Abstract
Although recommended approaches to CKD management are achieved less often in Hispanics than in non-Hispanics, whether long-term outcomes differ between these groups is unclear. In a prospective longitudinal analysis of participants enrolled into the Chronic Renal Insufficiency Cohort (CRIC) and Hispanic-CRIC Studies, we used Cox proportional hazards models to determine the association between race/ethnicity, CKD progression (50% eGFR loss or incident ESRD), incident ESRD, and all-cause mortality, and linear mixed-effects models to assess differences in eGFR slope. Among 3785 participants, 13% were Hispanic, 43% were non-Hispanic white (NHW), and 44% were non-Hispanic black (NHB). Over a median follow-up of 5.1 years for Hispanics and 6.8 years for non-Hispanics, 27.6% of all participants had CKD progression, 21.3% reached incident ESRD, and 18.3% died. Hispanics had significantly higher rates of CKD progression, incident ESRD, and mean annual decline in eGFR than did NHW (P<0.05) but not NHB. Hispanics had a mortality rate similar to that of NHW but lower than that of NHB (P<0.05). In adjusted analyses, the risk of CKD progression did not differ between Hispanics and NHW or NHB. However, among nondiabetic participants, compared with NHB, Hispanics had a lower risk of CKD progression (hazard ratio, 0.61; 95% confidence interval, 0.39 to 0.95) and incident ESRD (hazard ratio, 0.50; 95% confidence interval, 0.30 to 0.84). At higher levels of urine protein, Hispanics had a significantly lower risk of mortality than did non-Hispanics (P<0.05). Thus, important differences in CKD progression and mortality exist between Hispanics and non-Hispanics and may be affected by proteinuria and diabetes.
Keywords: chronic kidney disease, ethnicity, mortality
Hispanics are the largest racial/ethnic minority group in the United States,1 and they bear a substantial burden of ESRD. Compared with non-Hispanic whites (NHW), the age- and sex-adjusted prevalence rate of ESRD is approximately twofold greater in Hispanics.2 Although the prevalence of eGFR-defined CKD (e.g., eGFR<60 ml/min per 1.73 m2) is lower among Hispanics than non-Hispanics,3 Hispanic ethnicity is associated with higher levels of microalbuminuria and proteinuria, even after accounting for differences in diabetes prevalence.3–8 Moreover, well recognized hematologic and metabolic complications of CKD have been found to be more common in Hispanic than NHW adults with an eGFR<60 ml/min per 1.73 m2.2,9
In previous cross-sectional analyses from the Chronic Renal Insufficiency Cohort (CRIC) and Hispanic-CRIC (H-CRIC) Studies, we found that Hispanics with CKD had significantly lower eGFR, higher urine protein excretion, more unfavorable metabolic biomarkers, and a greater burden of left ventricular hypertrophy than non-Hispanics at study entry.10,11 Additionally, several recommended goals for CKD management were achieved less often among Hispanics compared with non-Hispanics, including BP control, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEi/ARB) use, and secondary prevention of cardiovascular disease.10,11
Differences in long-term outcomes between Hispanics and non-Hispanics with CKD have been infrequently examined prospectively.12 In several cohorts with CKD, Hispanic ethnicity was found to be associated with a lower risk of death relative to NHW,13–15 while others found similar mortality risk in Mexican Americans with CKD compared with NHW.16,17 The relationship between Hispanic ethnicity and CKD progression has received less attention, with one study finding Hispanics with diabetes to have a lower risk of incident ESRD,4 while others report higher rates of CKD progression among Hispanics.13,15,17 These observations are limited by lack of equally spaced, serial longitudinal outpatient measurements of kidney function; incomplete adjustment for potential confounders; or not accounting for the competing risk of death with incident ESRD. Hence, there is a critical need to more rigorously characterize the potential scope and reasons for racial/ethnic disparities in CKD progression and mortality in adults with CKD.
To further explore the racial/ethnic disparities in CKD progression and death of Hispanics, we studied participants in the CRIC and H-CRIC Studies, which are prospective multicenter observational studies of adults with CKD.
Results
Baseline Participant Characteristics
Among 3785 participants in the final analytic cohort, 43% (n=1638) were NHW, 44% (n=1650) were non-Hispanic black (NHB), and 13% (n=497) were Hispanic (Table 1). Focusing on the important factors involved in analyses and their baseline differences across racial/ethnic groups, Hispanics were younger, less well educated, and smoked cigarettes less often than NHW and NHB (P<0.05). Moreover, Hispanics less often had health insurance and had less frequently received nephrology care at baseline compared with non-Hispanics (P<0.05). In comparison to non-Hispanics, Hispanics were more likely to have diabetes and to have a higher systolic BP at baseline, as well as having more severe kidney disease as exemplified by lower eGFR and higher 24-hour urine protein (P<0.05).
Table 1.
Baseline characteristics among CRIC participants stratified by race/ethnicity.
