, hypertension, and heart disease).
Logistic Regression analyses of SRH
Race differences in SRH were analyzed using cumulative logistic regression models as conducted by SAS Proc LOGISTIC. In these models the predictive effects of race and the covariates were held constant across each successive SRH transition (i.e., from excellent to very good, from very good to good, from good to fair, and from fair to poor). This yielded one overall odds ratio (OR) that indicated the increased odds of a poorer SRH level in association with the corresponding change on the predictor variable. A covariate-adjusted cumulative logistic regression model was estimated that examined race differences in SRH after controlling for demographic and disease history covariates. After this model, an additional model was run that added a race*age group interaction effect. That interaction effect tested whether race differences in SRH were diminished in the older age groups, a finding that, if found to be statistically significant, would support a health-related selective survival effect for the African Americans in comparison to the Whites.
All-Cause Mortality Analyses
Cox proportional hazards models of the predictors of mortality were conducted using SAS Proc PHREG (Allison, 2010). These models were based on the number of days elapsed between the baseline interview date and the date of death for the deceased cases (median = 1,775 days). For 18,453 participants who completed at least one semi-annual follow-up interview since December 31, 2012 and for whom there was no confirmed death report, the date of that last semi-annual follow-up interview was used as a right censoring date. For 6,283 participants with no death reports from administrative databases and who were considered “inactive” in the REGARDS project because their last follow-up interview was prior to 2013, a right censoring date of December 31, 2012 was used. Inactive participants who died prior to that date were detected in administrative databases (e.g., Social Security index, National Death index) and were therefore analyzed as deceased cases in the analyses. Inactive participants without a confirmed date of death in the administrative databases were conservatively considered to still be living as of December 31, 2012. The median follow-up intervals for the active and inactive subgroups of living participants were 9.6 years and 8.1 years, respectively. The overall length of follow-up time for those who did not die during the follow-up period ranged from 5.2 years to 12.2 years (median = 9.1 years).
The primary all-cause mortality predictors examined in the present analyses were race, age group, and SRH. As with the cumulative logistic regression models of SRH, covariates included 5 sociodemographic factors (gender, region, marital status, education, income) and 5 disease history indicators (stroke, kidney disease, diabetes, hypertension, heart disease). Main effects for race and age group were examined first without any further adjustments (Model 1). Next, the 5 sociodemographic covariates were added (Model 2). In a third step, the 5 disease history covariates were added (Model 3) and then SRH was added to the analysis (Model 4).
The hypothesized crossover mortality effects were then examined by adding two-way interaction terms to the analysis (models 5 and 6). That is, race*SRH, race*age group, and age group*SRH multiplicative terms were added as predictors in individual models (model 5) and simultaneously in one overall model (model 6). In a final step (model 7), a three-way interaction term (race*age group*SRH) was added to the analytic model to examine whether this higher-order interaction effect would further qualify any significant two-way interaction effects. Such an interaction effect would detect statistically whether the age-related crossover mortality effect for African Americans (race*age group interaction) would itself be stronger or weaker at different levels of SRH-related mortality risk.
Wald’s chi-square statistics were used to conduct omnibus tests of the main effects of race, age group, SRH, and their interactive effects on mortality after accounting for covariates and for the other predictors in each model. In addition, in order to further aid in interpreting any statistically significant two-way interaction effects, separate proportional hazard models stratified by race and age were also examined.
Results
Descriptive Information
Table 1 depicts summary data by race group for the REGARDS participants included in the present analyses. Of the 29,617 participants included in the analyses, 4,881 (16.5%) died at some point during the follow-up period. Unadjusted differences by race were observed on all variables in Table 1 including mortality as determined by chi-square tests of association (all p values < 0.001).
