Abstract
Despite advances in immunosuppression, antibody-mediated rejection is a serious threat to allograft survival. Alloreactive memory helper T cells can induce potent alloantibody responses and often associate with poor graft outcome. Nevertheless, the ability of memory T cells to elicit well characterized manifestations of antibody-mediated rejection has not been tested. We investigated helper functions of memory CD4 T cells in a mouse model of renal transplantation. Whereas the majority of unsensitized C57Bl/6 recipients spontaneously accepted fully MHC–mismatched A/J renal allografts, recipients containing donor–reactive memory CD4 T cells rapidly lost allograft function. Increased serum creatinine levels, high serum titers of donor-specific alloantibody, minimal T cell infiltration, and intense C4d deposition in the grafts of sensitized recipients fulfilled all diagnostic criteria for acute renal antibody–mediated rejection in humans. IFNγ neutralization did not prevent the renal allograft rejection induced by memory helper T cells, and CD8 T cell depletion at the time of transplantation or depletion of both CD4 and CD8 T cells also did not prevent the renal allograft rejection induced by memory helper T cells starting at day 4 after transplantation. However, B cell depletion inhibited alloantibody generation and significantly extended allograft survival, indicating that donor-specific alloantibodies (not T cells) were the critical effector mechanism of renal allograft rejection induced by memory CD4 T cells. Our studies provide direct evidence that recipient T cell sensitization may result in antibody-mediated rejection of renal allografts and introduce a physiologically relevant animal model with which to investigate mechanisms of antibody-mediated rejection and novel therapeutic approaches for its prevention and treatment.
Keywords: transplantation, immunology, lymphocytes
Acute antibody–mediated rejection (AMR) and chronic AMR are among the leading causes of renal allograft loss.1–4 Whereas routine alloantibody screening, paired kidney exchange programs, and aggressive desensitization protocols diminish the risk of preexisting donor–specific alloantibody (DSA), de novo DSA development is hard to predict and often results in worse transplant outcome compared with preexisting alloantibodies.5,6 However, the cellular and molecular mechanisms of de novo alloantibody generation remain poorly understood, limiting therapeutic options for the prevention and treatment of AMR.
Production of pathogenic DSA requires interactions between antibody–secreting B cells and helper CD4 T cells specific for the same set of donor antigens.7 Although primary helper T cell responses are efficiently controlled by current immunosuppressive regimens, human transplant recipients contain memory CD4 T cells that may be alloreactive as a result of previous alloantigen exposure or heterologous immunity. Because of their enhanced survival, activation, and trafficking properties, memory T cells can precipitate allograft rejection, despite immunosuppression or conventional costimulatory blockade.8,9 Multiple clinical studies have shown the correlation between the presence of memory T cells before transplantation and poor transplant outcome.10–12 However, the mechanistic links between preexisting helper memory T cells, de novo DSA generation, and AMR of renal allografts have not been previously investigated.
We have previously reported that, compared with newly generated effector T cells, donor–specific memory CD4 T cells provide help for pathogenic alloantibody production in a mouse model of cardiac allograft rejection.13–16 However, the rejection in this model occurs primarily through cellular mechanisms, thus impeding investigation of alloantibody induction and the role in tissue injury. Furthermore, the diagnostic criteria are best developed for AMR of kidney transplants. The goal of this study was to test the effect of memory helper T cells after renal transplantation.
Results
