Abstract
Changes in metabolite levels of the kynurenine pathway have been observed in patients with CKD, suggesting involvement of this pathway in disease pathogenesis. Our recent genetic analysis in the mouse identified the kynurenine 3-mono-oxygenase (KMO) gene (Kmo) as a candidate gene associated with albuminuria. This study investigated this association in more detail. We compared KMO abundance in the glomeruli of mice and humans under normal and diabetic conditions, observing a decrease in glomerular KMO expression with diabetes. Knockdown of kmo expression in zebrafish and genetic deletion of Kmo in mice each led to a proteinuria phenotype. We observed pronounced podocyte foot process effacement on long stretches of the filtration barrier in the zebrafish knockdown model and mild podocyte foot process effacement in the mouse model, whereas all other structures within the kidney remained unremarkable. These data establish the candidacy of KMO as a causal factor for changes in the kidney leading to proteinuria and indicate a functional role for KMO and metabolites of the tryptophan pathway in podocytes.
Keywords: podocyte, proteinuria, genetic renal disease
The kynurenine pathway is a major catabolic route for L-tryptophan. Abnormalities in this pathway have been found in several disorders, including CKD. Pawlak et al. reported increases in plasma levels of kynurenine metabolites in CKD patients.1 Similar abnormalities can be seen in renal-insufficient animal models, such as rats with experimental chronic renal failure induced by 5/6 nephrectomy. In these animals, kynurenine metabolite levels increased parallel to the severity of renal failure.2,3 In vitro studies by Yoshimura et al. have shown that kynurenine metabolites affect mesangial cell proliferation in culture.4
More direct evidence for the significance of kynurenine metabolites in the progression of renal failure has been revealed by studies using mice deficient in arylformamidase, which catalyzes the reaction of N-formyl-L-kynurenine and water into formate and L-kynurenine. Plasma levels of kynurenine metabolites are several times higher in Afmid-deficient mice than wildtype mice, and renal failure is observed. Kidney glomeruli develop global diffuse glomerulosclerosis, characterized by increased mesangial matrix and decreased cellularity.5,6
We recently carried out genome-wide association mapping using 290 outbred NMRI mice to map loci affecting HDL cholesterol, systolic BP, triglyceride and glucose levels, and urinary albumin-to-creatinine ratios.7 One single-nucleotide polymorphism found to be associated with urinary albumin-to-creatinine ratios is located on Chr 1, at a locus linked to GN in mice.8 One of the genes within the small interval is Kmo, which encodes kynurenine-3-mono-oxygenase (KMO). KMO is an NADPH-dependent flavin mono-oxygenase that catalyzes the hydroxylation of the L-tryptophan metabolite L-kynurenine to form L-3-hydroxykynurenine, and is the next enzyme in the kynurenine pathway after arylformamidase. So far, it has only been described in relation to brain and neurologic phenotypes.9
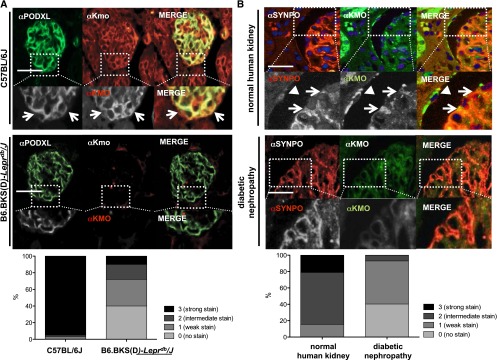
Because of its association with proteinuria, we wanted to assess whether KMO is expressed in glomerular cells of the mouse and human kidney, and in podocytes in particular. To accomplish this, we stained paraffin-embedded mouse and human kidney sections with anti-KMO antibodies and, to examine a potential colocalization with podocytes, with podocalyxin (PODXL) and synaptopodin as podocyte-specific markers. Due to very strong Kmo expression in the proximal tubules, it was challenging to demonstrate glomerular expression on complete cortex sections. Nevertheless, we found that KMO is clearly expressed in glomerular cells and podocytes in mice and humans (Figure 1, A and B). Of note, other glomerular cells in the endocapillary/mesangial compartment and parietal epithelial cells were also immunolabeled for KMO. Having confirmed KMO expression in podocytes, we sought to assess changes in podocytic KMO expression in the context of kidney disease by examining KMO expression in paraffin-embedded tissue sections of 28-week-old, diabetic, B6.BKS(D)-Leprdb/J mice and age-matched control C57BL/6J mice. We observed a decrease in glomerular KMO expression under diabetic conditions (Figure 1A). The slides were then subjected to systematic analysis for colocalization of KMO and PODXL, and semiquantitatively scored in a blinded manner. The scores ranged from 0 (no podocyte/KMO colocalization) to 3 (strong glomerular stain/podocyte-KMO colocalization). In C57BL/6J mice, we analyzed 60 glomeruli and found that 57 (95%) had a score of 3, one glomerulus had a score of 2, and two glomeruli had a score of 1. No glomerulus was rated as 0. In contrast, in the B6.BKS(D)-Leprdb/J mice, we analyzed 100 glomeruli and scored 40% as 0, 32% as 1, 18% as 2, and 10% as 3 (Figure 1A).