| Variable, n (%) | Hispanic (n=497) | NHW (n=1638) | NHB (n=1650) | Hispanic versus NHW P Value | Hispanic versus NHB P Value |
|---|---|---|---|---|---|
| Age, yra | 56.3±11.7 | 58.9±11.0 | 58.1±10.6 | <0.001 | 0.001 |
| Male | 288 (58.0) | 982 (60.0) | 806 (48.9) | 0.43 | 0.001 |
| Education | |||||
| Less than high school | 293 (59.0) | 90 (5.5) | 437 (26.5) | <0.001 | <0.001 |
| High school graduate | 71 (14.3) | 291 (17.8) | 366 (22.2) | ||
| Some college | 78 (15.7) | 467 (28.5) | 567 (34.4) | ||
| College graduate or more | 55 (11.1) | 790 (48.2) | 280 (17.0) | ||
| Income | |||||
| ≤$20,000 | 313 (63.0) | 254 (15.5) | 646 (39.2) | <0.001 | <0.001 |
| $20,001–$50,000 | 92 (18.5) | 416 (25.4) | 417 (25.3) | ||
| $50,000–$100,000 | 24 (4.8) | 455 (27.8) | 215 (13.0) | ||
| >$100,000 | 12 (2.4) | 295 (18.0) | 62 (3.8) | ||
| Don't wish to answer | 56 (11.3) | 218 (13.3) | 310 (18.8) | ||
| Presence of Health Insurance | |||||
| Yes | 329 (74.4) | 1446 (96.8) | 1340 (93.3) | <0.001 | <0.001 |
| No | 113 (25.6) | 48 (3.2) | 96 (6.7) | ||
| Type of Health Insurance | |||||
| Any Medicare | 84 (19.0) | 96 (6.4) | 327 (22.8) | <0.001 | <0.001 |
| Medicaid/public aid | 121 (27.4) | 564 (37.8) | 494 (34.4) | ||
| VA/military/CHAMPUS | 9 (2.0) | 73 (4.9) | 110 (7.7) | ||
| Private/commercial | 68 (15.4) | 284 (19.0) | 190 (13.2) | ||
| Unknown/incomplete | 47 (10.6) | 429 (28.7) | 219 (15.3) | ||
| None | 113 (25.6) | 48 (3.2) | 96 (6.7) | ||
| Nephrology Care | |||||
| Yes | 265 (53.3) | 1131 (69.0) | 1107 (67.1) | <0.001 | <0.001 |
| No | 232 (46.7) | 507 (31.0) | 543 (32.9) | ||
| History of Smoking | |||||
| Current smoking | 29 (5.8) | 155 (9.5) | 320 (19.4) | <0.001 | <0.001 |
| Past smoking | 189 (38.0) | 765 (46.7) | 635 (38.5) | ||
| BMI, kg/m2a | 31.6±6.6 | 31.2±7.6 | 33.4±8.3 | 0.23 | <0.001 |
| Hypertension | 443 (89.1) | 1293 (78.9) | 1533 (92.9) | <0.001 | 0.01 |
| Diabetes | 335 (67.4) | 649 (39.6) | 848 (51.4) | <0.001 | <0.001 |
| MI/Prior revascularization | 90 (18.1) | 376 (23.0) | 361 (21.9) | 0.02 | 0.07 |
| Congestive heart failure | 37 (7.4) | 117 (7.1) | 217 (13.2) | 0.82 | 0.001 |
| Peripheral vascular disease | 35 (7.0) | 105 (6.4) | 117 (7.1) | 0.62 | 0.97 |
| Systolic BP, mmHga | 136.1±23.7 | 121.8±18.6 | 132.9±23.1 | <0.001 | 0.01 |
| Controlled BP, <130/80 mmHgb | 142 (32.1) | 744 (57.5) | 589 (38.4) | <0.001 | 0.02 |
| HbA1ca | 7.0±1.7 | 6.3±1.3 | 6.9±1.7 | <0.001 | 0.18 |
| Controlled diabetes, HbA1c<7%c | 48 (14.3) | 101 (15.6) | 93 (11.0) | 0.61 | 0.12 |
| ACEi/ARB use | 332 (67.3) | 1089 (66.9) | 1164 (71.1) | 0.87 | 0.11 |
| eGFR, ml/min per m2a | 39.0±15.2 | 47.7±17.1 | 43.5±16.3 | <0.001 | <0.001 |
| Urine protein, g/24 hd | 0.71 (0.12, 3.34) | 0.12 (0.07, 0.5) | 0.24 (0.08, 1.07) | <0.001 | <0.001 |
VA, veterans affairs; CHAMPUS, civilian health and medical program of the uniformed services; MI, myocardial infarction.
Mean±SD.
Denominator is participants with hypertension only.
Denominator is participants with diabetes only.
Median (interquartile range).
Rates of CKD Progression, ESRD, and All-Cause Mortality by Race/Ethnicity
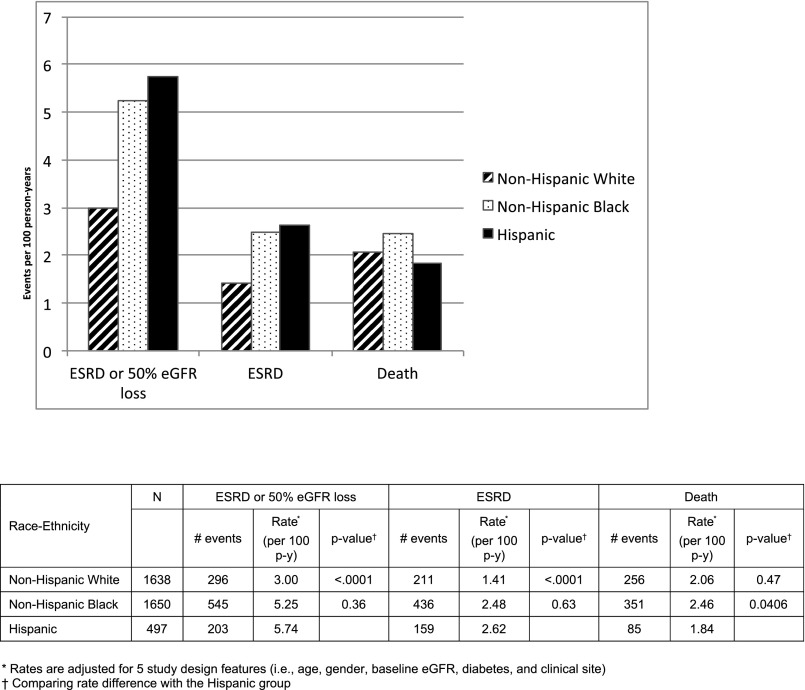
During an overall median of 6.6 years of follow-up (5.1 years for Hispanics and 6.8 years for non-Hispanics), 27.6% of participants experienced CKD progression (defined as ESRD or 50% eGFR loss), 21.3% developed incident ESRD, and 18.3% died (Figure 1). Across racial/ethnic groups, participant retention was similar at around 90%. After controlling for study design features (i.e., clinical center, age, sex, baseline eGFR, diabetes), Hispanics had significantly higher rates of CKD progression (5.74 per 100 person-years) compared with NHW (3.00 per 100 person-years; P<0.001) but not NHB (5.25 per 100 person-years; P=0.36). Similarly, in comparison to the incident ESRD rate of Hispanics (2.62 per 100 person-years), NHW had lower rates (1.41 per 100 person-years; P<0.001) but NHB had similar rates (2.48 per 100 person-years; P=0.63). All-cause mortality rates for Hispanics (1.84 per 100 person-years) were not significantly different than NHW (2.06 per 100 person-years; P=0.47), but lower than that of NHB per (2.46 per 100 person-years; P=0.04).