We used a mouse model in which recipients are transplanted with a single kidney allograft and the remaining native kidney is removed. In contrast to heterotopic heart transplantation, recipient survival depends on renal graft function, which can be monitored by serum creatinine levels like in clinical transplantation. Consistent with previously published data, the majority of B6 (H-2b) recipients spontaneously accept fully MHC–mismatched A/J (H-2a) kidney allografts for >60 days and develop low–level cellular and humoral immune responses against donor antigens.17 Recipients sensitized with A/J skin allografts 4–6 weeks before renal transplantation rapidly reject A/J kidney allografts (MST of 6 days versus >60 days in nonsensitized recipients). However, the contributions of cellular versus humoral alloimmunity to renal tissue injury are hard to discern in these settings, because skin allograft rejection generates memory CD4 and CD8 T cells, memory B cells, high–affinity DSA–secreting plasma cells, and circulating DSA.18
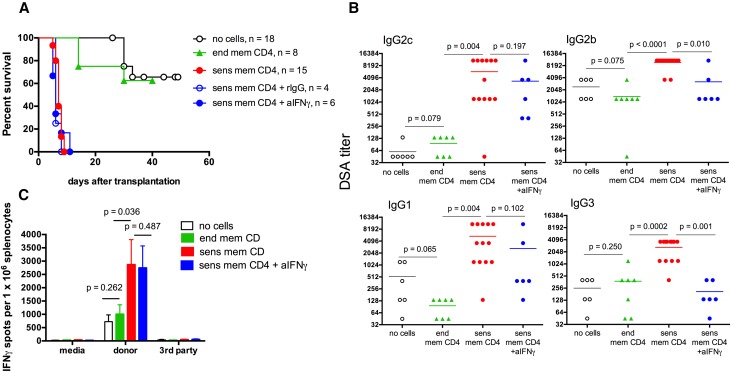
To specifically focus on the role of donor–reactive memory CD4 T cells in de novo DSA responses, we isolated CD4+ T cells from A/J skin–sensitized B6 recipients or naïve B6 mice and injected 5×106 CD4+CD44hi cells into naïve B6 mice followed by A/J renal transplantation; 60% of control recipients injected with nonsensitized (endogenous) memory CD4 T cells maintained renal allograft function for >50 days, with serum creatinine levels of 0.4±0.1 mg/dl (versus <0.4 mg/dl in nontransplanted mice). In contrast, all recipients with A/J–sensitized memory CD4 T cells had a rapid rise of serum creatinine >1 mg/dl (1.7±0.6 mg/dl by 6–8 days post-transplant) and had to be euthanized because of renal transplant failure with MST of 7 days (Figure 1A). At rejection, these recipients had high serum titers of A/J–reactive IgG DSA and increased frequencies of donor–reactive T cells producing IFNγ compared with the controls euthanized at matching time points (Figure 1, B and C). To test the potential role of IFNγ during allograft rejection, we treated recipients with sensitized memory CD4 T cells with mouse IFNγ neutralizing mAb throughout the duration of the experiment. The efficacy of IFNγ neutralization in vivo was confirmed by a significant decrease in the intragraft expression of IFNγ–dependent chemokine CXCL919 (Supplemental Figure 1A). As anticipated, anti–IFNγ mAb treatment did not affect the ability of donor spleen T cells to secrete IFNγ after in vitro restimulation (Figure 1C). Consistent with the well established role of IFNγ in antibody isotype switching,20,21 blocking IFNγ significantly decreased serum titers of IgG2b and IgG3 DSAs, whereas high levels of IgG2c and IgG1 DSAs were still produced (Figure 1B). Most importantly, IFNγ neutralization did not alter the kinetics of renal allograft rejection (MST of 6 days; serum creatinine of 1.9±0.8 mg/dl at the time of euthanasia) compared with those in control rat IgG–treated or –untreated recipients injected with donor–sensitized memory CD4 T cells (Figure 1A).
Figure 1.
Donor–reactive memory CD4 T cells induce rapid rejection of kidney allografts. B6 mice were injected with 5×106 CD4+CD44hi cells isolated from either A/J skin–sensitized B6 mice (sensitized memory CD4 T cells) or naïve B6 mice (endogenous memory CD4 T cells) and transplanted with A/J renal allografts 2 days later. Groups of recipients injected with sensitized memory CD4 T cells were treated with either anti– IFNγ neutralizing mAb XMG1.2 or control rat IgG (0.5 mg intraperitoneally every other day starting at the time of transplantation). Control B6 mice were transplanted with A/J kidney allografts without CD4 T cell injection (no cells group). (A) Renal allograft survival. *P<0.001 versus recipients injected with endogenous memory CD4 T cells. (B) Serum DSA titers and (C) the frequencies of donor–reactive IFNγ–secreting spleen T cells determined on day 7 post-transplant. Antidonor immune responses in recipients injected with sensitized memory CD4 T cells and treated with control rat IgG were similar to those in untreated recipients containing sensitized memory CD4 T cells (not shown). Graphs represent (A and B) individual animals or (C) means±SDs for 6–13 animals per group. aIFNγ, anti-IFNg antibody; end mem, endogenous memory; rIgG, rat IgG; sens mem, sensitized memory.