Figure 1.
Glomerular KMO expression is reduced in murine and human diabetes. (A) Paraffin-embedded kidney cortex sections of mouse kidneys were stained for KMO expression. Podocyte-specific marker PODXL and KMO-staining show coexpression in the glomeruli in wildtype C57BL/6J mice (white arrows, upper panels), whereas in diabetic B6.BKS(D)-Leprdb/J mice the podocytic KMO staining is mostly absent (lower panels). Comparative quantification of podocytic KMO expression in mice reveals costaining of KMO and PODXL in 95% of examined glomeruli in C57BL/6J mice, in contrast to 10% in the diabetic B6.BKS(D)-Leprdb/J mice. (B) In paraffin-embedded human kidney cortex sections, white arrows mark podocytic KMO expression, as identified by coexpression with the podocyte-specific marker synaptopodin and arrowhead marks positive parietal epithelial cell (upper panels). In comparison, KMO expression is absent in biopsy tissues of patients with diabetic nephropathy. Quantification reveals strong or intermediate KMO expression in 85% of all healthy glomeruli examined in contrast to only a weak or intermediate staining in 60% of glomeruli in diabetic kidneys. No glomerulus in the diabetic group showed a strong (score 3) stain.
Next, we examined KMO expression in a series of biopsies of patients with diagnosed diabetic nephropathy and compared them with sections of morphologically healthy kidneys. Again, we observed significantly reduced expression of KMO in diabetic tissue. Blinded scoring of 265 control glomeruli showed a score of 3 in 21%, a score of 2 in 63%, and a score of 1 in 16%. No glomerulus scored 0. In contrast, of 57 glomeruli from patients with diabetic nephropathy, 40% were scored as 0, 53% were rated with a score of 1, and 7% reached a score of 2, whereas no glomerulus received a score of 3. These data indicate that podocytic KMO expression is decreased in diabetic conditions in both mice and humans.