Race Differences in Self-Rated Health
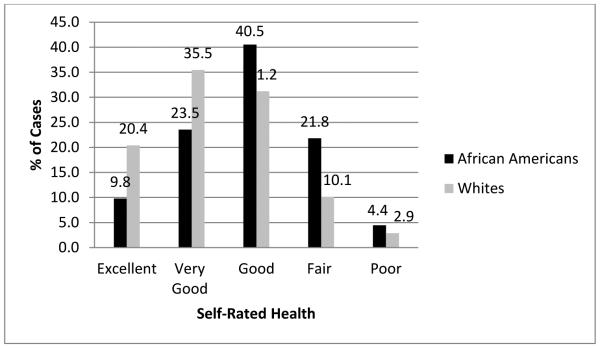
Unadjusted differences in SRH as a function of race are described in Table 1 and illustrated graphically in Figure 1. Whites were more than twice as likely as African Americans to rate their health as “excellent,” whereas African Americans disproportionately described their overall health as good, fair, or poor. An unadjusted cumulative logistic regression analysis indicated that African Americans had a much higher odds of endorsing a worse state of SRH in comparison with Whites (OR = 2.437, 95% confidence interval (CI) = 2.334 – 2.544). The results of the cumulative logistic regression analysis that included demographic (age group, gender, region, marital status, education, income) and health history (stroke, kidney disease, diabetes, hypertension, heart disease) covariates are summarized in Table 2. A statistically significant race difference on SRH persisted even after adjusting for these covariates (OR = 1.571, 95% CI = 1.498 – 1.646). Based on the natural logarithms of the ORs from the unadjusted and adjusted models, only 49% of the unadjusted race difference in SRH could be explained by the demographic and disease history covariates collectively, leaving 51% of the race effect on SRH unaccounted for by these covariates. Interestingly, after adjusting for covariates including health history, older age was generally associated with better SRH.
Figure 1.
Bar chart of self-rated health by race.
Table 2.
Results of the Cumulative Logistic Regression Analysis Predicting SRH.
| Effect | Odds Ratio (OR) | 95% CI |
|---|---|---|
| Race (African American vs. White) | 1.571*** | 1.498 – 1.646 |
| Gender (Female vs. Male) | 1.129*** | 1.078 – 1.190 |
| Age Group: 45-54 (referent) | 1.000 | |
| 55-64 | 0.816*** | 0.761 – 0.874 |
| 65-74 | 0.584*** | 0.543 – 0.629 |
| 75-84 | 0.603*** | 0.553 – 0.658 |
| 85+ | 0.569*** | 0.481 – 0.672 |
| Region: Non-belt (referent) | 1.000 | --- |
| Stroke Belt | 1.100*** | 1.048 – 1.154 |
| Stroke Buckle | 1.114** | 1.053 – 1.178 |
| Marital Status: Married (referent) | 1.000 | --- |
| Widowed | 0.954 | 0.895 – 1.017 |
| Divorced | 0.992 | 0.929 – 1.060 |
| Single | 1.199** | 1.086 – 1.324 |
| Other | 1.137 | 0.985 – 1.312 |
| Education: College graduate and above (referent) | 1.000 | --- |
| Some college | 1.318*** | 1.247 –1.394 |
| High school graduate | 1.572*** | 1.482 – 1.668 |
| Less than high school | 2.235*** | 2.067 – 2.418 |
| Income: $75,000 or more (referent) | 1.000 | --- |
| Less than $20,000 | 2.632*** | 2.409 – 2.876 |
| $25,000 – $34,999 | 1.871*** | 1.733 – 2.019 |
| $35,000 – $74,999 | 1.412*** | 1.320 – 1.511 |
| Refused | 1.927*** | 1.768 – 2.100 |
| Health History: | ||
| Stroke | 2.063*** | 1.888 – 2.253 |
| Kidney disease | 2.359*** | 2.010 – 2.768 |
| Diabetes | 2.191*** | 2.077 – 2.312 |
| Hypertension | 2.126*** | 2.031 – 2.226 |
| Heart disease | 2.228*** | 2.094 – 2.371 |
Note: Odds ratios > 1.0 indicate increased odds of poorer SRH for the indicated group relative to the referent group.
p < .05
p < .001
p < .0001
The addition of the race*age group interaction effect to the logistic regression model revealed that this interaction effect was not statistically significant (Wald’s X2 (df = 4) = 3.40, p = 0.49). The race differences in SRH, therefore, were consistent across age group and did not diminish for the older age groups, an effect that would be expected if significant health-related selective mortality had occurred.
