Abstract
The single leading cause of mortality on hemodialysis is sudden cardiac death. Whether measures of electrophysiologic substrate independently associate with mortality is unknown. We examined measures of electrophysiologic substrate in a prospective cohort of 571 patients on incident hemodialysis enrolled in the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease Study. A total of 358 participants completed both baseline 5-minute and 12-lead electrocardiogram recordings on a nondialysis day. Measures of electrophysiologic substrate included ventricular late potentials by the signal-averaged electrocardiogram and spatial mean QRS-T angle measured on the averaged beat recorded within a median of 106 days (interquartile range, 78–151 days) from dialysis initiation. The cohort was 59% men, and 73% were black, with a mean±SD age of 55±13 years. Transthoracic echocardiography revealed a mean±SD ejection fraction of 65.5%±12.0% and a mean±SD left ventricular mass index of 66.6±22.3 g/m2.7. During 864.6 person-years of follow-up, 77 patients died; 35 died from cardiovascular causes, of which 15 were sudden cardiac deaths. By Cox regression analysis, QRS-T angle ≥75° significantly associated with increased risk of cardiovascular mortality (hazard ratio, 2.99; 95% confidence interval, 1.31 to 6.82) and sudden cardiac death (hazard ratio, 4.52; 95% confidence interval, 1.17 to 17.40) after multivariable adjustment for demographic, cardiovascular, and dialysis factors. Abnormal signal–averaged electrocardiogram measures did not associate with mortality. In conclusion, spatial QRS-T angle but not abnormal signal–averaged electrocardiogram significantly associates with cardiovascular mortality and sudden cardiac death independent of traditional risk factors in patients starting hemodialysis.
Keywords: Electrocardiogram, QRS-T angle, Arrhythmia, mortality, End stage renal disease, Sudden cardiac death
CKD has an estimated prevalence in the general population of 12.1%. Approximately 20% of these patients progress to ESRD, with a 3-year mortality of nearly 50%.1–3 The most common cause of death is cardiovascular disease, with sudden cardiac death (SCD) being the largest proportion of these events.4,5 Understanding the mechanisms and risk factors of cardiovascular causes of mortality in ESRD is critical to improving survival.
Despite the increased risk of mortality from SCD, there are few systematic investigations of the electrophysiologic substrate in ESRD with longitudinal follow-up. Most studies focus on traditional cardiovascular risk factors, such as cholesterol and hypertension, which are not consistently associated with sudden death in the context of hemodialysis unlike in the general population, thus highlighting the need to better understand the pathogenesis of arrhythmias and SCD among patients on dialysis.6,7
A number of parameters probe the electrophysiologic substrate and have been used to risk stratify those at highest risk for SCD. The presence of late potentials on a signal-averaged electrocardiogram (SAECG) characterizes a scar-related area of slow conduction in the ventricles of the heart and is associated with re-entrant ventricular tachycardia.8 Wide QRS-T angle characterizes abnormal ventricular conduction and global electrical heterogeneity in patients at risk of SCD.9 Often, the presence of these abnormal electrocardiogram (ECG) metrics is amplified in those with left ventricular hypertrophy (LVH), which is also independently linked to SCD.10
The goal of this study is to determine and compare the association of spatial QRS-T angle and late potentials on SAECG with mortality in a large prospective cohort of patients on incident hemodialysis.11 Evaluations of electrophysiologic markers specifically in an incident rather than prevalent dialysis population may yield more promising targets for interventions to decrease risk of mortality, which is highest in the first few months on dialysis.
Results
In total, 571 patients on incident hemodialysis were enrolled in the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) Study, and 400 completed the initial cardiovascular study visit. After exclusion of participants without complete digital SAECG measurement, 358 patients comprised the final study population. Demographic and clinical characteristics of patients are presented in Table 1. The cohort had a mean age of 55±13 years, 73% were black, 59% were men, 58% were patients with diabetes, and only 37% had prevalent coronary artery disease.
Table 1.