Figure 1.
Overall rates of ESRD, CKD progression and all-cause mortality by race/ethnicity.
When stratified by important baseline factors (e.g., eGFR, proteinuria, diabetes, age, sex, and ACEi/ARB use), rates of CKD progression, ESRD, and mortality for racial/ethnic groups were consistent with the aggregate results for racial/ethnic groups (Supplemental Table 1). CKD progression, ESRD, and mortality rates were notably higher in participants with low eGFR (<45 ml/min per 1.73 m2), elevated urine protein (protein-to-creatinine ratio >0.5 g/g), and diabetes. The highest rates were observed in the presence of both elevated urine protein and diabetes.
Change in eGFR over Time by Race/Ethnicity
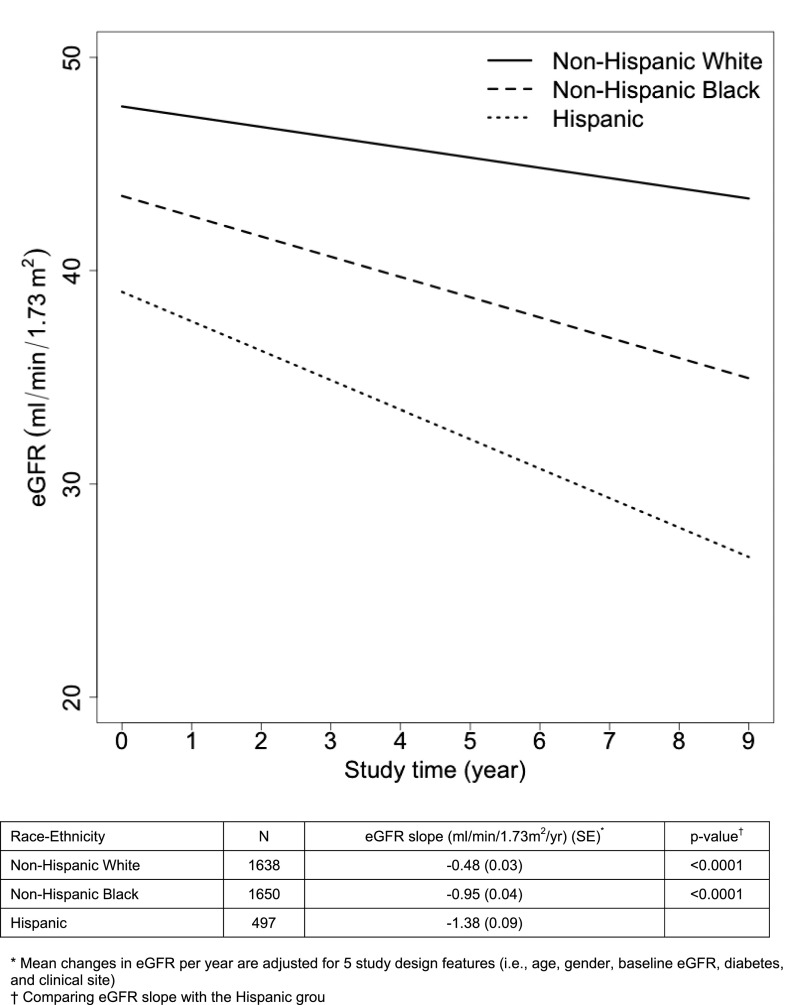
During follow-up, Hispanics had significantly higher rates of eGFR decline (−1.38 ml/min per 1.73 m2 per year) compared with that of NHW (−0.48 ml/min per 1.73 m2 per year; P<0.001) and that of NHB (−0.95 ml/min per 1.73 m2 per year; P<0.001) (Figure 2).
Figure 2.
Mean change in eGFR by race/ethnicity during study follow up.
Multivariable Analyses of CKD Progression, ESRD, and Differences in eGFR Slope
In analyses using death as a competing risk (ESRD outcome) and adjusted for clinical site, age, sex, education, health insurance, nephrology care, smoking, baseline eGFR, systolic BP, ACEi/ARB use, diabetes, body mass index (BMI), and hemoglobin A1c (HbA1c) (Table 2, Model 3), Hispanic ethnicity was associated with an increased risk of CKD progression (hazard ratio [HR], 1.90; 95% confidence interval [95% CI], 1.46 to 2.48) and incident ESRD (HR, 1.81; 95% CI, 1.34–2.45) relative to NHW race. After further adjustment for 24-hour urine protein excretion (Model 4), this increased risk of CKD progression (HR, 1.28; 95% CI, 0.96 to 1.70) and incident ESRD (HR, 1.32; 95% CI, 0.96 to 1.81) was attenuated and did not reach statistical significance at the P=0.05 level. In the fully adjusted model (Model 4), Hispanic ethnicity was not significantly associated with CKD progression (HR, 0.88; 95% CI, 0.68 to 1.13) and incident ESRD (HR, 0.94; 95% CI, 0.71 to 1.25) relative to NHB race.
Table 2.