Unadjusted All-Cause Mortality Findings
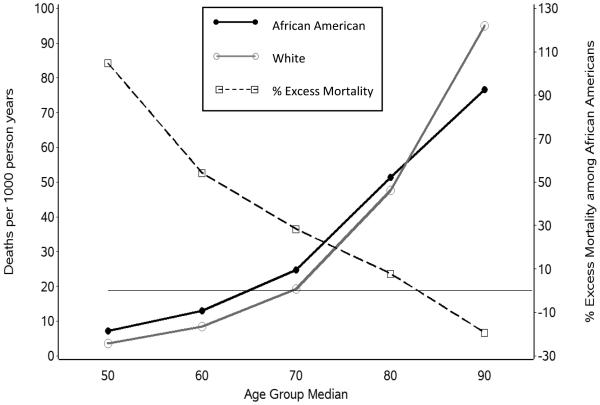
Unadjusted mortality rates over 9 years for African Americans and for Whites by age group at the time of REGARDS enrollment and by SRH at REGARDS enrollment are displayed in Figures 2 and 3, respectively. In addition to the raw mortality rates per 1000 person-years, the percentage of excess mortality for African Americans compared to Whites was calculated and displayed for each age group and SRH classification. This excess mortality percentage was determined by 1) calculating the difference in mortality rates (African American minus White), 2) dividing this difference by the White mortality rate, and 3) multiplying that quotient by 100. As indicated in Figure 2, the excess mortality for African Americans was particularly high among the youngest age group in REGARDS (45-54) and diminished in a remarkably linear fashion until there was no excess mortality for African Americans in the older age groups. Indeed, African Americans in the oldest age group in REGARDS (85 or more years of age at enrollment) had lower mortality rates than Whites, and the age-based crossover mortality effect appeared to occur at approximately age 82 in REGARDS (see Figure 2).
Figure 2.
Race differences in mortality by age.
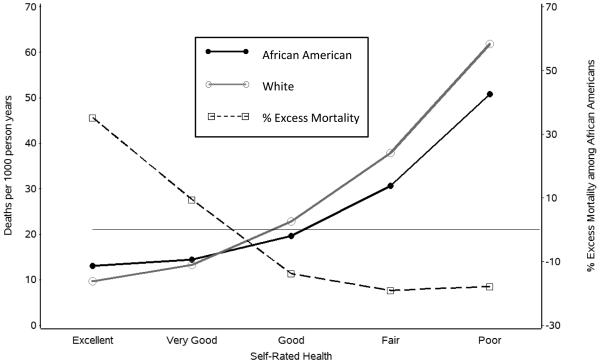
Figure 3.
Race differences in mortality by self-rated health.
Figure 3 conveys a similar pattern for SRH. The highest excess mortality for African Americans compared to Whites was among the participants who rated their health as “excellent.” Diminished excess mortality for African Americans was observed for those who characterized their health as “very good,” and African Americans had lower mortality rates than Whites among the participants who rated their health as “good,” “fair,” or “poor.”
Multivariable All-Cause Mortality Models
The omnibus Wald’s chi-square tests of the different effects from the all-cause mortality models are summarized in Table 3. The omnibus statistical tests for the combined effects across all categories within a given predictor (e.g., age group) are presented in Table 3 whereas selected individual hazard ratios for specific group comparisons are summarized in the text below. When only race and age group were included as predictors (Model 1), African Americans showed significantly elevated mortality in comparison with Whites (HR = 1.307, 95% CI = 1.235 – 1.383). However, after adding the five sociodemographic covariates as predictors (Model 2), the elevated mortality rate for African Americans was largely diminished, though still statistically significant (HR = 1.074, 95% CI = 1.010 – 1.142). When the five health history indicators were then added to the analysis (Model 3), the adjusted mortality effect for race was no longer statistically significant (HR = 1.009, 95% CI = 0.948 – 1.075). Adding SRH to the model on the next step (Model 4) revealed that SRH had a highly significant effect in predicting mortality even after controlling for sociodemographic and health history covariates. The effect for race continued to be non-significant (HR = 0.957, 95% CI = 0.898 – 1.019).
Table 3.
