Abstract
Trials designed to assess the effect of interventions on death and graft failure in kidney transplant recipients are not feasible, because these are predominantly late events. Here, we examined the potential of percentage decline in eGFR as a surrogate for hard outcomes. We obtained deidentified data from the Australia and New Zealand Dialysis and Transplant Registry and studied 7949 transplants performed from 1995 to 2009, including 71,845 patient-years of follow-up, 1121 graft losses, and 1192 deaths. We used adjusted Cox proportional hazards models to determine risks of death or death–censored graft failure related to percentage change in eGFR between years 1 and 3 after transplant. Percentage change in eGFR was modeled as a restricted cubic spline. Rate of eGFR decline associated with exponentially increased risks of graft failure and death. Compared with stable eGFR, a ≥30% decline in eGFR, detected in 10% of patients, strongly associated with subsequent death (hazard ratio, 2.20; 95% confidence interval, 1.87 to 2.60) and death–censored graft failure (hazard ratio, 5.14; 95% confidence interval, 4.44 to 5.95). Decline in eGFR was superior to other surrogates, including acute rejection, doubling of serum creatinine level, and eGFR at year 1 or year 2. We conclude that 30% decline in eGFR between years 1 and 3 after kidney transplant is common and strongly associated with risks of subsequent death and death–censored graft failure, which mirrors findings in CKD. Percentage decline in eGFR should be considered for use as a surrogate outcome in kidney transplant trials.
Keywords: glomerular filtration rate, mortality, renal transplantation, transplant outcomes
Kidney transplantation provides the optimal form of RRT for the majority of people with ESRD. Although 1-year patient and graft survival now exceeds 95% in major transplanting centers, long-term outcomes have failed to improve over time. Beyond the first post–transplant year, an annual attrition rate of 4%–5% has been reported in the United States, Australia, and other regions, which is caused in equal parts by graft failure and patient death with a functioning graft.1,2
The majority of clinical trials in kidney transplantation have focused primarily on outcomes during the first 1–3 years after transplantation, including the incidence of acute rejection and patient and graft survival. Because of the low rates of death or graft loss during the first 3 years after transplantation, current trials provide little insight into the effect of therapy on such outcomes over the longer term. Trials of therapies seeking to improve long-term outcomes require either very large numbers or extended follow-up duration. Although desperately needed, high cost and challenging logistics have resulted in a paucity of such trials.3
The use of surrogate outcomes for mortality and late graft failure may enable investigators to design trials that are more affordable and feasible.4 In this regard, kidney transplantation has much in common with CKD.5 Trials in CKD have traditionally addressed primary outcomes of death, ESRD, or doubling of baseline serum creatinine. Using the large, multinational CKD Prognosis Consortium dataset, Coresh et al.6 explored use of percentage reduction in eGFR as a surrogate for hard outcomes. Compared with traditional end points, the investigators reported that a ≥30% decline in eGFR over the typical trial durations of 1, 2, or 3 years was substantially more frequent but also, strongly predictive of ESRD and death on longer–term follow-up.6 We, therefore, examined the relationship between eGFR decline and subsequent hard outcomes after kidney transplantation.
Results
The analysis included 7949 grafts, with a median follow-up of 8.5 years and 71,845 patient-years in total. There were 1121 graft failures, 863 deaths with a functioning graft, and another 329 deaths after graft failure. Sixty-nine (0.87%) patients were lost to follow-up after a median of 9 years. The baseline characteristics of the patients are shown in Table 1.
Table 1.