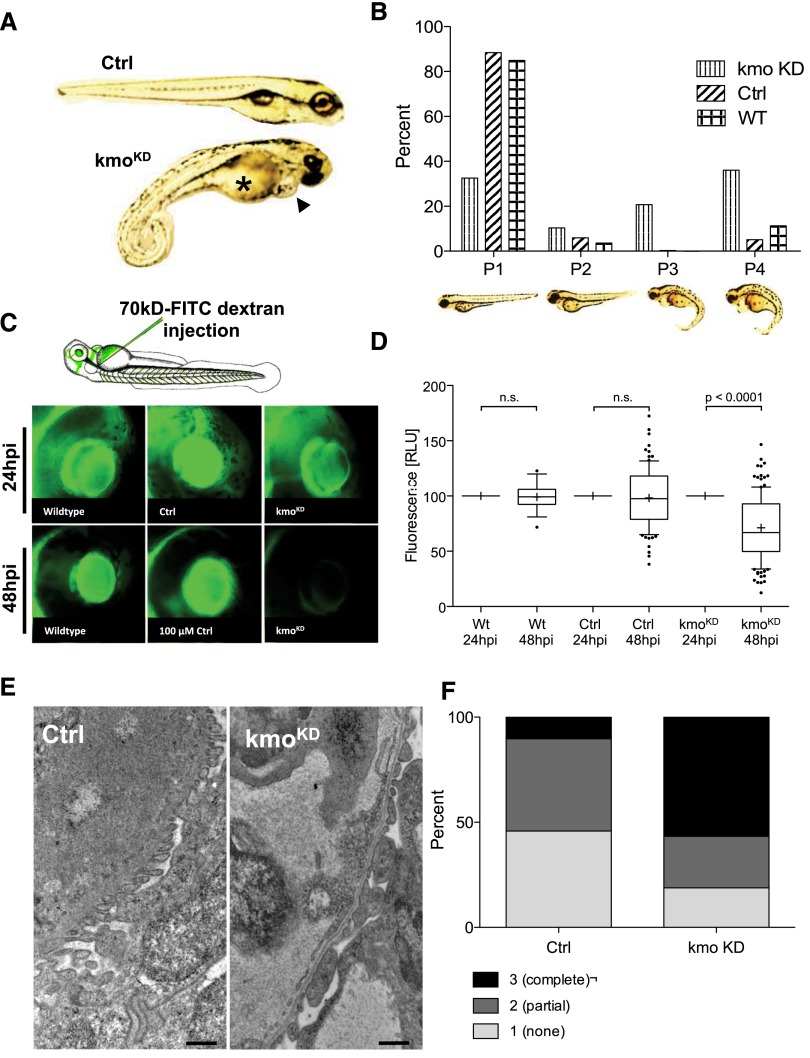
To examine the relevance of kmo function in vivo, we performed morpholino knockdown in zebrafish. In the kmo knockdown (kmoKD) group, using an ATG-blocking morpholino, we found a significant amount of individuals developed edema with pericardial effusion and yolk sac edema, which was monitored for up to 120 hours postfertilization (hpf) (Figure 2A). The fish were rated as normal (P1), mildly edematous (P2), severely edematous (P3), or very severely edematous (P4). The kmoKD fish showed a tenfold increase in edematous phenotypes P3 and P4 compared with animals injected with a scrambled control morpholino (Figure 2B). Since edema in fish is not conclusive evidence of renal damage, we carried out functional assays to determine whether the glomerular filtration barrier was intact. We injected 70 kDa FITC-labeled dextran into the cardiac venous sinus of the zebrafish and measured the fluorescent signal in the eye. KmoKD fish lost 28.9% of their fluorescence over the 24 hours between the baseline reading and the second measurement, a significant (P<0.001) increase in excretion of protein compared with uninjected fish, which lost 1.0%, and our control-injected fish, which lost 0.5% of their fluorescence (Figure 3, C and D). The knockdown experiments were repeated in an independent experimental setting using a second, splice-donor morpholine, which resulted in identical development of edema phenotypes and loss of high molecular weight fluorescence from the circulation. Knockdown was confirmed by quantitative PCR and resulted in a dose-dependent knockdown from 28% (using 100 μM morpholino) up to 61% (using 400 μM morpholino) (Supplemental Figure 1). To further verify our findings, we fixed and embedded 120 hpfkmoKD larvae showing a P3 or P4 phenotype for ultrastructural analysis by transmission electron microscopy (TEM). We found that endothelial cells, as well as the glomerular basement membrane, looked unremarkable in all fish; however, the podocytes in kmoKD larvae displayed a partial foot process effacement, whereas the control animals showed long stretches of the characteristic pearl-string pattern of podocytic foot processes, as expected of a healthy kidney (Figure 3E). To quantify our findings, we graded the appearance of the podocyte foot processes, ranging from normal (1), partially/intermediately effaced (2), to effaced (3) in 500 nm increments. We examined a total of more than 1000 μm of basement membrane in knockdown and control animals and found a 5.7-fold increase in severely effaced podocyte foot processes in kmoKD larvae compared with control-injected fish (Figure 3F). These data indicate a significant loss of high molecular mass proteins from the circulation in the absence of functional kmo and demonstrates disruption of the glomerular filtration barrier with a clear podocyte phenotype, thereby validating kmoKD zebrafish as an in vivo proteinuria model.