Wald’s Chi-Square Statistics of Independent Prediction of All-Cause Mortality
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5a | Model 6 | ||
|---|---|---|---|---|---|---|---|
| Effect | Degrees of freedom |
X2 | X2 | X2 | X2 | X2 | X2 |
| Race | 1 | 85.52*** | 5.19* | 0.08 | 1.87 | 1.47 | 8.39* |
| Age group | 4 | 2562.15*** | 1701.51*** | 1548.87*** | 1679.53*** | 1683.40*** | 323.27*** |
| SRH | 4 | ---- | ---- | ---- | 349.09*** | 352.50*** | 65.26*** |
| Race*Age group | 4 | ---- | ---- | ---- | ---- | 27.69*** | 20.65** |
| Race*SRH | 4 | ---- | ---- | ---- | ---- | 15.43* | 23.13** |
| Age group*SRH | 16 | ---- | ---- | ---- | ---- | 53.94*** | 49.90*** |
p < .05
p < .001
p < .0001
Model 5 consists of three separate analyses for each 2-way interaction effect examined individually. The main effects reported for model 5 are for the predictor not involved in the interaction effect (e.g., the race effect is from the model that included the age group*SRH interaction).
Note: Model 1 has no covariaes. Model 2 is adjusted for the effects of participant gender, region of residence, marital status, education, and income. Models 3-6 are adjusted for the effects of participant gender, region of residence, marital status, education, income, and self-reported history of stroke, diabetes, kidney disease, hypertension, and heart disease.
The race*age group, race*SRH and age group*SRH interaction effects were all found to be statistically significant, both when added to the model individually (model 5) and collectively (model 6). The model adding the three-way race*age group*SRH interaction effect (model 7) indicated that this effect was not statistically significant (Wald’s X2 (df = 16) = 11.19, p = 0.80). Because this three-way interaction effect was not statistically significant and was the only effect in model 7 that was not also included in the more parsimonious model 6, model 7 was no longer considered or summarized in Table 3.
Table 4 summarizes the results of the proportional hazards models that were estimated separately for African Americans and Whites. These analyses further aid in the interpretation of the race*SRH and race*age interaction effects from models 5 and 6. The hazard ratios from these models confirm that older age and poorer SRH were more strongly associated with increased mortality for Whites than for African Americans. Conversely, the protective effects of excellent SRH and relative youth are weaker for African Americans than for Whites. Separate models for participants less than 65 and for those 65 years of age or older revealed no significant covariate-adjusted race effect for the younger subgroup (HR = 1.028, 95% CI = 0.907 – 1.165). This suggests that the excess mortality of African Americans among younger ages (Figure 2) can be largely explained by demographic differences and their increased prevalence of chronic disease. Interestingly, after adjusting for demographic, disease history, and SRH covariates, African Americans older than age 65 had significantly lower mortality rates than their White counterparts (HR = 0.872, 95% CI = 0.810 – 0.939). In addition, the age-specific proportional hazards models indicated that poor SRH, relative to excellent SRH, was a slightly stronger predictor of subsequent mortality for younger participants (HR = 3.117, 95% CI = 2.323 – 4.184) than for older participants (HR = 2.816, 95% CI = 2.360 – 3.359).
Table 4.
Effects of Age and SRH on All-Cause Mortality in Race-Specific Analyses
| African American | White | |||
|---|---|---|---|---|
| Effect | HR | 95% CI | HR | 95% CI |
| Age Group | ||||
| 45-54 (referent) | 1.000 | ---- | 1.000 | ---- |
| 55-64 | 1.583 | 1.272 – 1.970 | 2.055 | 1.560 – 2.708 |
| 65-74 | 2.777 | 2.234 – 3.453 | 4.268 | 3.255 – 5.595 |
| 75-84 | 5.175 | 4.127 – 6.490 | 9.968 | 7.588 – 13.094 |
| 85+ | 8.357 | 6.224 – 11.219 | 20.228 | 15.003 – 27.275 |
| SRH | ||||
| Excellent (referent) | 1.000 | ---- | 1.000 | ---- |
| Very Good | 1.056 | 0.865 – 1.289 | 1.182 | 1.032 – 1.352 |
| Good | 1.262 | 1.048 – 1.520 | 1.735 | 1.520 – 1.980 |
| Fair | 1.611 | 1.328 – 1.954 | 2.505 | 2.154 – 2.913 |
| Poor | 2.267 | 1.797 – 2.860 | 3.748 | 3.088 – 4.548 |
Note: All models adjusted for the effects of participant gender, region of residence, marital status, education, income, and self-reported history of stroke, diabetes, kidney disease, hypertension, and heart disease.