Baseline characteristics
| Characteristic | Value |
|---|---|
| N | 7949 |
| Age at transplant, yr, mean (SD) | 45.3 (12.9) |
| Recipient, men | 4957 (62%) |
| Race | |
| White | 6699 (84%) |
| Australian indigenous | 186 (2%) |
| Asian | 666 (8%) |
| Māori | 172 (2%) |
| Pacific People | 133 (2%) |
| Other | 93 (1%) |
| Primary renal disease | |
| GN | 4020 (51%) |
| Polycystic kidney disease | 1076 (14%) |
| Reflux nephropathy | 892 (11%) |
| Hypertension | 313 (4%) |
| Diabetic nephropathy | 562 (7%) |
| Other | 1086 (14%) |
| Diabetes | 808 (10%) |
| Coronary artery disease | 935 (12%) |
| Peripheral vascular disease | 469 (6%) |
| Cerebrovascular disease | 299 (4%) |
| Chronic lung disease | 389 (5%) |
| Living donor | 3135 (39%) |
| Repeat transplant | 946 (12%) |
| Donor age, yr, median (interquartile range) | 46.0 (33.0–55.0) |
| HLA mismatches | |
| 0–2 | 3262 (41%) |
| 3–4 | 2908 (37%) |
| 5–6 | 1779 (22%) |
| Peak panel–reactive antibody, median (interquartile range) | 3.0% (0.0%–18.0%) |
| Transplant era | |
| 1995–1997 | 1252 (16%) |
| 1998–2000 | 1388 (17%) |
| 2001–2003 | 1577 (20%) |
| 2004–2006 | 1733 (22%) |
| 2007–2009 | 1999 (25%) |
| eGFR at 1 yr, mean (SD) | 54.4 (18.0) |
| eGFR at 3 yr, mean (SD) | 53.0 (19.5) |
| Change in eGFR, year 3 versus 1, mean (SD) | 0.5% (25.3%) |
Excluded patients (n=3994) were slightly older (mean [SD] =45.3 [12.9] versus 48.6 [13.2] years old; P<0.001), had a higher prevalence of comorbidities pretransplant (diabetes, 10% versus 17%; coronary disease, 12% versus 19%; both P<0.001), had higher donor age (median [interquartile range] =46 [33–55] versus 50 [39–59] years old; P<0.001), had a modestly greater degree of HLA mismatch (5–6 mis-match, 22% versus 30%), and had lower eGFR at both year 1 (mean [SD] =54.4 [18.0] versus 53.1 [20.9]; P<0.01) and year 3 when available (53.0 [19.5] versus 48.7 [24.4]; P=0.03), with no substantive differences in sex or race. Because the major reasons for exclusion were graft loss or death during the first 3 post-transplant years, it is expected that those excluded would exhibit a higher risk profile.