Figure 2.
Knockdown of kmo in zebrafish leads to edema, proteinuria, and foot process effacement. (A) Zebrafish at 120 hpf develop, after knockdown of kmo, a phenotype with yolk sac edema (asterisk) and pericardial effusion (black arrowhead). (B) Percentage of phenotypes graded from P1 (no edema) to P4 (very severe edema) at 120 hpf after kmo knockdown compared with 100 μM control morpholino injection (Ctrl) and uninjected wildtype (WT) larvae. In the kmoKD group, 47% exhibit the severe phenotypes P3 and P4 compared with 4.5% in the Ctrl group and 8% in the WT group. (C) Eye assay in WT, Ctrl-injected and kmoKD animals after cardinal vein injection of high–molecular mass (70 kD) FITC-labeled-dextran. Fluorescence was measured 24 hours postinjection as baseline and measured 48 hours postinjection as readout. (D) The fluorescence measurements in the retinal vessel plexus of WT and Ctrl-injected fish do not show any significant decrease in fluorescence over time, whereas the kmoKD animals exhibit a significant loss of fluorescence from the circulation, indicating disturbance of size selectivity of the filtration barrier. (E) Electron microscopy shows a clear podocyte effacement phenotype in the pronephros of the kmoKD larvae (right) compared with Ctrl-injected fish (left), whereas basement membrane and fenestrated endothelium look unremarkable. (F) Scoring of the podocyte effacement reveals a complete effacement (score 3) in 56% of the examined filtration barrier in the kmoKD larvae (a total of 1000 μm was scored in 500 nm increments).
Figure 3.
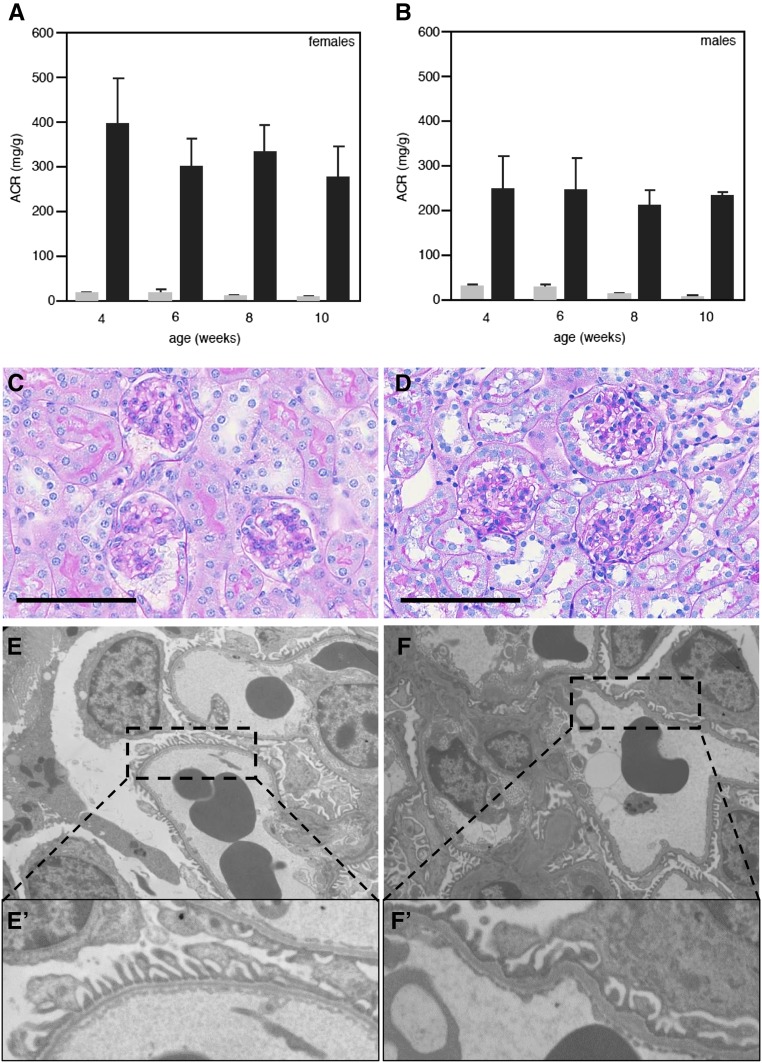
Kmo-KO mice develop albuminuria and foot process effacement. Female (A) and male (B) Kmo-KO mice (C57BL/6N-Kmotm1a(KOMP)Wtsi) were examined for development of albuminuria (black bars). Both groups develop albuminuria as early as 4 weeks after birth compared with wildtype animals (gray bars). Albuminuria does not change significantly between 4 and 10 weeks. Morphologic analysis using light microscopy revealed no obvious differences between wildtype (C) and Kmo-KO animals (D) at 10 weeks of age (periodic acid–Schiff staining; size bar represents 50 μm). TEM analysis showed only a partial podocyte foot process effacement in some areas in the KO mice (panels F/F′), whereas the majority of examined glomeruli displayed normal foot processes comparable to the wildtype animals (panels E/E′).