Discussion
The present analyses provide a more comprehensive and integrated picture of the multiple associations that have been reported previously among age, SRH, race, and all-cause mortality. The findings for age and SRH in relation to subsequent mortality were remarkably similar, with both predictors showing significant, monotonic relationships with mortality that were, nonetheless, significantly attenuated for African Americans compared to Whites. Our paper contributes to the literature by further examining the health-related selective mortality explanation for some of the previously observed age-based race crossover effects. That explanation predicts that the age-based mortality differences between African Americans and Whites can be explained by health-related variables, including potential race differences in the strength of the SRH – mortality association. However, by examining all three of the two-way interaction effects among SRH, age, and race simultaneously in the same analysis (model 6), we were able to confirm that these two mortality risk factors (age and SRH) have attenuated associations with mortality in African Americans that are largely distinct and not overlapping with each other. Thus, both relative youth and excellent SRH confer stronger survival advantages for Whites than for African Americans, and these advantages appear to reflect independent and separate causal pathways.
The results of our analyses are consistent with several previous population-based studies of SRH and mortality. After controlling for numerous demographic and medical history covariates, we found SRH differences between African Americans and Whites that are similar to those reported from the Health and Retirement Study (Spencer et al., 2009). We also found that these race differences in SRH were not diminished in the older age groups, a finding that also failed to support a health-related selective mortality phenomenon for African Americans in comparison to Whites in the REGARDS sample. Furthermore, consistent with previous studies (Johnson, 2000; Manton, Poss, & Wing, 1979; Wing et al., 1985; Yao & Robert, 2011), we found that the excess mortality of African Americans in comparison to Whites diminishes for the older age groups, and that the association between SRH and mortality is also weaker for African Americans than it is for Whites (Lee et al., 2007). The present study is unique in that it is the first to synthesize these various individual findings into one integrated report of the relationships among these important mortality risk variables.
The mechanisms for these distinct age- and SRH-based mortality crossover effects for African Americans are undoubtedly complex. While age progresses consistently across time at the same rate for everyone, SRH can remain stable, improve, or decline across time. In addition, different patterns of SRH change can be observed for different individuals (Wolinsky et al., 2008).Older adults from the Health and Retirement Study with unstable SRH over time, either improving or declining, have been found to have a greater risk of death than those with stable SRH (Vogelsang, 2014). Our analyses were restricted to SRH measured at baseline, and thus, more studies examining change in SRH over time are needed. In addition, more research is needed to delineate the factors that affect SRH and on how beliefs about health might vary across race and other sociodemographic factors. Wolinsky and colleagues (2008), for example, found that SRH was relatively stable over a 4-year period for a sample of middle-aged African Americans, showing patterns of stability and change that were comparable to Whites. However, potential race differences in health beliefs, health pessimism or fatalism, domains considered relevant for an overall rating of health, and the frames of reference for good health, may all contribute to subtle differences in SRH across race groups and consequently, the degree to which SRH is linked to other health outcomes (Bailis et al., 2003; Galenkamp, Husiman, Braam, & Deeg, 2012; Krause & Jay, 1994; Spencer et al., 2009).
A number of other factors and conditions, such as adverse childhood events, social support and integration, and racial discrimination, also differ between race groups and might differ across age-based cohorts. These factors could impact both objective health and health perceptions that affect SRH. Causes of death that are not directly health-related, such as accidents or acts of violence, also contribute to the selective survival effects and the statistical risk associated with other predictors. One contributor to the disproportionately elevated mortality rate for younger African American adults is their increased risk of being a victim of homicide (Heron, 2013). In general, when more individuals of one demographic subgroup die disproportionately due to tragic events that are not directly related to health conditions, then this can have the statistical effect of attenuating the effects of other age-related and health-related risk factors for mortality for that demographic subgroup. Such dynamic competing outcomes mechanisms may contribute to the complex inter-relationships among age, SRH, race, and mortality that have been observed in REGARDS and other large population-based studies.