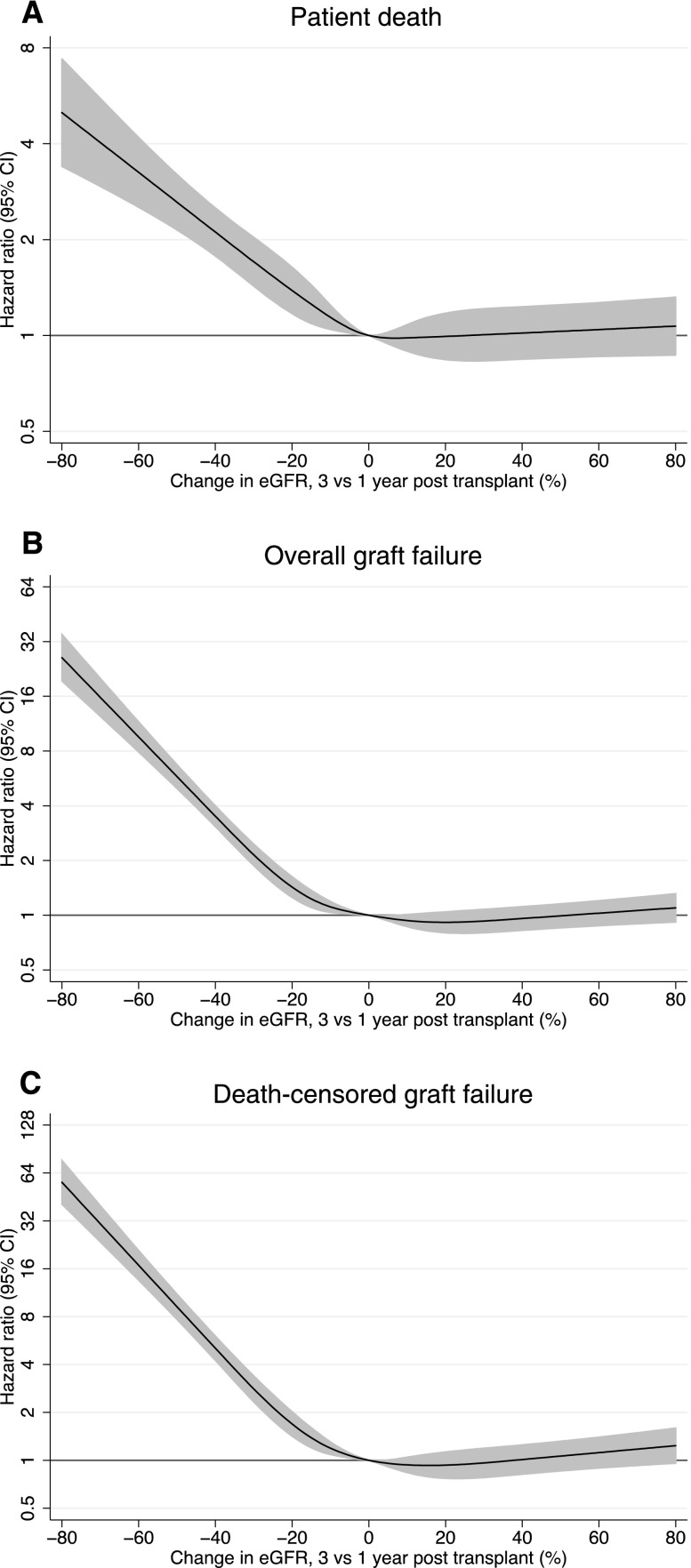
Percentage change in eGFR between the end of years 1 and 3 post-transplant was significantly predictive of patient survival (Figure 1A). Greater reductions in eGFR decline were exponentially associated with increases in risk of death, whereas improvement in eGFR was not associated with death (Figure 1A). A decline in eGFR of ≥30% occurred in 10% of patients and was associated with a 2.2-fold increase in death compared with in those with stable eGFR (hazard ratio [HR], 2.20; 95% confidence interval [95% CI], 1.87 to 2.60).
Figure 1.
Relationship between decline in eGFR between years 1 and 3 post-transplant and hard outcomes. (A) Patient death, (B) overall graft failure, and (C) death-censored graft failure. The lines represent hazard ratios compared with no change in eGFR and the shaded regions are 95% CIs. Note the different scales on the y axes. All results are adjusted for the confounders reported in the text.
Overall, graft loss and in particular, death–censored graft failure were also strongly and exponentially associated with percentage reduction in eGFR, whereas improvement in eGFR was not associated with graft loss (Figure 1, B and C, respectively). A decline in eGFR of ≥30% was associated with a 3.5-fold increase in graft failure (HR, 3.58; 95% CI, 3.16 to 4.05) and a fivefold increase in death–censored graft failure (HR, 5.14; 95% CI, 4.44 to 5.95).
Separate sensitivity analyses restricted to first graft recipients using the Modification of Diet in Renal Disease 4 (MDRD-4) equation rather than the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to estimate GFR7,8 and examining change in eGFR between years 3 and 5 post-transplant yielded similar results. Stratification of the cohort by recipient age, presence or absence of diabetes, panel–reactive antibody percentage, donor source, year 1 eGFR, or cause of graft failure showed relatively consistent relationships between decline in eGFR and all–cause graft failure (Table 2).
Table 2.