Multivariable analyses of risk of CKD progression (ESRD or 50% eGFR loss and ESRD), differences in eGFR slope, and mortality (baseline and time-updated covariates)
| Model | Survival Modelsa | Mixed-Effects Models | Survival Modelsa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESRD or 50% eGFR Loss | ESRDb | Difference in eGFR Slope (ml/min per 1.73 m2 per yr) | Death | |||||||||||||
| Hispanic versus NHW | Hispanic versus NHB | Hispanic versus NHW | Hispanic versus NHB | Hispanic versus NHW | Hispanic versus NHB | Hispanic versus NHW | Hispanic versus NHB | |||||||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | Mean (95% CI) | P Value | Mean (95% CI) | P value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model 1 | 2.08 (1.65 to 2.62) | <0.001 | 1.16 (0.94 to 1.43) | 0.16 | 1.83 (1.40 to 2.38) | <0.001 | 1.04 (0.82 to 1.32) | 0.76 | −0.45 (−0.66 to −0.23) | <0.001 | −0.07 (−0.29 to 0.14) | 0.49 | 0.96 (0.71 to 1.30) | 0.80 | 0.81 (0.61 to 1.07) | 0.13 |
| Model 2 | 2.22 (1.73 to 2.84) | <0.001 | 1.24 (0.99 to 1.54) | 0.06 | 2.28 (1.71 to 3.03) | <0.001 | 1.22 (0.95 to 1.58) | 0.12 | −0.42 (−0.65 to −0.20) | 0.001 | −0.09 (−0.31 to 0.13) | 0.41 | 1.15 (0.78 to 1.69) | 0.48 | 1.06 (0.75 to 1.50) | 0.74 |
| Model 3 | 1.90 (1.46 to 2.48) | <0.001 | 1.26 (0.997 to 1.58) | 0.05 | 1.81 (1.34 to 2.45) | <0.001 | 1.17 (0.89 to 1.53) | 0.25 | −0.41 (−0.66 to −0.17) | 0.001 | −0.21 (−0.45 to 0.03) | 0.08 | 1.16 (0.78 to 1.71) | 0.47 | 1.07 (0.75 to 1.53) | 0.70 |
| Model 4 | 1.28 (0.96 to 1.70) | 0.09 | 0.88 (0.68 to 1.13) | 0.31 | 1.32 (0.96 to 1.81) | 0.08 | 0.94 (0.71 to 1.25) | 0.67 | −0.09 (−0.33 to 0.16) | 0.48 | 0.09 (−0.15 to 0.33) | 0.46 | 0.89 (0.59 to 1.35) | 0.58 | 0.89 (0.61 to 1.30) | 0.54 |
| Model 4a | 1.10 (0.67 to 1.81) | 0.70 | 0.61 (0.39 to 0.95) | 0.03 | 0.92 (0.51 to 1.64) | 0.77 | 0.50 (0.30 to 0.84) | 0.01 | −0.06 (−0.41 to 0.30) | 0.76 | 0.15 (−0.20 to 0.50) | 0.40 | c | c | ||
| Model 4b | 1.31 (0.93 to 1.84) | 0.12 | 1.04 (0.77 to 1.40) | 0.81 | 1.43 (0.97 to 2.10) | 0.07 | 1.16 (0.83 to 1.63) | 0.38 | −0.23 (−0.56 to 0.11) | 0.19 | −0.05 (−0.37 to 0.27) | 0.76 | c | c | ||
Model 1: age, sex, baseline eGFR, diabetes, clinical site. Model 2: Model 1 + education, health insurance, nephology care, smoking. Model 3: Model 2 + systolic BP, ACEi/ARB use, BMI, HbA1c. Model 4: Model 3 + log (24-hour urine protein + 1). Model 4a: Model 4 participants without diabetes only. Model 4b: Model 4 participants with diabetes only.
All models with time-updated nephrology care, systolic BP, ACEi/ARB use, BMI, and HbA1c.
Death treated as a competing risk using the method of Fine and Gray.43
Interaction between race and diabetes in Model 4 for death is not significant (P>0.05).
A significant interaction was found between race/ethnicity and diabetes for the CKD progression outcomes. Further models stratified by diabetes status (Models 4a and b) did not show a significant association of Hispanic ethnicity with these outcomes relative to NHW race. However, among participants without diabetes, Hispanic ethnicity was associated with a significantly lower risk of CKD progression (HR, 0.61; 95% CI, 0.39 to 0.95) and incident ESRD (HR, 0.50; 95% CI, 0.30 to 0.84) relative to NHB race.
In regression analyses adjusted for clinical site, age, sex, education, health insurance, nephrology care, smoking, baseline eGFR, systolic BP, ACEi/ARB use, diabetes, BMI, and HbA1c (Table 2, Model 3), Hispanics had a significantly greater rate of eGFR decline over time than that of NHW (mean annual adjusted difference in eGFR slope, −0.41; 95% CI, −0.66 to −0.17), but not than that of NHB (mean annual adjusted difference in eGFR slope, −0.21; 95% CI, −0.45 to +0.03). After additional adjustment for 24-hour urine protein excretion (Model 4), the higher rate of eGFR loss for Hispanics relative to NHW was no longer statistically significant (P>0.05). Moreover, in analyses stratified by diabetes status, there were also no significant associations between race/ethnicity and eGFR slope.
Multivariable Analyses of All-Cause Mortality
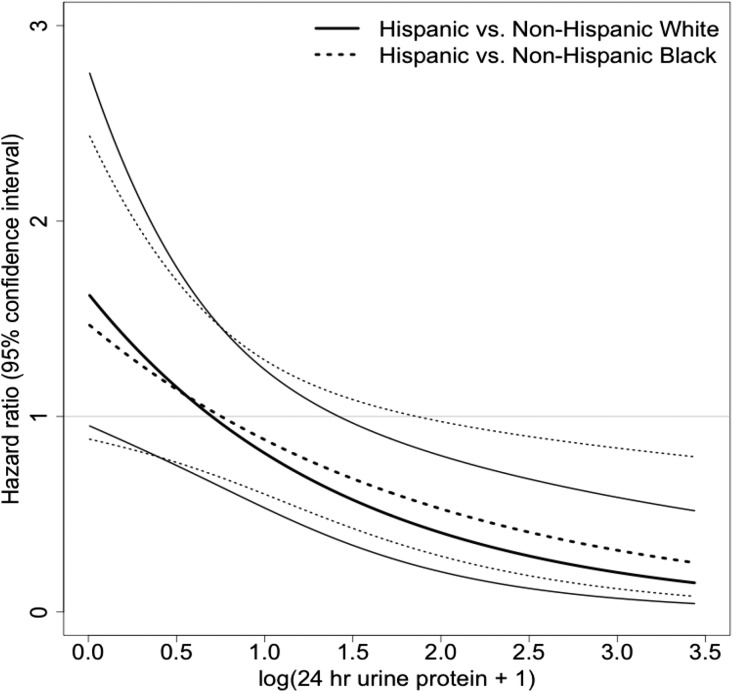
In iterative and fully adjusted regression analyses (clinical site, age, sex, education, health insurance, nephrology care, smoking, baseline eGFR, systolic BP, ACEi/ARB use, diabetes, BMI, HbA1c, and 24-hour urine protein) (Table 2, Model 4), the risk of all-cause mortality among Hispanics was similar compared with NHW (HR, 0.89; 95% CI, 0.59 to 1.35) and NHB (HR, 0.89; 95% CI, 0.61 to 1.30). A significant interaction between race/ethnicity and 24-hour urine protein was observed. The risk of death for Hispanics relative to NHW and NHB across levels of 24-hour urine protein was nonlinear, and significantly lower at higher levels of 24-hour urine protein (Figure 3).