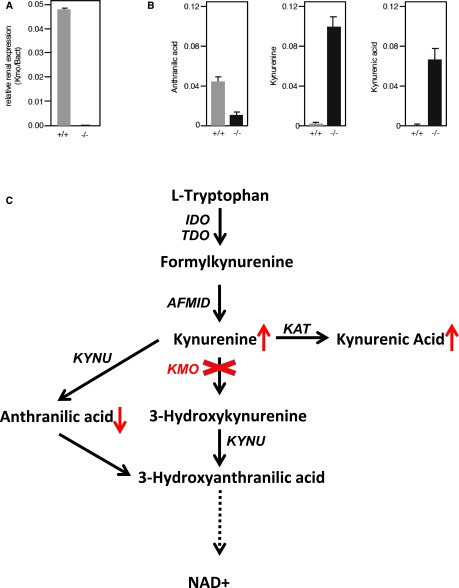
To further examine Kmo in a mammalian in vivo model, C57BL/6N-Kmotm1a(KOMP)Wtsi mouse embryos were obtained from the knockout mouse project (KOMP) Repository (University of California, Davis) and transferred to CByB6F1/J foster females. RNA was isolated from the kidneys and used for real-time PCR. There was no expression of Kmo detectable in the homozygous KO mice (Figure 4A). We found that both female and male KO animals had increased albumin in their urine, at as early as 4 weeks of age, compared with wildtype animals. Albuminuria did not change significantly between 4 and 10 weeks (Figure 3, A and B). Morphologic analysis by light microscopy revealed no obvious differences (Figure 3, C and D). With TEM, we were only able to detect partial effacement of podocyte foot processes in KO mice, whereas the majority of examined glomeruli displayed foot processes comparable to wildtype animals (Figure 3, E–F′). Of note, proximal tubular cells were completely unremarkable on examination by light microscopy as well as by TEM in wildtype and KO animals, without any signs of distress, and displayed intact brush borders (data not shown). To determine the effect of Kmo deletion on the tryptophan pathway, we measured several metabolites from serum. There was an increase in levels of both kynurenine (approximately 60-fold) and kynurenic acid (approximately 300-fold), but a decrease in anthranilic acid (approximately fourfold) in KO compared with wildtype animals (Figure 4B). There was no difference in plasma tryptophan levels (data not shown).
Figure 4.
Characterization of the kynurenine pathway in Kmo-KO mice. (A) Real-time PCR on kidney RNA shows a complete absence of Kmo expression in the KO. (B) The result of the KO is a decrease in anthranilic acid and an increase of kynurenine and kynurenic acid. (C) Overview of the kynurenine pathway indicating the changes caused by the Kmo-KO.