HRs for the association between ≥30% decline in eGFR from years 1 to 3 and subsequent graft failure by subgroup
| Subgroup | HR (95% CI) |
|---|---|
| Cause of graft failure | |
| Death with function | 1.75 (1.42 to 2.16) |
| Acute rejection | 4.48 (1.39 to 14.39) |
| Chronic allograft nephropathy | 5.27 (4.46 to 6.22) |
| GN | 5.04 (3.24 to 7.83) |
| Noncompliance | 3.35 (1.86 to 6.03) |
| Other | 5.08 (2.84 to 9.12) |
| Comorbid diabetes mellitus | |
| Yes | 3.10 (2.22 to 4.33) |
| No | 3.78 (3.30 to 4.31) |
| Donor source | |
| Deceased | 3.39 (2.92 to 3.94) |
| Living | 4.12 (3.31 to 5.13) |
| Prior transplant | |
| Yes | 3.41 (2.48 to 4.70) |
| No | 3.68 (3.22 to 4.20) |
| Cause of ESRD | |
| GN | 4.22 (3.54 to 5.04) |
| Polycystic | 3.53 (2.25 to 5.54) |
| Reflux | 3.33 (2.26 to 4.89) |
| Hypertension | 3.57 (1.83 to 6.98) |
| Diabetes | 2.32 (1.58 to 3.40) |
| Other | 4.14 (3.06 to 5.60) |
| Peak panel–reactive antibodies | |
| 0%–49% | 3.72 (3.25 to 4.27) |
| 50%–79% | 3.28 (2.09 to 5.15) |
| 80%–100% | 3.17 (2.12 to 4.74) |
| eGFR at year 1, ml/min per 1.73 m2 | |
| 0–29 | 6.14 (4.25 to 8.89) |
| 30–44 | 5.14 (4.03 to 6.56) |
| 45–59 | 3.85 (3.04 to 4.86) |
| 60–89 | 3.41 (2.68 to 4.33) |
| >90 | 5.12 (2.28 to 11.52) |
To choose the optimal decline in eGFR between years 1 and 3 for use as a surrogate outcome, we compared various thresholds in terms of risk prediction (Table 3). All cut points from ≥10% to ≥50% decline in eGFR were predictive of both graft and patient survival. Predictably, the higher cut points were more strongly predictive but less common. Overall, no cut point was superior to others as assessed by c statistics. We favor a ≥30% decline as being both clinically plausible and exhibiting an acceptable tradeoff between prevalence and predictive power.
Table 3.
Relationships between percentage eGFR decline between years 1 and 3 post-transplant and outcome
| eGFR Decline | Prevalence, % | Graft Failure | Patient Death | ||
|---|---|---|---|---|---|
| HR (95% CI) | c Statistic | HR (95% CI) | c Statistic | ||
| ≥10% | 33 | 2.09 (1.91 to 2.29) | 0.68 | 1.52 (1.35 to 1.71) | 0.75 |
| ≥20% | 19 | 2.50 (2.26 to 2.77) | 0.69 | 1.84 (1.62 to 2.10) | 0.75 |
| ≥30% | 10 | 3.58 (3.16 to 4.05) | 0.70 | 2.20 (1.87 to 2.60) | 0.75 |
| ≥40% | 5 | 5.24 (4.43 to 6.20) | 0.69 | 2.57 (2.04 to 3.22) | 0.75 |
| ≥50% | 3 | 7.90 (6.21 to 10.06) | 0.67 | 2.96 (2.17 to 4.04) | 0.75 |
A comparison of decline in eGFR between years 1 and 3 post-transplantation with various other potential surrogate markers of death and graft failure is shown in Table 4. Decline in eGFR of ≥30% between month 6 and year 2 post-transplantation was only modestly less predictive and less frequent. Doubling of serum creatinine between years 1 and 3 post-transplantation, the equivalent of a 57% decline in eGFR,6 was more strongly predictive of both death and graft failure but less frequent than a ≥30% decline over the same period, occurring in only 1.9% of patients. Year 1 eGFR has been proposed9 as a surrogate for trials; however, eGFR at years 1 or 2 post-transplantation was less strongly predictive than decline in eGFR. Acute rejection was common but only weakly predictive of death or graft failure.
Table 4.