Future inquiry into the mechanisms that might underlie the persistent excess of early mortality experienced by African Americans would benefit from more comprehensive multivariable analyses based on large population-based samples with sufficient numbers of outcome occurrences (e.g., deaths). A limitation of the present analyses is its reliance on all-cause mortality only, and additional analyses with more death events than observed here would be necessary to further explore the statistical impact of certain specific causes of death. Additional population-based studies are needed not only to further refine predictive models but also to inform possible avenues for extending longevity and reducing health disparities. Although age is not a modifiable risk factor, SRH is potentially modifiable, and researchers have called for interventions to address the “health pessimism” of African Americans (Spencer et al., 2009). However, the weaker SRH – mortality association in this race group suggests that such interventions would not necessarily convert to substantial population survival advantages. The persistent finding of increased mortality of younger African Americans cannot be explained by poorer SRH, just as previous analyses have shown that it cannot be explained by neighborhood socioeconomic indicators (Yao & Robert, 2011). It is possible that other modifiable factors that influence SRH may impact mortality in ways that have not yet been elucidated. For example, if the opportunity to engage in stimulating social activities is weighted more heavily by Whites than by African Americans in making SRH ratings, and if socially active individuals are less likely to die than socially isolated individuals (Berkman & Syme, 1979; Perissinotto, Cenzer, & Covinsky, 2012), then increasing one’s social activity and better connecting social activity with perceptions of health might represent one avenue for improving SRH and reducing mortality among younger African Americans.
Differential mortality by race may also serve in some ways to perpetuate health disparities and contribute to the stubborn persistence of the problem (Rodriguez et al., 2015). The relative lack of minority individuals who survive into old age and the relative robustness of this group due to selective survival mechanisms might also explain other findings, such as why African Americans are less likely to use nursing homes and other sources of more formal support in old age (Markides & Machalek, 1984). The solutions to the high mortality rates experienced by African Americans at younger ages are likely to be complex, multifaceted, and require commitments of resources that extend over multiple generations. Those solutions will also ultimately alter the composition of the population of surviving older adults and require additional adjustments in health policies and resources for that population.
In summary, the present analyses demonstrate that both younger age and excellent SRH confer greater survival advantages for Whites than for African Americans, and that these “crossover” relationships reflect distinct and separate pathways. Future work to corroborate these findings using national population-based studies that extend further to include behavioral, social, cultural, and biological factors on mortality is warranted. Ideally, such investigations will include measures of the cumulative disadvantages experienced by African Americans and will incorporate time-varying predictors of mortality when feasible. Further investigation of these relationships, as well as consideration of other factors that can influence mortality, may prove to be worthwhile for better understanding and ultimately reducing health disparities.
References
- Allison PD. Survival Analysis Using SAS: A Practical Guide. 2nd The SAS Institute; Cary, NC: 2010. [Google Scholar]
- Bailis DS, Segall A, Chipperfield JG. Two views of self-rated general health status. Social Science & Medicine. 2003;56(2):203–217. doi: 10.1016/s0277-9536(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Blumstein T, Lusky A, Modan B. Gender differences in the self-rated health-mortality association: Is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival? Gerontologist. 2003;43(3):396–405. doi: 10.1093/geront/43.3.396. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Idler EL. Community studies reporting association between self-rated health and mortality: additional studies, 1995-1998. Research on Aging. 1999;21(3):392–401. [Google Scholar]