In conclusion, these findings lend support to the link between KMO expression and kidney health and function. We found that glomerular KMO expression is decreased in both mouse and human kidneys in a diabetic environment. Colocalization with podocyte-specific markers show that this decrease is primarily restricted to podocytes. Knockdown of expression in zebrafish and genetic deletion of Kmo in mice results in proteinuria, but the clear histologic changes we found in zebrafish were less pronounced in mice. Measurements of tryptophan metabolites in the Kmo-KO mice showed an increase in kynurenine and kynurenic acid and a decrease in anthranilic acid, suggesting redirection of the pathway. Because the Kmo-KO mice have a distinctly different renal phenotype from the Afmid-mutant mice,5,6 we do not believe the phenotype in Kmo-KO mice is due to an increase in formylkynurenine. Whether it is caused by an increase in kynurenic acid levels or a decrease in levels of a downstream product within the pathway remains to be investigated. It is tempting to speculate that the tryptophan metabolism leading to NAD+ generation (Figure 4C) could be essential for podocytic microtubule formation and cytoskeleton rearrangements, as NAD+ is essential for these processes.10 Since NAD+ is also a cofactor for impairment of insulin sensitivity,11 and insulin is essential for podocyte function,12 the reduced KMO activity in diabetes might contribute to proteinuria development in diabetic nephropathy. Additionally, as glomerular podocytes are highly differentiated and specialized cells with no significant potential to regenerate, these cells might react more sensitive to changes in metabolic pathways and have less capacity to compensate for missing pathway components compared with other epithelial cell types.13
Concise Methods
Immunohistochemistry in Human and Mouse
Renal tissue from humans was obtained from the archives of the Department of Cellular Pathology, John Radcliffe Hospital, Oxford, UK. Paraffin-embedded specimens of normal kidneys from nephrectomies performed for cancer treatment, and renal biopsy samples from adults with diabetic nephropathy, were included in this study. Ethics approval was obtained from the Oxford Research Ethics Committee B (reference no. C02.062) for the use of human tissue. All patients gave written consent at hospital admission to experiments on their anonymized archived tissue samples. For immunohistochemistry on mice, paraffin-embedded kidney cortex sections of healthy C57BL/6J animals and diabetic B6.BKS(D)-Leprdb/J mice from the Jackson Laboratory were used. After a standard deparaffinization procedure, the slides were microwaved in citric acid monohydrate buffer (pH 6.0) and incubated with polyclonal rabbit anti-KMO antibody (10698-1-AP, Proteintech, Chicago, IL) or monoclonal mouse anti-KMO antibody (60029-1-Ig) at a 1:50 dilution in 1x PBS overnight at 4°C. For double-staining, goat anti-PODXL (1:500) or mouse anti-synaptopodin (undiluted tissue culture supernatant) antibody was used. Secondary antibodies were Alexa-fluor-labeled donkey–anti-rabbit 488, donkey–anti-goat 488, or donkey–anti-mouse 555. Slides were examined using a Leica DMLB fluorescence microscope; images were taken using a Leica DFC425 camera (both Leica Microsystems, Buffalo Grove, IL). The images were processed with Adobe Photoshop to merge the fluorescence channels.
Zebrafish Stocks and Injections
Zebrafish (AB) were grown and mated at 28.5°C, and embryos were kept and handled in standard E3 solution as described.14 Morpholinos were injected into fertilized eggs between the one- and four-cell stage using a Nanoject II injection device (Drummond Scientific, Broomall, PA). The following morpholinos were designed and ordered from GeneTools (Philomath, OR): control, 5′-CCTCTTACCTCAGTTACAATTTATA-3′, kmo, 5′-GATGTGAGAAAGCTGTCTCCATGTT-3′ as ATG-blocker, and kmo, 5′-ATGGATTATGTATTAACCTTCCCGA -3′ as splice donor morpholino to skip exon 2. Morpholino injections were carried out with concentrations ranging from 50 to 250 μM, with an injection volume of 4.6 nl in injection buffer (100 mM potassium chloride, 0.1% phenol red). Embryos were monitored for the development of phenotype until 120 hpf. The phenotype was scored from 1 to 4, relative to the amount of edema present.