Associations between different eGFR–based surrogate outcomes and hard outcomes
| Outcome | Prevalence, % | Graft Failure | Death–Censored Graft Failure | Patient Death | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | c Statistic | HR (95% CI) | c Statistic | HR (95% CI) | c Statistic | ||
| ≥30% decline eGFR 1–3 yr | 9.9 | 3.58 (3.16 to 4.05) | 0.70 | 5.14 (4.44 to 5.95) | 0.75 | 2.20 (1.87 to 2.60) | 0.75 |
| ≥30% decline eGFR 1–2 yr | 6.1 | 3.51 (3.01 to 4.09) | 0.68 | 4.69 (3.92 to 5.61) | 0.72 | 2.33 (1.91 to 2.86) | 0.75 |
| ≥30% decline eGFR 6 mo to 2 yr | 8.7 | 2.94 (2.59 to 3.35) | 0.68 | 4.16 (3.59 to 4.83) | 0.73 | 1.99 (1.68 to 2.36) | 0.75 |
| eGFR at 1 yr <45 ml/min per 1.73 m2 | 32.3 | 1.85 (1.69 to 2.02) | 0.67 | 2.60 (2.31 to 2.93) | 0.73 | 1.39 (1.24 to 1.56) | 0.74 |
| eGFR at 2 yr <45 ml/min per 1.73 m2 | 33.7 | 2.21 (2.01 to 2.42) | 0.68 | 3.16 (2.78 to 3.58) | 0.74 | 1.68 (1.49 to 1.89) | 0.75 |
| Rejection first 6 mo | 24.4 | 1.34 (1.21 to 1.47) | 0.66 | 1.37 (1.21 to 1.55) | 0.69 | 1.27 (1.12 to 1.44) | 0.75 |
| Double creatinine 1–3 yr | 1.9 | 9.87 (7.27 to 13.42) | 0.66 | 15.20 (11.18 to 20.67) | 0.70 | 2.81 (1.84 to 4.29) | 0.75 |
| ΔeGFR 1–3 yr <−15 ml/min per 1.73 m2 | 12.0 | 2.48 (2.20 to 2.81) | 0.68 | 3.28 (2.84 to 3.80) | 0.72 | 1.77 (1.50 to 2.09) | 0.75 |
All models are adjusted for age at transplant, sex, race, primary disease, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, chronic lung disease, donor type, prior transplant, donor age, HLA mismatch, peak panel–reactive antibodies, and era.
We explored the effect of eGFR decline as a surrogate end point on power calculation for future clinical trials. If a treatment were to achieve an improvement of 5 ml/min per 1.73 m2 in eGFR at year 3, on the basis of our data, 5% of treated subjects versus 10% of controls would experience a ≥30% decline in years 1–3 eGFR. To show this with 80% power, a trial enrolling 435 to each arm would be required, rounded to 500 per arm to allow for dropouts. Such a difference in eGFR at 3 years has been achieved in recently reported trials comparing belatacept or everolimus with cyclosporin–based control groups.10,11
Use of inclusion criteria to enrich for patients at higher risk of eGFR decline, if possible, could reduce the numbers required for inclusion by ≤50%.
Discussion
This registry analysis of a large cohort of kidney transplant recipients has shown that percentage decline in eGFR exhibits characteristics that would support its use as a surrogate marker for the important, hard, long–term outcomes of death and graft failure. Analogous to the situation in CKD, selecting a decline in eGFR over a 2-year trial period of ≥30% as a primary trial end point would, because of the higher frequency of this end point compared with ESRD or doubling of serum creatinine, facilitate the design of trials enrolling relatively smaller numbers of patients followed for 2 years. Such changes would reduce funding requirements and enhance feasibility, and they may thereby enable the conduct of trials now required to underpin improvements in long-term outcomes for kidney transplant recipients.4
A symposium conducted at the 2014 World Transplant Congress, “The Future of Transplantation Immunosuppression R&D: Problems and Solutions from Clinical, Biopharmaceutical and Regulatory Perspectives,” concluded that use of surrogate outcomes should be considered to improve the feasibility of trials required to address the key unmet needs in clinical kidney transplantation (R. Morris, D. Kuypers, and P. O’Connell, personal communications).