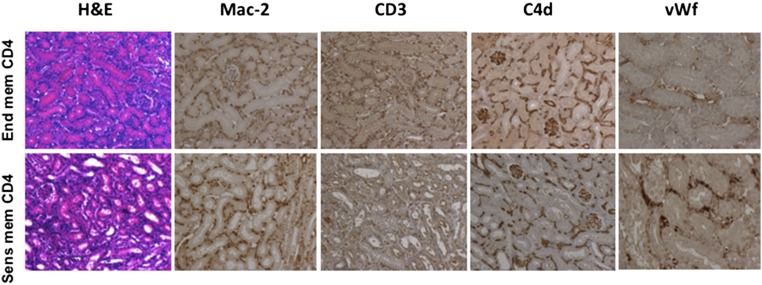
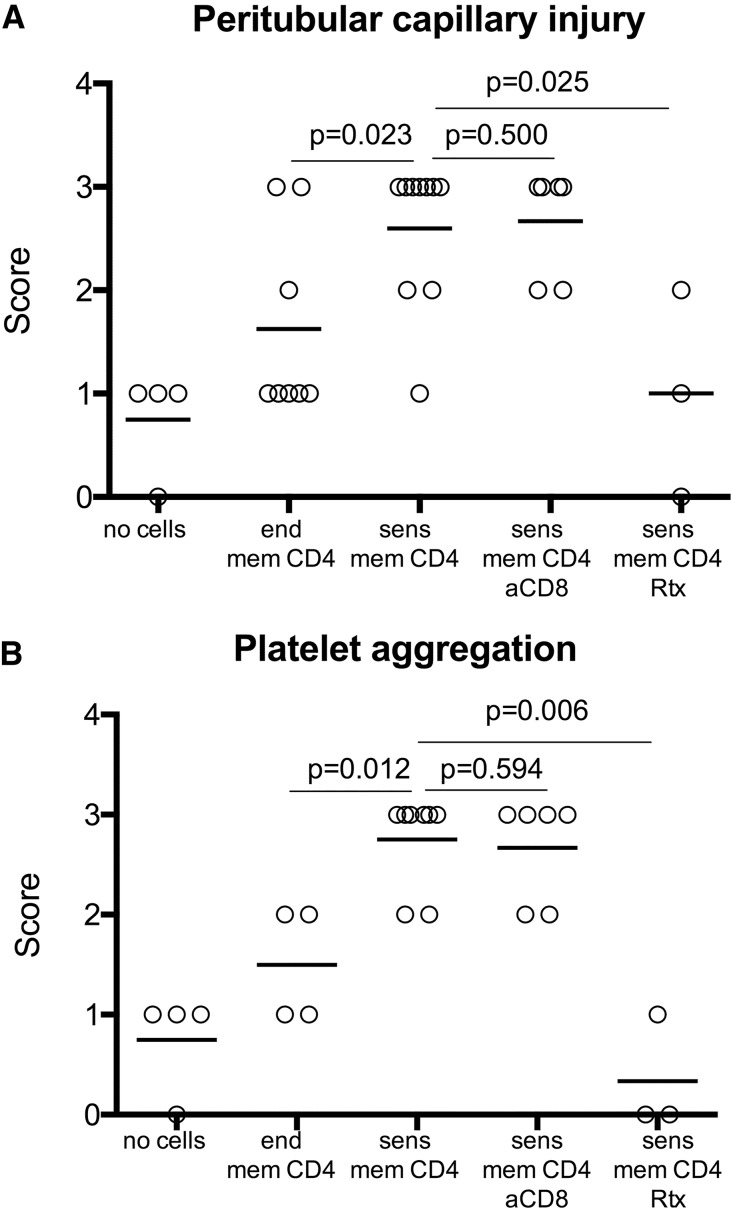
Although injection of sensitized memory CD4 T cells increased priming of donor–reactive T cells in the spleen compared with transfer of endogenous memory CD4 T cells, cellular infiltrates into rejecting allografts consisted mainly of macrophages with few CD3+ cells (Figure 2). Recipients containing either donor–sensitized or endogenous memory CD4 T cells had diffuse deposition of C4d in peritubular and glomerular capillaries. However, vascular changes commonly associated with antibody–mediated tissue injury, such as peritubular capillary dilation, endothelial cell swelling, and margination of monocytes,1,22,23 were significantly more prominent in renal allografts rejected by recipients containing sensitized donor–reactive memory CD4 T cells (Figure 3A). Staining for vWf revealed recruitment and aggregation of platelets within graft capillaries of rejecting but not control allografts (Figures 2 and 3B). Histologic findings in the grafts from anti–IFNγ mAb–treated recipients at the time of rejection were similar to those observed in rat IgG–treated or –untreated recipients (Supplemental Figure 1B). Thus, adoptive transfer of donor–reactive memory CD4 T cells caused kidney graft loss consistent with all diagnostic criteria for AMR3: (1) increased serum creatinine levels, (2) high serum DSA titers, (3) histologic evidence of acute tissue injury without prominent T cell infiltration, and (4) C4d deposition and evidence of vascular injury in the graft capillaries.
Figure 2.
Renal allografts in recipients containing donor-sensitized memory CD4 T cells have characteristic features of antibody-mediated rejection. Renal allografts were harvested at 7 days post-transplant and analyzed by hematoxylin and eosin staining and immunoperoxidase stains for Mac-2, CD3, C4d, and vWf. The photographs are representative of six to eight animals in each group. end mem, Endogenous memory; sens mem, sensitized memory. Original magnification, ×200 in hematoxylin and eosin, Mac-2, CD3, and C4d; ×400 in vWf.
Figure 3.
Alloantibody induced by donor-reactive memory CD4 T cells facilitate vascular injury and platelet aggregation within renal allografts. Renal allografts were harvested at indicated time points after transplantation. Immunohistology for C4d and vWf was scored in a blinded fashion as outlined in Concise Methods. AMR was assessed on evidence of (A) vascular injury and (B) platelet aggregation. aCD8, anti-CD8 antibody; end mem, endogenous memory; sens mem, sensitized memory.