- Berkman LF, Syme SL. Social networks, host-resistance, and mortality: a nine-year follow-up study of Alameda County residents. American Journal of Epidemiology. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Boardman JD. Health pessimism among black and white adults: the role of interpersonal and institutional maltreatment. Social Science & Medicine. 2004;59(12):2523–2533. doi: 10.1016/j.socscimed.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Crawford ND. Race, ethnicity, and self-rated health status in the Behavioral Risk Factor Surveillance System Survey. Hispanic Journal of Behavioral Sciences. 2006;28(3):387–403. [Google Scholar]
- DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self-rated health question. A meta-analysis. Journal of General Internal Medicine. 2006;21(3):267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody MM, Hayes HM, Bilgrad R. Comparability of national death index plus and standard procedures for determining causes of death in epidemiologic studies. Annals of Epidemiology. 2001;11(1):46–50. doi: 10.1016/s1047-2797(00)00177-0. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A. Does the predictive power of self-rated health for subsequent mortality risk vary by socioeconomic status in the US? International Journal of Epidemiology. 2007;36(6):1214–21. doi: 10.1093/ije/dym214. [DOI] [PubMed] [Google Scholar]
- Ferraro KF. Are Black older adults health-pessimistic? Journal of Health and Social Behavior. 1993;34(3):201–214. [PubMed] [Google Scholar]
- Ferraro KF, Kelley-Moore JA. Self-rated health and mortality among black and white adults: Examining the dynamic evaluation thesis. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2001;56(4):S195–205. doi: 10.1093/geronb/56.4.s195. [DOI] [PubMed] [Google Scholar]
- Galenkamp H, Huisman M, Braam A, Deeg D. Estimates of prospective change in self-rated health in older people were biased owing to potential recalibration response shift. Journal of Clinical Epidemiology. 2012;65(9):978–988. doi: 10.1016/j.jclinepi.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Halanych JH, Shuaib F, Parmar G, Tanikella R, Howard VJ, Roth DL, Safford MM. Agreement on cause of death between proxies, death certificate, and clinician adjudicators in the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study. American Journal of Epidemiology. 2011;173(11):1319–26. doi: 10.1093/aje/kwr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M. National Vital Statistics Reports. Vol. 62. National Center for Health Statistics; Hyattsville, MD: 2013. Deaths: Leading causes for 2010. [PubMed] [Google Scholar]
- Hoyert DL, Xu JQ. National Vitalist Statistics Reports. Vol. 61. National Center for Health Statistics; Hyattsville, MD: 2012. Deaths: Preliminary data for 2011. [PubMed] [Google Scholar]
- Howard G, Anderson R, Johnson NJ, Sorlie P, Russell G, Howard VJ. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke. 1997;28:936–940. doi: 10.1161/01.str.28.5.936. [DOI] [PubMed] [Google Scholar]
- Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Howard VJ. Traditional risk factors as the underlying cause of racial disparities in stroke: Lessons from the half-full (empty?) glass. Stroke. 2011;42(12):3369–75. doi: 10.1161/STROKEAHA.111.625277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S, Wilmoth JR. Deceleration in the age pattern of mortality at older ages. Demography. 1998;35(4):391–412. [PubMed] [Google Scholar]
- Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Howard G. The Reasons for Geographic And Racial Differences in Stroke study: Objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38(1):21–37. [PubMed] [Google Scholar]
- Johnson N. The racial crossover in comorbidity, disability, and mortality. Demography. 2000;37(3):267–283. [PubMed] [Google Scholar]
- Jylha M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Social Science & Medicine. 2009;69(3):307–16. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Krause NM, Jay GM. What do global self-rated health items measure? Medical Care. 1994;32(9):930–942. doi: 10.1097/00005650-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Moody-Ayers SY, Landefeld CS, Walter LC, Lindquist K, Segal MR, Covinsky KE. The relationship between self-rated health and mortality in older black and white Americans. Journal of the American Geriatric Society. 2007;55(10):1624–1629. doi: 10.1111/j.1532-5415.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- Lima-Costa MF, Cesar CC, Chor D, Proietti FA. Self-rated health compared with objectively measured health status as a tool for mortality risk screening in older adults: 10-year follow-up of the Bambui Cohort Study of Aging. American Journal of Epidemiology. 2012;175(3):228–235. doi: 10.1093/aje/kwr290. [DOI] [PubMed] [Google Scholar]