Various measures of urinary protein excretion are predictive of long-term death and graft failure and have been considered as surrogate outcomes for trial purposes; however, lack of specificity9 and test variability may limit the utility of single measures, such as spot urine protein-to-creatinine or albumin-to-creatinine ratios.12,13
Kidney function at 1 year post-transplant has previously been considered as a potential surrogate.4,9,14 Hariharan et al.14 studied 105,742 kidney recipients reported to United Network for Organ Sharing between 1988 and 1998 and described a strong, inverse relationship between 1-year serum creatinine and 5-year death–censored graft survival.12 Using a cut point of 1.5 mg/dl, higher creatinine was most commonly associated with indicators of relative kidney mass at transplantation, such as men recipients, black recipients, women donors, older donors, and delayed graft function, whereas markers of alloimmune risk, such as incidence of acute rejection, HLA matching, and previous transplantation, seemed less strongly associated. On this basis, it can be argued that a single measure of graft function may be more reflective of endowment rather than ongoing immunologic processes.4 Consistent with this hypothesis, the authors also examined “delta-creatinine” and found that change between month 6 and 1 year was superior to 1-year creatinine.14 Limitations with this study include the lack of standardization of creatinine measurement, common use of cyclosporin and azathioprine rather than tacrolimus and mycophenolate, and higher rates of acute rejection in that era.14 Kasiske et al.9 examined the relationship between CKD stage as defined by MDRD eGFR at 1-year post-transplant and graft failure at 10 years among 13,671 patients transplanted between 1990 and 2007 and captured within the Patient Outcomes in Renal Transplant Study. Significantly increased hazards for graft loss were observed for those with CKD stage 3b (eGFR=30–45 ml/min per 1.73 m2), with successive increases in hazards for stages 4 and 5. Similar limitations to those in the work by Hariharan et al.14 apply to this data, and ΔGFR was not examined. In our dataset, eGFR<45 ml/min per 1.73 m2 was present in 32.3% of the cohort at year 1, although it was less strongly associated with both graft and patient survival than ≥30% decline in eGFR between 1 and 3 years (Table 4).
Measures of alloimmune processes are another potential surrogate and have the advantage of measuring the target of immunosuppressive therapy. However, practical tests of alloimmunity are limited and have not been adequately predictive of long-term outcomes. Development of donor-specific antibodies is an infrequent event, and the relationship between donor–specific antibody development and graft and patient survival requires clarification.15 Biopsy–proven acute rejection (BPAR) is clear demonstration of destructive alloimmunity; however, BPAR has become relatively infrequent, and the relationship between BPAR and graft and patient survival has been attenuated in recent eras. In our cohort, at 24.4% incidence, BPAR was more commonly observed than in current trials in kidney transplantation; however, it was less strongly associated with either patient or graft survival than was a ≥30% decline in eGFR between 1 and 3 years (Table 4).
Multiple potential causes of graft loss after kidney transplantation exist, including rejection, calcineurin inhibitor toxicity, hypertension, progression of donor-derived lesions, and recurrence of primary disease, and decline in eGFR as a surrogate provides limited insight into which of these is at play. Because a causal understanding is important, particularly in trials of immunosuppressive drugs, the use of dual or composite primary end points may provide greater insight. Use of composite primary end points has been common in clinical trials in transplantation, typically combining infrequent hard outcomes of death and graft loss with a more frequent surrogate, such as acute rejection. Combining the incidence of acute rejection with eGFR decline, death, or graft loss has significant appeal because of its frequency (incidence of 30% eGFR decline and/or acute rejection was 31.7% of our cohort), association with hard outcomes, and documentation of pathology.
The magnitude and timing of eGFR decline as an outcome measure may require tailoring to the specific trial requirements. We selected a 30% decline in eGFR between years 1 and 3, because it provided the best balance between frequency, clinical significance, and strength of association with hard outcomes. This timeframe would be best suited to switch trials,11 and given the subtly different characteristics of different magnitudes of eGFR decline within the range of ≥20% to ≥40% (Table 3), we would encourage exploration of different cut points in designing such trials. Regarding de novo trials, ≥30% decline in eGFR between 6 months and 2 years post-transplant has great appeal, because this was only modestly less frequent and predictive of hard outcomes compared with ≥30% eGFR decline between years 1 and 3 (Table 4) but offers a far more pragmatic timeframe for de novo studies.