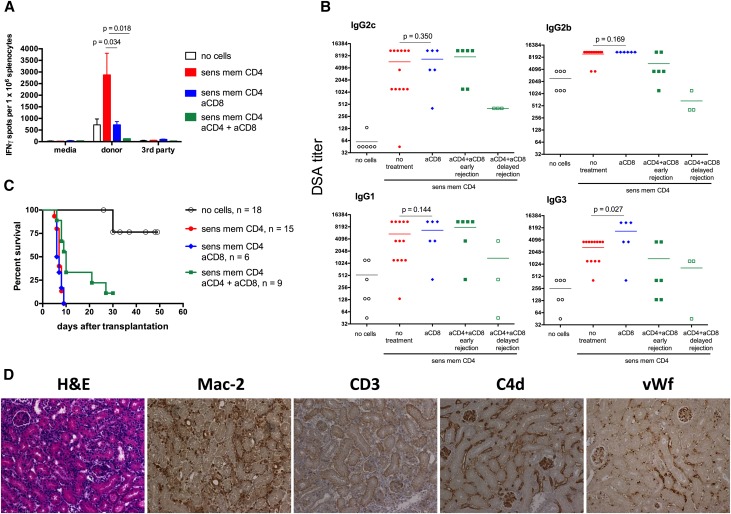
To investigate the potential role for CD8 effector T cells during allograft rejection, recipients containing memory CD4 T cells were injected with anti–CD8–depleting mAb before transplantation. CD8 T cell depletion significantly reduced the frequencies of donor–reactive T cells secreting IFNγ (Figure 4A) and slightly but significantly increased serum titers of donor-reactive IgG3 but did not alter IgG2c, IgG2b, and IgG1 DSA serum titers (Figure 4B) and graft rejection kinetics (Figure 4C) (MST of 7 days; serum creatinine levels at euthanasia of 1.3±0.2 mg/dl; n=6). Histologic appearance of rejecting allografts was similar to that observed in CD8 cell–sufficient B6 recipients injected with memory CD4 T cells, including an intense macrophage infiltrate, few CD3+ cells, diffuse C4D deposition, prominent capillary injury, and large quantities of aggregated platelets (Figure 4D).
Figure 4.
Effector T cell depletion does not prevent renal allograft rejection induced by donor–reactive memory CD4 T cells. B6 mice injected with A/J–reactive memory CD4 T cells were treated with a cocktail of anti–mouse CD8 depletion mAb before transplantation, treated with anti–mouse CD8 and anti–mouse CD4 depletion mAbs starting on day 4 post-transplant, or left untreated. The control group received no memory CD4 T cells (no cells group). (A) The frequencies of donor–reactive IFNγ–secreting spleen T cells on day 7 post-transplant. Graphs represent means±SDs for 7–12 animals per group. (B) Serum DSA titers determined on day 14 post-transplant for the aCD4 + aCD8 delayed rejection group (open green squares) and day 7 post-transplant for all remaining groups. Graphs represent individual animals. (C) Renal allograft survival. (D) Renal allografts were harvested from CD8 T cell–depleted recipients containing memory CD4 T cells at the time of rejection (days 6–9) and analyzed by hematoxylin and eosin staining and immunohistochemistry. The photographs are representative of at least six grafts per group. Endogenous memory; sens mem, sensitized memory. Original magnification, ×200. aCD4, anti-CD4 antibody; aCD8, anti-CD8 antibody.
To entirely rule out the role of effector T cells during renal allograft rejection in our model, recipients injected with memory CD4 T cells were treated with anti–CD8– and anti–CD4–depleting mAbs starting on day 4 after transplantation. We reasoned that this depletion regimen would allow transferred memory helper T cells to induce DSA. Indeed, six of nine recipients in this group rapidly developed high DSA titers and promptly rejected renal allografts (serum creatinine of 1.4±0.8 mg/dl) (Figure 4). However, 3 days before T cell depletion were not sufficient for donor–sensitized memory CD4 T cells to uniformly induce high DSA titers in all recipients. Two of three remaining recipients had intermediate DSA titers at day 14 post-transplant and rejected A/J renal allografts in a delayed fashion (serum creatinine >1 mg/dl at days 21 and 27 post-transplant), and one recipient had low DSA titers at day 14 and maintained serum creatinine of 0.4 mg/dl at 30 days post-transplant (Figure 4, B and C). Most importantly, all recipients in this group showed vast depletion of peripheral CD4 and CD8 T cells (Supplemental Figure 2A), extremely low levels of donor–specific T cell priming in the spleen compared with those in nondepleted recipients (Figure 4A), and minimal infiltration of CD3+ T cells within the graft at the time of rejection (Supplemental Figure 2B). Thus, in the absence of effector T cells, the magnitude of antidonor humoral responses inversely correlated with the renal allograft survival, further confirming the predominant role of DSA in the rejection process.