Figure 3.
Risk of death by race/ethnicity and urine protein.
Discussion
In a large, racially and ethnically diverse prospective cohort study of adults with CKD, we found important differences in outcomes between Hispanics and non-Hispanics, and observed a profound impact on these relationships by proteinuria and diabetes. Although Hispanics had substantially higher crude rates of CKD progression and eGFR loss than NHW, this elevated risk did not achieve traditional statistical significance after adjusting for important characteristics and proteinuria. While Hispanics had similar crude rates of CKD progression and eGFR slope compared with NHB, Hispanic ethnicity was independently associated with an approximately 40% lower risk of CKD progression in nondiabetic participants after accounting for other contributing factors. Proteinuria significantly modified the relationship between race/ethnicity and mortality. While at lower levels of urine protein Hispanics had a similar risk of death compared with non-Hispanics, their risk of death was significantly lower at higher levels of urine protein.
Despite their increased risk for ESRD, there is scant information regarding decline in kidney function and other measures of CKD progression among Hispanics with CKD because they have not been included in sufficient numbers in prior major observational studies and clinical trials of CKD.18–23 Moreover, there is little understanding of factors that potentially impact outcomes in Hispanics with CKD such as literacy, acculturation, access to care, and immigration status.12,24 The CRIC and H-CRIC Studies assembled the first large longitudinal cohort of Hispanics with CKD in the United States by overcoming known barriers to recruiting and evaluating Hispanics into clinical studies, including bilingual recruitment strategies and study instruments.25,26 Through robust primary and secondary data collection on clinical exposures and events, these studies offer an excellent opportunity to examine understudied relations between race/ethnicity, CKD, and outcomes.27,28 Furthermore, the CRIC Study has employed rigorous methodologic approaches to study CKD epidemiology, including the refinement of outcome measures and analytic strategies (e.g., complementary time to event and slope analyses, competing risk analyses) for CKD progression.27,29 Toward that end, our study contributes robust findings to improve our understanding of CKD-related outcomes and mortality among Hispanics with CKD.
Previous studies of the relation between Hispanic ethnicity and CKD progression have yielded heterogeneous results. One prior study examining change in eGFR over time in a cohort of approximately one million patients of Kaiser Permanente of Southern California cohort with generally preserved eGFR, noted that the annual percentage change in eGFR was similar among Hispanics, whites, and blacks; however, these analyses were not adjusted for comorbidities or proteinuria.15 Prior studies have more frequently employed time to event analyses to examine CKD progression. A study of United States veterans with diabetes found Hispanics to have a lower risk of ESRD compared with whites4; however, only 10% of Hispanics had CKD at baseline. In contrast, in a study by Peralta et al. of nearly 40,000 patients with stage 3–4 CKD enrolled in Kaiser Permanente Northern California and in a study by Hall et al. of nearly 15,000 patients with nondialysis-dependent CKD in a community health network in San Francisco,13,17 Hispanic ethnicity was also associated with an increased risk for ESRD compared with NHW. Importantly, participants in both of these studies had a much lower prevalence of diabetes, which may have accounted for the lower observed ESRD event rates compared with ours. Furthermore, neither of these studies incorporated detailed urine protein quantification in their analyses, which appeared to account for a significant portion of the higher crude rates of CKD progression in Hispanics compared with non-Hispanics in our study.
In the two prior studies that included diabetic and nondiabetic participants as well as diverse racial/ethnic groups with Hispanics, NHB, and NHW, blacks had the highest risk of eGFR decline15 and incident ESRD.17 However, neither study directly compared rates of CKD progression of Hispanics with NHB or examined effect modification by diabetes status. Hence, our finding of a significantly lower risk of CKD progression in nondiabetic Hispanics versus NHB is novel and warrants further explanation. One potential reason for this observation includes differences in the etiology of nondiabetic CKD, which was not collected in this study.
It is well established that proteinuria has a profound negative impact on CKD progression,30 and proteinuria did explain a substantial component of the increased risk among the Hispanics whom we studied. Similar to our findings, Hispanics with diabetic nephropathy enrolled in a multinational interventional trial (i.e., the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan Study) studying the impact of losartan on CKD progression had more baseline proteinuria and a higher rate of progression to ESRD than NHW with diabetic nephropathy.31 In addition, Hispanics as well as the other racial/ethnic groups who experienced a significant decrement in proteinuria after 6 months of treatment had a more favorable outcome than individuals who did not experience a decrement in proteinuria, suggesting that proteinuria is a modifiable risk factor across racial/ethnic groups.31 Future studies are needed to investigate whether more aggressive targeting of proteinuria might be associated with more favorable outcomes in Hispanics. An important related question is the underpinning of higher proteinuria in Hispanics. It is not known if Hispanics are intrinsically more predisposed to proteinuric kidney disease or if it is a reflection of other risk factors. Although it is difficult to isolate the effect of any given factor, it is worth noting that the addition of traditional modifiable risk factors (e.g., systolic BP, HbA1c, ACEi/ARB use), socioeconomic parameters (e.g., education), and access to care (e.g., health insurance) did not significantly alter the relationship between race/ethnicity and CKD progression in any of our regression models. Further work is needed to explore this susceptibility as it has implications regarding potential causal pathways between Hispanic ethnicity and CKD progression.
It is important to recognize that Hispanics have diverse racial and ethnic origins which possess key differences in acculturation, customs, cultural beliefs, and social structure.24,32 In the Multiethnic Study of Atherosclerosis cohort, Dominicans and Puerto Ricans had faster rates of kidney function decline than whites; however, Mexicans, South Americans, and other Hispanic groups had similar rates of decline compared with whites.33 The authors speculated that the higher degree of African ancestry and presence of APOL1 alleles in Dominicans and Puerto Ricans may explain these differences.33,34 Since the H-CRIC cohort was predominantly Mexican American, it is likely that the prevalence of the high-risk APOL1 variant will be significantly lower than in Hispanic background groups with a higher degree of African ancestry.35 Lastly, although we adjusted for several important factors (e.g., biologic, socioeconomic) in our analyses that have been previously posited to contribute to disparities,16,24,36 we cannot exclude residual confounding as accounting for observed differences in race/ethnicity and CKD progression.