A surrogate end point is only useful in a trial context if the effect of intervention on the surrogate is subsequently mirrored by similar effects on hard outcomes. Recently, support for the use of either doubling of serum creatinine (the equivalent of a 57% decline in eGFR) or proteinuria as a surrogate for ESRD has been provided in this regard by calculation of a treatment effect ratio in a meta-analysis of 27 trials including 97,458 participants with CKD stages 1–4, a minority of whom were transplant recipients. The analysis found that treatment effects on both measures were consistent with the effects on ESRD and concluded that doubling of serum creatinine is generally a good surrogate for ESRD.16 Demonstration of this in a purely transplant context may be difficult; however, registry–based long–term follow-up of trial participants may offer a means of confirming such a relationship in this context.17,18
The findings of this study seem robust given the large dataset examined and the consistency of results across sensitivity analyses examining patient mix, transplant number, different eGFR equations, and timing post-transplant. Indeed, the striking similarity to the results obtained from similar analyses in CKD cohorts6 provides additional weight to the findings after kidney transplantation. However, extending these findings across populations of differing racial mix and comorbidity profiles and across recipients of different immunosuppressive regimens (steroid-free maintenance and use of lymphocyte–depleting induction therapy were both uncommon in this cohort) should be undertaken to ensure generalizability. Furthermore, a decline in kidney function post-transplant may be caused by alloimmune events, such as acute or chronic rejection, but also, other processes, including recurrent disease, drug toxicity, and hypertension. Another limitation in our study is the absence of data on proteinuria, donor-specific antibody, and graft histology, which precluded direct comparison of these potential surrogates with decline in eGFR. As discussed, a composite end point of decline in GFR and a measure of alloimmunity, such as BPAR, may represent a logical direction for immunosuppressive drug trial design.
We conclude that, as was the case in CKD,6 a ≥30% decline in eGFR over 2 years was strongly associated with both death and graft loss among kidney transplant recipients, supporting consideration of this as a new surrogate end point for trials in kidney transplantation.
Concise Methods
We analyzed deidentified data from the Australia and New Zealand Dialysis and Transplant Registry. The registry collects data on all patients receiving RRT in Australia and New Zealand. We included kidney transplant recipients in Australia and New Zealand over 1995–2009 (n=13,199) with follow-up until December 31, 2012. We excluded recipients of multiorgan transplants (n=553) and patients aged <18 years old at the time of transplant (n=703). We further excluded those whose grafts functioned for <3 years (n=3810), those missing data for eGFR at 1 and/or 3 years (n=121), and those missing data for confounders (n=63), yielding a final cohort of 7949.
We used Cox proportional hazards models to examine the relationship between percentage change in eGFR calculated using the CKD-EPI equation8 between the end of years 1 and 3 post-transplant and subsequent patient, graft, and death–censored graft survival. Patient survival was censored at retransplantation. Percentage change in eGFR was modeled as a restricted cubic spline. All models were adjusted for recipient age, sex, race, primary disease, comorbidities (diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, and chronic lung disease), graft number (primary versus subsequent), donor type (living versus deceased), donor age, HLA mismatch, peak panel–reactive antibody, and transplant era (five eras of 3 years each). We used cluster robust variance estimators to account for patients with more than one graft during the study period. Model fit was assessed using scaled Schoenfeld residuals, Martingale residuals, Cox–Snell residuals, and Harrell c concordance statistics.
In a sensitivity analysis, we examined seven other potential eGFR–based surrogates: percentage change in eGFR from 6 months to 2 years post-transplant and from 1–2 years post-transplant, eGFR at 1 year of <45 ml/min per 1.73 m2, eGFR at 2 years of <45 ml/min per 1.73 m2, rejection within the first 6 months post-transplant, doubling of serum creatinine between 1 and 3 years post-transplant, and the absolute change in eGFR between 1 and 3 years post-transplant of <−15 ml/min per 1.73 m2. Each of these surrogates was examined using Cox models adjusted for the same confounders as the primary analysis. We compared the different predictors using prevalence, HRs, and Harrell c statistics.
Analyses were conducted in Stata/IC 14 (StataCorp., College Station, TX).