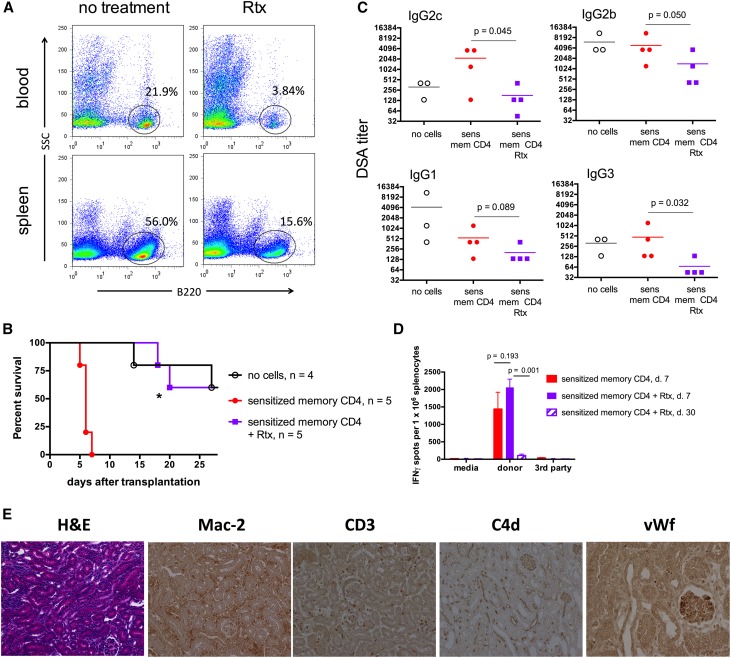
To test the role of B cells and antibody, we used B6 recipients expressing human CD20 under the B220 promoter (B6.huCD20). The injection of the anti–human CD20 mAb rituximab (Rtx) depleted >80% of circulating B cells and >70% of spleen B cells in these mice (Figure 5A), as previously published.24–26 Rtx treatment of hCD20tg recipients containing donor–reactive memory CD4 T cells efficiently inhibited DSA induction and markedly extended renal allograft survival (Figure 5, B and C). Notably, the numbers of donor–reactive IFNγ T cells at day 7 post-transplant were comparable between groups with or without Rtx treatment (Figure 5D), further indicating that DSAs and not T lymphocytes were the primary cause of early allograft rejection observed in control untreated recipients. The early T cell alloresponses gradually waned, because significantly lower numbers of donor–reactive IFNγ–producing spleen cells were detected in Rtx-treated recipients at day 30 than at day 7 post-transplant (Figure 5D). Surviving renal allografts analyzed at 30 days post-transplant had decreased mononuclear cell infiltration compared with acutely rejecting allografts. Consistent with decreased serum DSA titers, these recipients revealed moderate C4d deposition in graft capillaries and significantly reduced peritubular capillary injury and platelet aggregation (Figures 3 and 5E).
Figure 5.
Renal allograft rejection induced by memory CD4 T cells is prevented by B cell depletion. (A) B6.huCD20 mice were either injected with Rtx (0.5 mg intraperitoneally on 2 consecutive days) or left untreated. Percentages and numbers of B220+ cells were determined in peripheral blood and spleen by flow cytometry 48 hours after last Rtx injection. (B) Renal allograft survival. B6.huCD20 mice were injected with donor–reactive memory CD4 T cells, transplanted with A/J renal allografts, and either treated with Rtx (0.5 mg intraperitoneally on days −1 and 0 and every 5 days throughout the duration of the experiment) or left untreated. Control B6.huCD20 mice received A/J kidney transplants without memory CD4 T cell transfer (white circles). *P=0.003 for comparison between Rtx-treated and untreated recipients injected with memory CD4 T cells. (C) Serum DSA titers at rejection or on day 30 post-transplant if rejection did not occur earlier. (D) The frequencies of donor–reactive IFNγ–secreting spleen cells determined on day 7 or 30 post-transplant (n=3–5 animals per group). (E) Histologic analysis of renal allografts harvested from Rtx–treated B6.huCD20 recipients containing memory CD4 T cells at day 30 post-transplant. The photographs are representative of three animals per group. sens mem, Sensitized memory. Original magnification, ×200 in hematoxylin and eosin, Mac-2, CD3, and C4d; ×400 in vWf.
Discussion
To our knowledge, this is the first report of an AMR model in which pathogenic DSAs are generated by wild-type mice in the course of a physiologic immune response to a renal allograft. Most current information on AMR causes and mechanisms was gained in animal models using adoptive DSA transfer into immunodeficient recipients or genetically modified recipients with dysregulated immunity.27 Using complementary approaches, we have definitively shown that alloantibodies and not effector T cells are the primary cause of renal allograft failure in recipients containing donor–reactive memory helper T cells. Whereas targeting effector T cell responses had minimal effects on DSA production, allograft survival, and histology (Figures 1 and 4), B cell depletion decreased serum DSA titers and significantly prolonged allograft survival (Figure 5). We recently showed that Rtx treatment prolongs allograft survival in B6.huCD20.CCR5−/− recipients, which typically reject renal allografts with high serum DSA titers and marked C4d deposition in grafts.24 The results in our new mouse renal AMR model are in accord with this previous report. Furthermore, the use of recipients containing donor–reactive memory CD4 T cells provides a unique opportunity to approximate the clinical situation of de novo alloantibody development and investigate AMR mechanisms as well as therapies targeting B cells and antibody–mediated graft injury. Notably, the findings in this report are distinct from a role of memory helper T cells in murine cardiac allograft recipients, in which effector CD8 T cells mediate rejection before high–titer DSA generation.13,15 These results imply that the effector functions of donor–reactive T cells are determined by allograft type and that the results in heterotopic cardiac transplant models may not always translate directly to other solid–organ transplants.