In the general population, Hispanic ethnicity confers a survival advantage compared with NHW race despite a higher burden of cardiovascular risk factors among Hispanics, which has been described as the ‘Hispanic paradox’37. Several potential explanations for this observation include salmon bias (i.e., return migration of Hispanics to country of origin to die), healthy migrant effect (i.e., younger healthy Hispanic immigrants), and strong family-based social support and caregiving.38–40
Despite Hispanics in our study having a worse profile of baseline risk factors for death,10,11 we found that their risk of death was significantly lower than non-Hispanics at higher levels of urine protein after accounting for important factors. In five cohorts of adults with CKD, including those from Kaiser Permanente of Northern and Southern California, the National Health and Nutrition Examination Survey, the Kidney Early Evaluation Program, and a San Francisco-based community cohort, Hispanic ethnicity has been inconsistently associated with a lower risk of death relative to NHW.13–17 While some observed a lower mortality risk associated with Hispanic ethnicity,13,15 others did not.14,16,17 Potential explanations for the differing findings across studies include differences in CKD severity (i.e., eGFR, proteinuria), prevalence and inclusion of comorbidities into analyses (e.g., diabetes), measurement and incorporation of proteinuria into analyses, and other unmeasured characteristics reducing overall death rates in our cohort. To our knowledge, prior studies have not examined a modifying effect of proteinuria on the relationship between death and race/ethnicity, which needs further investigation.
Our study has limitations. First, our findings are subject to residual confounding and bias that may occur in observational studies, especially since baseline characteristics differ between racial/ethnic groups. Additionally, the median time of follow up for Hispanics (5.1 years) was slightly less than for non-Hispanics (6.8 years), which could potentially bias observed relationships between race/ethnicity and outcomes. However, by capitalizing on detailed data collection with minimal missing data and a very high participant retention across racial/ethnic groups (approximately 90%), we were able to adjust our analyses for a large number of important covariates to minimize these concerns.27,28 Second, as noted previously,11 the majority of Hispanics in our study were Mexican American (69%) and were recruited from the Chicago region (85%). While many characteristics of our Hispanic cohort, including background group, socioeconomic parameters, and primary language are similar to representative United States population-based samples such as those in National Health and Nutrition Examination Survey,16 we recognize that our findings may not be fully generalizable to all Hispanics with CKD in the United States. Because of prespecified enrollment targets in the CRIC/H-CRIC Studies, the prevalence of diabetes and degree of proteinuria were notably higher in Hispanics than non-Hispanics in our study compared with prior studies. Although we explicitly included these covariates in our risk-adjusted models, we cannot eliminate remaining selection bias. The ongoing Hispanic Community Health Study/Study of Latinos will likely provide insights into risk factors for CKD across the major Hispanic background groups in the United States.41 Lastly, because of exclusion criteria for the CRIC/H-CRIC Studies, polycystic kidney disease and active GN requiring immunosuppression were not studied. Furthermore, the CRIC Study was not designed to collect details regarding the etiology of kidney disease for enrolled participants. Nonetheless, the CRIC/H-CRIC cohorts are representative of the CKD and ESRD populations as described in National Health and Nutrition Examination Survey and United States Renal Data System, where diabetes and/or hypertension are the primary diagnoses for >65% of patients.2,16
In summary, in a large, racially and ethnically diverse cohort of adults with CKD, we found Hispanic ethnicity to be associated with a lower risk of CKD progression than NHB among participants without diabetes and a lower risk of death than NHB and NHW at high levels of urine protein. Proteinuria had an important role in the relationships between race/ethnicity and outcomes. The forces driving disparities for Hispanics with CKD remain incompletely elucidated. Moreover, Hispanics possess key differences in racial/ethnic origins, acculturation, customs, cultural beliefs, and social structure. Further study is needed to comprehensively examine the impact of this broad spectrum of factors on clinical outcomes and to identify modifiable characteristics.
Concise Methods
Study Sample and Design
We conducted a longitudinal analysis of participants of the CRIC and H-CRIC Studies. The CRIC Study is a prospective multicenter observational cohort study of adults with CKD. Details of the design and methods of the CRIC Study have been published previously.27,28 Major eligibility criteria for the CRIC Study included adults aged 21–74 years with mild to moderate CKD using age-based eGFR. Exclusion criteria included inability to consent, New York Heart Association class 3 or 4 heart failure, cirrhosis, HIV/AIDS, polycystic kidney disease, prior dialysis therapy or transplant, immunosuppressive therapy within 6 months, or chemotherapy for cancer within 2 years. The H-CRIC Study, an ancillary study to the CRIC Study, adopted eligibility and exclusion criteria identical to the parent study11 but recruited only participants of Hispanic ethnicity. As noted previously, 170 Hispanics and 3288 non-Hispanics were recruited into the CRIC Study at seven clinical centers from May of 2003 through to March of 2007, while H-CRIC included 327 Hispanics recruited at the University of Illinois at Chicago and the Chicago metropolitan area from October of 2005 through to June of 2008. Recruitment sites included university-based, community-based, and private health clinics.25 Both studies were approved by the institutional review boards of the participating centers, and the research was conducted in accordance with the principles of the Declaration of Helsinki. All study participants provided written informed consent.