Disclosures
W.H.L. has participated in industry–sponsored clinical trials and symposia and received educational grants, travel support, or honoraria from companies producing immunosuppressant drugs, including Novartis (Basel, Switzerland), Alexion, Genzyme (Sanofi US, Bridgewater, NJ), and Pfizer. S.J.C. has participated in industry–sponsored clinical trials and symposia and received travel support or honoraria from companies producing immunosuppressant drugs, including Novartis, Astellas, Alexion, Roche (Basel, Switzerland), and Pfizer. The other authors of this manuscript have no conflicts of interest to disclose.
Acknowledgments
We are grateful to the Australian and New Zealand renal units, patients, and staff for their cooperation and contributions to the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry.
The ANZDATA Registry is funded by the Australian Organ and Tissue Donation and Transplantation Authority, the New Zealand Ministry of Health, and Kidney Health Australia. P.A.C. is supported by a grant from the Medical Foundation, University of Sydney. P.A.C. and S.J.C. had full access to of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
The data reported here were supplied by the ANZDATA Registry. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the ANZDATA Registry.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.US Renal Data System: Transplantation. In: USRDS 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013, pp 283–294
- 2.Clayton P, Lim WH, Hurst K: Transplantation. In: ANZDATA Registry Report 2013, edited by McDonald CP, Hurst K, Adelaide, South Australia, Australia, Australia and New Zealand Dialysis and Transplant Registry, 2013, pp 1–28
- 3.Palmer SC, Sciancalepore M, Strippoli GF: Trial quality in nephrology: How are we measuring up? Am J Kidney Dis 58: 335–337, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Stegall MD, Gaston RS, Cosio FG, Matas A: Through a glass darkly: Seeking clarity in preventing late kidney transplant failure. J Am Soc Nephrol 26: 20–29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inrig JK, Califf RM, Tasneem A, Vegunta RK, Molina C, Stanifer JW, Chiswell K, Patel UD: The landscape of clinical trials in nephrology: A systematic review of Clinicaltrials.gov. Am J Kidney Dis 63: 771–780, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS CKD Prognosis Consortium : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasiske BL, Israni AK, Snyder JJ, Skeans MA Patient Outcomes in Renal Transplantation (PORT) Investigators : The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis 57: 466–475, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, Lang P, Urrea EM, Massari P, Mondragon-Ramirez G, Reyes-Acevedo R, Rice K, Rostaing L, Steinberg S, Xing J, Agarwal M, Harler MB, Charpentier B: Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant 12: 210–217, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Budde K, Lehner F, Sommerer C, Reinke P, Arns W, Eisenberger U, Wüthrich RP, Mühlfeld A, Heller K, Porstner M, Veit J, Paulus EM, Witzke O ZEUS Study Investigators : Five-year outcomes in kidney transplant patients converted from cyclosporine to everolimus: The randomized ZEUS study. Am J Transplant 15: 119–128, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Naresh CN, Hayen A, Weening A, Craig JC, Chadban SJ: Day-to-day variability in spot urine albumin-creatinine ratio. Am J Kidney Dis 62: 1095–1101, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Naresh CN, Hayen A, Craig JC, Chadban SJ: Day-to-day variability in spot urine protein-creatinine ratio measurements. Am J Kidney Dis 60: 561–566, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP: Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int 62: 311–318, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Jun M, Turin TC, Woodward M, Perkovic V, Lambers Heerspink HJ, Manns BJ, Tonelli M, Hemmelgarn BR: Assessing the validity of surrogate outcomes for ESRD: A meta-analysis. J Am Soc Nephrol 26: 2289–2302, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher MP, Hall B, Craig J, Berry G, Tiller DJ, Eris J Australian Multicenter Trial of Cyclosporine Withdrawal Study Group and the ANZ Dialysis and Transplantation Registry : A randomized controlled trial of cyclosporine withdrawal in renal-transplant recipients: 15-year results. Transplantation 78: 1653–1660, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Clayton PA, McDonald SP, Chapman JR, Chadban SJ: Mycophenolate versus azathioprine for kidney transplantation: A 15-year follow-up of a randomized trial. Transplantation 94: 152–158, 2012 [DOI] [PubMed] [Google Scholar]