As anticipated, de novo DSA generation in T cell–sensitized recipients corresponded with diffuse C4d deposition in the graft (Figure 2). However, control recipients with prolonged allograft survival had similar intensity of C4d staining, indicating that C4d deposition is not a conclusive marker of acute AMR. The correlation of serum DSA and AMR with C4d staining intensity and pattern is a matter of ongoing debate.28,29 Regardless of C4d deposition, we found that vascular changes commonly associated with antibody–mediated tissue injury, such as peritubular capillary dilation, endothelial cell swelling, and margination of monocytes,1,22,23 were significantly more prominent in renal allografts rejected by recipients containing sensitized but not endogenous memory CD4 T cells. In addition, our histologic findings raise the possibility that platelet accumulation and activation are critically determined by the kinetics and magnitude of DSA responses and implicate platelets as important mediators of graft tissue injury. Studies investigating the contribution of activated platelets and platelet-derived factors during acute AMR are currently ongoing in our group.
Previous studies of spontaneously accepted mouse renal allografts reported an important role for immune regulation.30,31 Consistent with these findings, control recipients containing endogenous memory CD4 T cells or those that received no T cell transfer had interstitial infiltrates of FoxP3+ T cells (data not shown). In contrast, allografts from T cell–sensitized recipients had minimal numbers of FoxP3+ cells, raising a possibility that pathogenic alloantibodies impede the migration and/or persistence of Tregs in the graft. These effects of DSA may not even be limited to Tregs, because very few graft–infiltrating T cells were detected in sensitized recipients, despite efficient T cell activation in the spleen.
Our results strongly suggest a mechanistic link between two of the most important problems in clinical transplantation: preexisting donor–reactive memory T cells and AMR. Although the recipient DSA sensitization status is usually known, there is no routine screening for donor–specific memory T cells before transplantation. Studies of the past two decades convincingly show that many PRA-negative individuals have alloreactive memory T cells.10–12 For the first time, we provide evidence that donor–reactive memory helper T cells can be potent inducers of de novo pathogenic DSAs and acute AMR in renal allograft recipients. Our novel physiologically relevant animal model of renal AMR will allow us to further investigate mechanisms underlying antibody–mediated graft tissue injury.
Concise Methods
Animals
Male C57BL/6J (B6, H-2b), A/J (H-2a), and SJL/J-Pde6brd1 (SJL, H-2s) mice ages 6–8 weeks old were purchased from The Jackson Laboratory (Bar Harbor, ME). Human CD20 transgenic mice (H-2b [B6.hCD20]) were provided by Genentech (South San Francisco, CA). All animal procedures were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Kidney Transplantation and Graft Evaluation
Murine kidney transplantation was performed as previously described.32 Briefly, the kidney with vascular supply and ureter were harvested en bloc, and the donor artery and vein were anastomosed to the recipient abdominal aorta and inferior vena cava. Urinary reconstruction was performed as described in the work by Han et al.33 The remaining native kidney was removed at the time of transplant, so that recipient survival was dependent on the kidney graft. Kidney graft survival was assessed by daily examination of overall animal health and measurement of serum creatinine levels using the VetScan i-STAT1 Analyzer (Abaxis, Union City, CA). Graft rejection was diagnosed when the mouse showed signs of illness and serum creatinine level was elevated to 0.8 mg/dl (<0.4 mg/dl in nontransplanted mice), at which time the recipient was euthanized. Grafts were harvested at the time of rejection, embedded in paraffin, and stained with hematoxylin and eosin and the following antibodies: rat anti–mouse Mac-2 (Cedarlane Laboratories, Burlington, NC), polyclonal rabbit anti–mouse CD3 (Abcam, Inc., Cambridge, MA), anti-mouse C4d,34 and rabbit polyclonal anti–human vWf (DAKO, Carpinteria, CA). Immunohistology for C4d and vWf was scored in a blinded fashion. Each stain was evaluated on two to three complete cross-sections. AMR was assessed on evidence of vascular injury. Each graft was scored on a scale of one to three as illustrated in Supplemental Figure 3 using clinically relevant criteria that included peritubular capillary dilation with endothelial cell swelling and margination of monocytes.1,22,23 Platelet aggregation was scored on a scale of one to three as illustrated in Supplemental Figure 4.