Variables and Data Sources
Sociodemographic characteristics (e.g., age, sex, race/ethnicity, education, and annual household income) and medical conditions (e.g., hypertension, high cholesterol level, chronic heart failure, peripheral arterial disease, diabetes, myocardial infarction, or coronary revascularization) were self-reported and recorded at the baseline visit.27,28 Self-reported race/ethnicity in the CRIC/H-CRIC Studies was categorized as NHW, NHB, and Hispanic.11 Anthropometric measurements (height and body weight) and BP were obtained by trained study personnel using a standardized protocol and recorded at enrollment. Current medications were reviewed and documented at study visits (i.e., participant medication bottles or updated medication list). Twenty-four-hour urine protein was measured for all participants at baseline, and serum creatinine was measured at baseline and annually. GFR was estimated for participants based on an equation developed in a subgroup of CRIC participants with an iothalamate GFR,42 which has been demonstrated to have superior accuracy in this cohort compared with other eGFR equations.42
We examined three primary time-to-event outcomes: incident ESRD, CKD progression, and all-cause mortality. Incident ESRD was defined by initiation of chronic dialysis or receipt of a kidney transplant, and was ascertained by self-report and United States Renal Data System data. CKD progression was defined as incident ESRD or loss of 50% of eGFR from baseline value. Because serum creatinine values for eGFR calculation were obtained annually, we imputed the time until reduction in 50% eGFR, assuming a linear decline between in-person annual study visits as described previously.29 Depending on the site, deaths were ascertained from reports of next of kin, death certificates, obituaries, hospital records, and linkage with the Social Security Death Master File and/or state death certificate files. We also examined mean annual rate of decline in kidney function (slope of eGFR over time) as a measure of CKD progression.
Statistical Analyses
Event rates (per 100 person-years) for the three time-to-event outcomes were calculated as the ratio of the number of participants reaching the event divided by the total person-years of follow-up before an event or until censoring by using Poisson regressions controlling for age, sex, baseline eGFR, diabetes, and clinical site to account for study design. In computations of event rates and in regression analyses, follow-up times for the ESRD and CKD progression outcomes were censored at time of all-cause death or withdrawal from study. Event rates are presented for the overall cohort and stratified by eGFR, proteinuria, and diabetes (Supplemental Table 1). Mean annual change in eGFR was calculated for each participant by averaging 12-month differences in eGFR measured at annual study visits throughout follow up.
Cox proportional hazards regression models were used to assess the association between race/ethnicity and the time-to-event outcomes, including incident ESRD and CKD progression and death, after adjusting for covariates. Violation of the assumption of proportional hazards in the Cox regression was evaluated using Schoenfeld residuals for all included covariates. For analyses of incident ESRD, death was treated as a competing risk using the method of Fine and Gray.43 In this framework, only loss to follow-up was treated as a censoring event. For eGFR slope analyses, linear mixed-effects models were used to assess the association between the difference in mean annual change in eGFR and race/ethnicity after adjusting for covariates. We compared NHB with Hispanic participants and NHW with Hispanic participants. We constructed serial nested models for each outcome, whereby covariates from each prior model are retained, and all covariates were chosen based on known clinical importance in prior literature. The final model included: age, sex, baseline eGFR, diabetes, clinical site, education, health insurance, nephology care, smoking, systolic BP, ACEi/ARB use, BMI, HbA1c, and proteinuria. The following covariates were included as time-updated covariates in standard Cox proportional hazards regression models: nephrology care, systolic BP, ACEi/ARB use, BMI, and HbA1c (Table 2). The time-updated covariates were collected on an annual basis in CRIC and the most recent value prior to event ascertainment was used in the Cox models. In addition, interactions between race/ethnicity and diabetes and between race/ethnicity and proteinuria were examined, and stratified analyses by these covariates were presented if the interaction was significant.
For all analyses, a two-sided P value of 0.05 was considered statistically significant. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc., Cary, NC).
Disclosures
Dr. Go has received a research grant from Astra Zeneca (London, United Kingdom).
Supplementary Material
Acknowledgments
Funding for the Chronic Renal Insufficiency Cohort Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award and the National Institutes of Health (NIH)/ National Center for Advancing Translational Sciences (NCATS) (UL1 TR-000003), Johns Hopkins University (UL1 TR-000424), University of Maryland General Clinical Research Center (M01 RR-16500), Clinical and Translational Science Collaborative of Cleveland, UL1 TR-000439 from the NCATS component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (UL1 TR-000433), University of Illinois at Chicago Clinical and Translational Science Award (UL1 RR-029879), Tulane University Translational Research in Hypertension and Renal Biology (P30GM103337), and Kaiser Permanente NIH/National Center for Research Resources, University of California-San Francisco Clinical & Translational Science Institute (UL1 RR-024131). Additional support was provided by the National Center for Minority Health and Health Disparities, the NIH, and Department of Veterans Affairs Health Services Research and Development Service (to M.J.F.). Support was provided from NIH/NIDDK K24-DK092290 (to J.P.L.), K23 DK091313 (to C.M.L.), K23 DK094829 (to A.C.R.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050570/-/DCSupplemental.