- Lima-Costa MF, Steptoe A, Cesar CC, De Oliveira C, Proietti FA, Marmot M. The influence of socioeconomic status on the predictive power of self-rated health for 6-year mortality in English and Brazilian older adults: the ELSA and Bambui cohort studies. Annals of Epidemiology. 2012;22(9):644–8. doi: 10.1016/j.annepidem.2012.06.101. [DOI] [PubMed] [Google Scholar]
- Manton KG, Poss SS, Wing S. The black/white mortality crossover: Investigation from the perspective of the components of aging. Gerontologist. 1979;19(3):291–300. doi: 10.1093/geront/19.3.291. [DOI] [PubMed] [Google Scholar]
- Markides KS, Machalek R. Selective survival, aging and society. Archives of Gerontology and Geriatics. 1984;3:207–222. doi: 10.1016/0167-4943(84)90022-0. [DOI] [PubMed] [Google Scholar]
- McGee DL, Liao Y, Cao G, Cooper RS. Self-reported health status and mortality in a multiethnic US cohort. American Journal of Epidemiology. 1999;149(1):41–46. doi: 10.1093/oxfordjournals.aje.a009725. [DOI] [PubMed] [Google Scholar]
- Ng-Mak DS, Dohrenwend BP, Abraido-Lanza AF, Turner JB. A further analysis of race differences in the national longitudinal mortality study. American Journal of Public Health. 1999;89:1748–1751. doi: 10.2105/ajph.89.11.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissinotto CM, Cenzer IS, Covinsky KE. Loneliness in older persons a predictor of functional decline and death. Archives of Internal Medicine. 2012;172(14):1078–83. doi: 10.1001/archinternmed.2012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SH, Elo IT. Black mortality at very old ages in official US life tables: A skeptical appraisal. Population and Development Review. 2006;32(3):557–565. [Google Scholar]
- Ren XS, Amick BC. Racial and ethnic disparities in self-assessed health status: Evidence from the national survey of families and households. Ethnicity and Health. 1996;1(3):293–303. doi: 10.1080/13557858.1996.9961798. [DOI] [PubMed] [Google Scholar]
- Rodriguez JM, Geronimus AT, Bound J, Dorling D. Black lives matter: Differential mortality and the racial composition of the U.S. electorate, 1970-2004. Social Science & Medicine. 2015:136–137. doi: 10.1016/j.socscimed.2015.04.014. 193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DL, Haley WE, Hovater M, Perkins M, Wadley VG, Judd S. Family caregiving and all-cause mortality: Findings from a population-based propensity-matched analysis. American Journal of Epidemiology. 2013;178(10):1571–1578. doi: 10.1093/aje/kwt225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarupski KA, Mendes de Leon CF, Bienias JL, Scherr PA, Zack MM, Moriarty DG, Evans DA. Black-white differences in health-related quality of life among older adults. Quality of Life Research. 2007;16(2):287–296. doi: 10.1007/s11136-006-9115-y. [DOI] [PubMed] [Google Scholar]
- Spencer SM, Schulz R, Rooks RN, Albert SM, Thorpe RJ, Jr., Brenes GA, Newman AB. Racial differences in self-rated health at similar levels of physical functioning: An examination of health pessimism in the health, aging, and body composition study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2009;64(1):87–94. doi: 10.1093/geronb/gbn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelsang EM. Self-rated health changes and oldest old mortality. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2014;69(4):612–621. doi: 10.1093/geronb/gbu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR. Miles to go before we sleep: Racial inequities in health. Journal of Health and Social Behavior. 2012;53(3):279–295. doi: 10.1177/0022146512455804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing S, Manton KG, Stallard E, Hames CG, Tryoler HA. The black/white mortality crossover: investigation in a community-based study. Journal of Gerontology. 1985;40(1):78–84. doi: 10.1093/geronj/40.1.78. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Miller TR, Malmstrom TK, Miller JP, Schootman M, Andresen EM, Miller DK. Self-rated health: Changes, trajectories, and their antecedents among African Americans. Journal of Aging and Health. 2008;20(2):143–158. doi: 10.1177/0898264307310449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Robert SA. Examining the racial crossover in mortality between African American and white older adults: A multilevel survival analysis of race, individual socioeconomic status, and neighborhood socioeconomic context. Journal of Aging Research. 2011:1–8. doi: 10.4061/2011/132073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajacova A, Burgard SA. Healthier, wealthier, and wiser: A demonstration of compositional changes in aging cohorts due to selective mortality. Population Research and Policy Review. 2013;32:311–324. doi: 10.1007/s11113-013-9273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]