Recipient Treatment
Recipient CD8 T cells were depleted using a cocktail of monoclonal anti–mouse CD8 antibodies (clones YTS169 and TIB105; Bio X Cell), with 0.2 mg each antibody injected intraperitoneally on days −3, −2, and −1 before transplantation and then, every 5 days throughout the duration of the experiment. The efficiency of CD8 T cell depletion (>98% compared with that in untreated mice) was confirmed by flow cytometry at the time of euthanasia. For delayed combined CD4 and CD8 T cell depletion, recipients were treated with anti–CD8 mAbs YTS169 and TIB105 on days 4–6 post-transplant and anti-CD4 mAb (clones GK1.5 and YTS191; 0.2 mg each intraperitoneally; Bio X Cell) on days 5–7 post-transplant. The efficacy of CD4 and CD8 T cell depletion was determined by flow cytometry of spleen cells at the time of euthanasia. For B cell depletion, B6.huCD20 mice were injected intraperitoneally with 0.5 mg anti–human CD20 mAb (Rtx; Biogen Idec Inc. and Genentech) on days 0 and 1 and then, every 5 days throughout the experiment. To neutralize IFNγ, recipients were treated with anti–mouse IFNγ mAb XMG1.2 (0.5 mg intraperitoneally; Bio X Cell) on days 0, 2, 4, 6, and 8 post-transplant.
Generation of Alloreactive Memory CD4 T Cells
To generate polyclonal alloreactive memory CD4 T cells, A/J skin allografts were placed onto B6 recipients. Six weeks after rejection, recipient spleen CD4+ cells were isolated using the EasySep Mouse CD4+ T Cell Isolation Kit (STEMCELL Technologies, Vancouver, BC, Canada). As a control, CD4+ T cells were isolated from naïve B6 mice. Regardless of donor sensitization status, 10%–15% of isolated CD4+ T cells were CD44hi. The equivalent of 5×106 sensitized or endogenous CD4+CD44hi cells was intravenously injected into naïve B6 mice. Adoptive cell transfers were performed 2 days before renal allograft transplantation.
Measurement of Serum Alloantibody Titers
Donor– and third party–reactive IgG1, IgG2c, IgG2b, and IgG3 antibody titers in recipient serum were determined by a flow cytometry–based assay as previously published.18 Donor A/J and third party SJL thymocytes were isolated, and 1×106 cell aliquots were incubated with 100 μl serially diluted recipient serum. FITC–conjugated goat anti–mouse IgG, phycoerythrin–conjugated anti–mouse IgG1, FITC–conjugated anti–mouse IgG2a (with reactivity to IgG2c), FITC–conjugated anti–mouse IgG3, and biotinylated anti–mouse IgG2b followed by phycoerythrin–streptavidin conjugate were used as detecting antibodies at 1:50–1:100 dilutions (BD Pharmingen). Cells were washed, fixed in 1% paraformaldehyde, and analyzed by flow cytometry. For every sample and every IgG isotype, the mean fluorescence intensity of each dilution was determined. The dilution that returned the mean fluorescence intensity to the level observed when thymocytes were stained with a 1:90 dilution of naïve B6 serum was divided by two and reported as the titer. The titers of third party SJL–reactive alloantibody were <135 for all IgG isotypes in all tested samples.
ELISPOT Assay
Assays were performed as previously described using capture and detecting anti–mouse IFNγ antibody from BD Pharmingen.13,15,16 Recipient spleen cells were stimulated with mitomycin C–treated donor A/J or third party SJL spleen cells for 24 hours. The resulting spots were analyzed using an ImmunoSpot Series 4 Analyzer (Cellular Technology, Cleveland, OH).
RNA Isolation and Quantitative Real–Time PCR Analyses
Portions of kidney allografts were frozen in liquid nitrogen and stored at −80°C. Total RNA was isolated from individual samples using TriZol Reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), and quantitative real–time PCR was done on a 7500 Fast Real-Time PCR System instrument using Taqman Fast Universal PCR Master Mix (2×; Applied Biosystems) and No AmpEraseUNG (Applied Biosystems). Probes and primers were from Taqman gene expression assay reagents (Applied Biosystems): CXCL9 (Mm00434946_m1) and β-actin (Mm02619580_g1). Data were normalized to Mrpl32 (Mm00777741-sH) RNA amplification and calculated relative to the expression of the target gene in kidneys harvested from naïve B6 mice.
Flow Cytometry
Fluorochrome-conjugated antibodies were purchased from BD Biosciences (San Jose, CA) or eBioscience (San Diego, CA). Cells were isolated from peripheral blood and spleen and stained as previously described35; ≥200,000 events per sample were acquired on a BD Biosciences LSRII followed by data analysis using FlowJo software (TreeStar, Inc., Ashland, OR).
Statistical Analyses
Renal allograft survival was compared between groups by Kaplan–Meier analysis. Differences between groups during recall immune responses were analyzed using a nonparametric equivalent of one-way ANOVA: the Kruskal–Wallis test. When the overall P value was <0.05, pairwise comparisons were carried out using the Mann–Whitney test. A value of P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Ms. Nina Dvorina for expert technical assistance in this study.
This work was supported by grant 1P01 AI087586 from NIAID (to R.F., W.M.B., and A.V.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Bad Memory: CD4 T Cell Presensitization Fosters Antibody-Mediated Kidney Transplant Rejection,” on pages 3231–3233.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015080848/-/DCSupplemental.