Zebrafish Proteinuria Assay
At 50–55 hours after ATG-blocker morpholino injection, remaining chorions were manually removed from all embryos. Cardinal vein injections were performed as initially described.14 Briefly, 4.6 μl of FITC-labeled 70-kD dextran (Molecular Probes, Eugene, OR) was injected into the cardiac venous sinus. For this injection, zebrafish were anesthetized with a 1:20–1:100 dilution of 4 mg/ml Tricaine (MESAB: ethyl-m-aminobenzoate methanesulfonate, 20mM Tris, pH 7.0; Sigma-Aldrich, St. Louis, MO) and positioned on their backs in a 1.5% agarose injection mold. After the injection, fish were returned to egg water, where they quickly regained motility. Splice donor was injected in Tg(l-fabp:DBP:EGFP) fish larvae. These animal protocols were approved by the Mount Desert Island Biological Laboratory Animal Care Committee. For a detailed review of the methods, see Hanke et al.15 For eye assay measurements, zebrafish larvae were transferred into individual wells of a 96-well plate (Thermo Fisher Scientific, Vernon Hills, IL). Fish were anesthetized with Tricaine and sequential images of live fish were generated using a Zeiss inverted microscope (Axiovert 200) connected to an AxioCam MRm charge-coupled device camera, and images were taken with fixed exposure times and gain using the Axio Vision release 4.5 SP1 software package. The maximum fluorescence intensities of images of the pupils of the fish were measured using the National Institutes of Health ImageJ application, and are reported in relative units of brightness.
Mice and Sample Collection
C57BL/6N-Kmotm1a(KOMP)Wtsi embryos were obtained from the KOMP Repository (University of California, Davis) and transferred to CByB6F1/J females (JAX #100009). Pups were designated C57BL/6N-Kmotm1a(KOMP)Wtsi/RkorJ (JAX #18939) according to standard nomenclature. Mice were housed in a climate-controlled facility with a 12-hour light/dark cycle and provided free access to food and water throughout the experiment. After weaning, mice were maintained on a chow diet (LabDiet 5K52, PMI Nutritional International, Bentwood, MO). Spot urine was collected in the mornings. Albumin and creatinine concentrations were measured on a Beckman AU680 Chemistry Analyzer. Actual mouse albumin concentrations were calculated by linear regression from a standard curve generated with mouse albumin standards (Kamiya Biomedical Company, Seattle, WA). The left kidney was flash frozen in liquid nitrogen and stored at −80°C, whereas the right kidney was fixed in Bouin fixative followed by embedding in paraffin. All experiments were approved by The Jackson Laboratory’s Animal Care and Use Committee.
Phenotyping of Mice
Paraffin-embedded kidneys were sectioned at 3 μm and stained with periodic acid–Schiff. For TEM, tissues were fixed overnight in 2% paraformaldehyde and 2% glutaraldehyde. Sections (90-nm thick) were stained with 2% uranyl acetate and Reynolds lead and viewed with the JEOL 1230 Transmission Electron Microscope.
For mass spectrometry of each 50 μl plasma sample, proteins were removed and the sample was resuspended in 100 μl of 50:50 H2O/acetonitrile. Samples were then analyzed on an Agilent 6530 Quadrupole Time-of-Flight mass spectrometer.
Real-Time PCR in Mice and Zebrafish
RNA was isolated from kidney samples using the Trizol method. For real-time PCR, samples were diluted and 2 μg were used for cDNA synthesis using the QuantiTect RT kit (Qiagen, Germantown, MD). Renal mRNA levels for Kmo were determined using a primer set designed by Primerdesign Ltd (Southampton, UK) (forward: 5′-CCTCTACCTTTATCCCTCTCTATAC-3′, reverse: 5′-GAGT CCTCTGTTTATCACCTTTTT-3′). Real-time PCR was performed using the Applied Biosystems ViiA 7 Real-Time PCR System (Life Technologies, Carlsbad, CA) with SYBR green. mRNA levels were expressed relative to those of the β-actin gene (Bact) (forward: 5′-GCTTCTTTGC AGCTCCTTCG-3′, reverse: 5′-CCCACGATGGAGGGGAATAC-3′). Knockdown of zebrafish kmo by splice donor morpholino was confirmed using specific zebrafish primer sets (kmo-forward-e2: TGCTTTCTTGCCAAACGAGGAT, kmo-reverse-e2: ACTTTAGCCTGCCGAATA TCTTCCC) and tbp-forward: CGGTGGATCCTGCGAATTA; tbp-reverse: TGACAGGTTATG AAGCAAAACAACA as housekeeper message.16
Disclosures
None.