Contributor Information
Collaborators: Lawrence J. Appel, Harold I. Feldman, Jiang He, Mahboob Rahman, and Raymond R. Townsend
References
- 1.US Census Bureau: The Hispanic Population: 2010, 2011. Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. Accessed: February 25, 2014
- 2.US Renal Data System : USRDS 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 3.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Young BA, Maynard C, Boyko EJ: Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care 26: 2392–2399, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Jones CA, Francis ME, Eberhardt MS, Chavers B, Coresh J, Engelgau M, Kusek JW, Byrd-Holt D, Narayan KM, Herman WH, Jones CP, Salive M, Agodoa LY: Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis 39: 445–459, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bryson CL, Ross HJ, Boyko EJ, Young BA: Racial and ethnic variations in albuminuria in the US Third National Health and Nutrition Examination Survey (NHANES III) population: associations with diabetes and level of CKD. Am J Kidney Dis 48: 720–726, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bhalla V, Zhao B, Azar KM, Wang EJ, Choi S, Wong EC, Fortmann SP, Palaniappan LP: Racial/ethnic differences in the prevalence of proteinuric and nonproteinuric diabetic kidney disease. Diabetes Care 36: 1215–1221, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jolly SE, Burrows NR, Chen SC, Li S, Jurkovitz CT, Narva AS, Norris KC, Shlipak MG: Racial and ethnic differences in albuminuria in individuals with estimated GFR greater than 60 mL/min/1.73 m(2): results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 55[Suppl 2]: S15–S22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foley RN, Wang C, Ishani A, Collins AJ: NHANES III: influence of race on GFR thresholds and detection of metabolic abnormalities. J Am Soc Nephrol 18: 2575–2582, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Ricardo AC, Lash JP, Fischer MJ, Lora CM, Budoff M, Keane MG, Kusek JW, Martinez M, Nessel L, Stamos T, Ojo A, Rahman M, Soliman EZ, Yang W, Feldman HI, Go AS; CRIC and HCRIC Investigators : Cardiovascular disease among hispanics and non-hispanics in the chronic renal insufficiency cohort (CRIC) study. Clin J Am Soc Nephrol 6: 2121–2131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP; CRIC and H-CRIC Study Groups : CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis 58: 214–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lora CM, Daviglus ML, Kusek JW, Porter A, Ricardo AC, Go AS, Lash JP: Chronic kidney disease in United States Hispanics: a growing public health problem. Ethn Dis 19: 466–472, 2009 [PMC free article] [PubMed] [Google Scholar]
- 13.Peralta CA, Shlipak MG, Fan D, Ordoñez J, Lash JP, Chertow GM, Go AS: Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol 17: 2892–2899, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Jolly SE, Burrows NR, Chen SC, Li S, Jurkovitz CT, Norris KC, Shlipak MG: Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the Kidney Early Evaluation Program (KEEP). Clin J Am Soc Nephrol 6: 1858–1865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derose SF, Rutkowski MP, Crooks PW, Shi JM, Wang JQ, Kalantar-Zadeh K, Kovesdy CP, Levin NW, Jacobsen SJ: Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. Am J Kidney Dis 62: 236–244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehrotra R, Kermah D, Fried L, Adler S, Norris K: Racial differences in mortality among those with CKD. J Am Soc Nephrol 19: 1403–1410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall YN, Choi AI, Chertow GM, Bindman AB: Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol 5: 828–835, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER 3rd, Norris K, O’Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley-Brown D, Tisher CC, Toto RD, Wright JT Jr, Xu S; African American Study of Kidney Disease and Hypertension (AASK) Study Group : Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 285: 2719–2728, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT Jr, Whelton PK, Barzilay J, Batuman V, Eckfeldt JH, Farber M, Henriquez M, Kopyt N, Louis GT, Saklayen M, Stanford C, Walworth C, Ward H, Wiegmann T: Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med 165: 936–946, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Appel LJ, Middleton J, Miller ER 3rd, Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT: The rationale and design of the AASK cohort study. J Am Soc Nephrol 14[Suppl 2]: S166–S172, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Lora CM, Gordon EJ, Sharp LK, Fischer MJ, Gerber BS, Lash JP: Progression of CKD in Hispanics: potential roles of health literacy, acculturation, and social support. Am J Kidney Dis 58: 282–290, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lora CM, Ricardo AC, Brecklin CS, Fischer MJ, Rosman RT, Carmona E, Lopez A, Balaram M, Nessel L, Tao KK, Xie D, Kusek JW, Go AS, Lash JP: Recruitment of Hispanics into an observational study of chronic kidney disease: the Hispanic Chronic Renal Insufficiency Cohort Study experience. Contemp Clin Trials 33: 1238–1244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricardo AC, Hacker E, Lora CM, Ackerson L, DeSalvo KB, Go A, Kusek JW, Nessel L, Ojo A, Townsend RR, Xie D, Ferrans CE, Lash JP; CRIC Investigators : Validation of the Kidney Disease Quality of Life Short Form 36 (KDQOL-36) US Spanish and English versions in a cohort of Hispanics with chronic kidney disease. Ethn Dis 23: 202–209, 2013 [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI: Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, Hsu CY, Fink JC, He J, Lash JP, Ojo A, Rahman M, Nessel L, Kusek JW, Feldman HI; CRIC Study Investigators : Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 63: 236–243, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turin TC, James M, Ravani P, Tonelli M, Manns BJ, Quinn R, Jun M, Klarenbach S, Hemmelgarn BR: Proteinuria and rate of change in kidney function in a community-based population. J Am Soc Nephrol 24: 1661–1667, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Zeeuw D, Ramjit D, Zhang Z, Ribeiro AB, Kurokawa K, Lash JP, Chan J, Remuzzi G, Brenner BM, Shahinfar S: Renal risk and renoprotection among ethnic groups with type 2 diabetic nephropathy: a post hoc analysis of RENAAL. Kidney Int 69: 1675–1682, 2006 [DOI] [PubMed] [Google Scholar]
- 32.González Burchard E, Borrell LN, Choudhry S, Naqvi M, Tsai HJ, Rodriguez-Santana JR, Chapela R, Rogers SD, Mei R, Rodriguez-Cintron W, Arena JF, Kittles R, Perez-Stable EJ, Ziv E, Risch N: Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am J Public Health 95: 2161–2168, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peralta CA, Katz R, DeBoer I, Ix J, Sarnak M, Kramer H, Siscovick D, Shea S, Szklo M, Shlipak M: Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol 22: 1327–1334, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzur S, Rosset S, Skorecki K, Wasser WG: APOL1 allelic variants are associated with lower age of dialysis initiation and thereby increased dialysis vintage in African and Hispanic Americans with non-diabetic end-stage kidney disease. Nephrol Dial Transplant 27: 1498–1505, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ; AASK Study Investigators; CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powe NR: Let’s get serious about racial and ethnic disparities. J Am Soc Nephrol 19: 1271–1275, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Franzini L, Ribble JC, Keddie AM: Understanding the Hispanic paradox. Ethn Dis 11: 496–518, 2001 [PubMed] [Google Scholar]
- 38.Abraído-Lanza AF, Dohrenwend BP, Ng-Mak DS, Turner JB: The Latino mortality paradox: a test of the “salmon bias” and healthy migrant hypotheses. Am J Public Health 89: 1543–1548, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao Y, Cooper RS, Cao G, Durazo-Arvizu R, Kaufman JS, Luke A, McGee DL: Mortality patterns among adult Hispanics: findings from the NHIS, 1986 to 1990. Am J Public Health 88: 227–232, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundquist J, Winkleby M: Country of birth, acculturation status and abdominal obesity in a national sample of Mexican-American women and men. Int J Epidemiol 29: 470–477, 2000 [PubMed] [Google Scholar]
- 41.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, Lavange L, Chambless LE, Heiss G: Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 20: 629–641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI; CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.