References
- 1.Colvin RB: Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol 18: 1046–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Colvin RB, Smith RN: Antibody-mediated organ-allograft rejection. Nat Rev Immunol 5: 807–817, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M: Diagnosis and management of antibody-mediated rejection: Current status and novel approaches. Am J Transplant 14: 255–271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Hidalgo LG, Campbell PM, Sis B, Einecke G, Mengel M, Chang J, Sellares J, Reeve J, Halloran PF: De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant 9: 2532–2541, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Okada T, Cyster JG: B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol 18: 278–285, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Valujskikh A: Targeting T-cell memory: Where do we stand? Curr Opin Organ Transplant 13: 344–349, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Valujskikh A, Li XC: Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. J Am Soc Nephrol 18: 2252–2261, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE: Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. Am J Transplant 5: 1971–1975, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M: Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol 163: 2267–2275, 1999 [PubMed] [Google Scholar]
- 12.Poggio ED, Augustine JJ, Clemente M, Danzig JM, Volokh N, Zand MS, Hricik DE, Heeger PS: Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation 83: 847–852, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Heeger PS, Valujskikh A: In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol 172: 5456–5466, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Rabant M, Gorbacheva V, Fan R, Yu H, Valujskikh A: CD40-independent help by memory CD4 T cells induces pathogenic alloantibody but does not lead to long-lasting humoral immunity. Am J Transplant 13: 2831–2841, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A: Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J Immunol 176: 770–777, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Zhang QW, Rabant M, Schenk A, Valujskikh A: ICOS-Dependent and -independent functions of memory CD4 T cells in allograft rejection. Am J Transplant 8: 497–506, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bickerstaff A, Nozaki T, Wang JJ, Pelletier R, Hadley G, Nadasdy G, Nadasdy T, Fairchild RL: Acute humoral rejection of renal allografts in CCR5(-/-) recipients. Am J Transplant 8: 557–566, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Sicard A, Phares TW, Yu H, Fan R, Baldwin WM 3rd, Fairchild RL, Valujskikh A: The spleen is the major source of antidonor antibody-secreting cells in murine heart allograft recipients. Am J Transplant 12: 1708–1719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Famulski KS, Einecke G, Reeve J, Ramassar V, Allanach K, Mueller T, Hidalgo LG, Zhu LF, Halloran PF: Changes in the transcriptome in allograft rejection: IFN-gamma-induced transcripts in mouse kidney allografts. Am J Transplant 6: 1342–1354, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Finkelman FD, Holmes J, Katona IM, Urban JF Jr., Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE: Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol 8: 303–333, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Snapper CM, Paul WE: Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236: 944–947, 1987 [DOI] [PubMed] [Google Scholar]
- 22.Lefaucheur C, Nochy D, Hill GS, Suberbielle-Boissel C, Antoine C, Charron D, Glotz D: Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant 7: 832–841, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Racusen LC, Halloran PF, Solez K: Banff 2003 meeting report: New diagnostic insights and standards. Am J Transplant 4: 1562–1566, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Abe T, Ishii D, Gorbacheva V, Kohei N, Tsuda H, Tanaka T, Dvorina N, Nonomura N, Takahara S, Valujskikh A, Baldwin WM 3rd, Fairchild RL: Anti-huCD20 antibody therapy for antibody-mediated rejection of renal allografts in a mouse model. Am J Transplant 15: 1192–1204, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ: Depletion of B cells in murine lupus: Efficacy and resistance. J Immunol 179: 3351–3361, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC: Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 174: 817–826, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Baldwin WM 3rd, Valujskikh A, Fairchild RL: Antibody-mediated rejection: Emergence of animal models to answer clinical questions. Am J Transplant 10: 1135–1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colvin RB: Dimensions of antibody-mediated rejection. Am J Transplant 10: 1509–1510, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loupy A, Hill GS, Suberbielle C, Charron D, Anglicheau D, Zuber J, Timsit MO, Duong JP, Bruneval P, Vernerey D, Empana JP, Jouven X, Nochy D, Legendre CH: Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA). Am J Transplant 11: 56–65, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Bickerstaff AA, Wang JJ, Pelletier RP, Orosz CG: Murine renal allografts: Spontaneous acceptance is associated with regulated T cell-mediated immunity. J Immunol 167: 4821–4827, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della Pelle P, Benichou G, Graham JA, Madsen JC, Russell PS, Colvin RB: Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol 178: 1635–1645, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R: Improved techniques for kidney transplantation in mice. Microsurgery 16: 103–109, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Han WR, Murray-Segal LJ, Mottram PL: Modified technique for kidney transplantation in mice. Microsurgery 19: 272–274, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Murata K, Fox-Talbot K, Qian Z, Takahashi K, Stahl GL, Baldwin WM 3rd, Wasowska BA: Synergistic deposition of C4d by complement-activating and non-activating antibodies in cardiac transplants. Am J Transplant 7: 2605–2614, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Ayasoufi K, Yu H, Fan R, Wang X, Williams J, Valujskikh A: Pretransplant antithymocyte globulin has increased efficacy in controlling donor-reactive memory T cells in mice. Am J Transplant 13: 589–599, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.