Supplementary Material
Acknowledgments
This work was funded by grant no. GM076468 from the National Institute of General Medical Sciences (to R.K.), and AG038070 from the National Institute on Aging (to R.K.), and the National Cancer Institute Cancer Core grant (CA034196) to The Jackson Laboratory, as well as by an Institutional Development Award from the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers P20GM104318 and P20GM103423 to Mount Desert Island Biological Laboratory and the German Research Council grants SCHI587/3 and 4, and rare disease consortium FSGS grant 01GM1518D funded by Bundesministerium für Bildung und Forschung (to M.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015070835/-/DCSupplemental.
References
- 1.Pawlak D, Pawlak K, Malyszko J, Mysliwiec M, Buczko W: Accumulation of toxic products degradation of kynurenine in hemodialyzed patients. Int Urol Nephrol 33: 399–404, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Pawlak D, Tankiewicz A, Buczko W: Kynurenine and its metabolites in the rat with experimental renal insufficiency. J Physiol Pharmacol 52: 755–766, 2001 [PubMed] [Google Scholar]
- 3.Pawlak D, Tankiewicz A, Matys T, Buczko W: Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J Physiol Pharmacol 54: 175–189, 2003 [PubMed] [Google Scholar]
- 4.Yoshimura H, Sakai T, Kuwahara Y, Ito M, Tsuritani K, Hirasawa Y, Nagamatsu T: Effects of kynurenine metabolites on mesangial cell proliferation and gene expression. Exp Mol Pathol 87: 70–75, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Dobrovolsky VN, Bucci T, Heflich RH, Desjardins J, Richardson FC: Mice deficient for cytosolic thymidine kinase gene develop fatal kidney disease. Mol Genet Metab 78: 1–10, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Dobrovolsky VN, Bowyer JF, Pabarcus MK, Heflich RH, Williams LD, Doerge DR, Arvidsson B, Bergquist J, Casida JE: Effect of arylformamidase (kynurenine formamidase) gene inactivation in mice on enzymatic activity, kynurenine pathway metabolites and phenotype. Biochim Biophys Acta 1724: 163–172, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Korstanje R, Thaisz J, Staedtler F, Harttman N, Xu L, Feng M, Yanas L, Yang H, Valdar W, Churchill GA, DiPetrillo K: Genome-Wide Association Mapping of Quantitative Traits in Outbred Mice. G3: Genes|Genomes|Genetics 2: 167–174, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK: Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity 1: 219–229, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Maddison DC, Giorgini F: The kynurenine pathway and neurodegenerative disease. Semin Cell Dev Biol 40: 134–141, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Harkcom WT, Ghosh AK, Sung MS, Matov A, Brown KD, Giannakakou P, Jaffrey SR: NAD+ and SIRT3 control microtubule dynamics and reduce susceptibility to antimicrotubule agents. Proc Natl Acad Sci U S A 111: E2443–E2452, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang J, Cui J, Gong H, Xi C, Zhang T-M: Effect of NAD on PARP-mediated insulin sensitivity in oleic acid treated hepatocytes. J Cell Physiol 230: 1607–1613, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, Tavaré JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJM: Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 12: 329–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cellesi F, Li M, Rastaldi MP: Podocyte injury and repair mechanisms. Curr Opin Nephrol Hypertens 24: 239–244, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, Bonventre JV, Haller H, Schiffer M: Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol 293: F1746–F1750, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Hanke N, Staggs L, Schroder P, Litteral J, Fleig S, Kaufeld J, Pauli C, Haller H, Schiffer M: “Zebrafishing” for novel genes relevant to the glomerular filtration barrier. BioMed Res Int 2013: 658270,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blum N, Begemann G: Retinoic acid signaling spatially restricts osteoblasts and controls ray-interray organization during zebrafish fin regeneration. Development 142: 2888–2893, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.