Comparison of clinical and electrocardiographic characteristics of participants on hemodialysis with QRS-T angle ≥75° and participants on hemodialysis with QRS-T angle <75°
| Characteristic | N | Overall, n=358 | QRS-T Angle <75°, n=152 | QRS-T Angle ≥75°, n=206 |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Mean age (SD), yr | 358 | 55 (13) | 55 (14) | 55 (13) |
| Men, n (%) | 358 | 212 (59) | 83 (55) | 129 (63) |
| Black, n (%) | 358 | 262 (73) | 106 (70) | 156 (76) |
| Mean BMI (SD), kg/m2 | 357 | 29.5 (7.9) | 29.0 (0.7) | 29.8 (0.5) |
| Diabetes, n (%) | 358 | 207 (58) | 76 (50) | 131 (64) |
| Hypertension, n (%) | 358 | 358 (100) | 152 (100) | 206 (100) |
| Coronary artery disease, n (%) | 358 | 132 (37) | 48 (32) | 84 (41) |
| Family history of CHD, n (%) | 358 | 153 (43) | 68 (45) | 85 (41) |
| Family history of sudden death, n (%) | 358 | 35 (10) | 16 (11) | 19 (9) |
| Ever smoke, n (%) | 358 | 220 (61) | 89 (59) | 131 (64) |
| Ever alcohol use, n (%) | 354 | 287 (81) | 125 (83) | 162 (79) |
| β-Blocker, n (%) | 322 | 226 (70) | 90 (65) | 136 (74) |
| ACE inhibitor, n (%) | 322 | 110 (34) | 42 (30) | 68 (37) |
| Angiotensin receptor blocker, n (%) | 322 | 33 (10) | 13 (9) | 20 (11) |
| Calcium channel blocker, n (%) | 322 | 196 (61) | 85 (62) | 111 (60) |
| 3-mo Averaged calcium (SD), mg/dl | 358 | 8.6 (0.6) | 8.6 (0.6) | 8.6 (0.7) |
| 3-mo Averaged potassium (SD), mEq/L | 356 | 4.3 (0.4) | 4.3 (0.5) | 4.3 (0.4) |
| 3-mo Averaged magnesium (SD), mg/dl | 355 | 2.1 (5.3) | 2.4 (8.2) | 1.8 (0.2) |
| 3-mo Averaged albumin (SD), g/dl | 356 | 3.6 (0.5) | 3.6 (0.5) | 3.5 (0.5) |
| 3-mo Averaged creatinine (SD), mg/dl | 340 | 6.7 (2.4) | 6.7 (2.4) | 6.8 (2.4) |
| 3-mo Averaged intradialytic weight change (SD), kg | 349 | 2.3 (0.9) | 2.1 (0.9) | 2.4 (0.9) |
| Mean nondialysis seated systolic BP (SD), mmHg | 357 | 137.3 (24.6) | 132.5 (23.3) | 140.9 (24.9) |
| Mean nondialysis seated diastolic BP (SD), mmHg | 357 | 74.8 (14.8) | 71.9 (13.6) | 76.9 (15.3) |
| Dialysate calcium (%), mEq/La | 349 | |||
| 2 | 145 (41) | 67 (46) | 78 (38) | |
| 2.25 | 15 (4) | 5 (3) | 10 (5) | |
| 2.5 | 187 (54) | 72 (50) | 115 (56) | |
| 3 | 2 (1) | 1 (1) | 1 (1) | |
| Dialysate potassium (%), mEq/La | 350 | |||
| 1 | 4 (1) | 2 (1) | 2 (1) | |
| 2 | 294 (84) | 120 (82) | 174 (85) | |
| 3 | 52 (15) | 24 (17) | 28 (14) | |
| LV function by ECG | ||||
| Mean LV ejection fraction (SD) | 351 | 65.5 (12.0) | 66.0 (11.2) | 65.2 (12.6) |
| Mean LV mass index (SD), g/m2 | 349 | 146.3 (48.6) | 132.4 (37.7) | 156.7 (53.1) |
| Mean LV mass index (SD), g/m2.7 | 350 | 66.6 (22.3) | 60 (17.7) | 72 (23.9) |
| LVH,b n (%) | 350 | 262 (75) | 104 (69) | 158 (79) |
| Mean LV end diastolic diameter (SD), cm | 351 | 5.4 (0.7) | 5.3 (0.6) | 5.4 (0.7) |
| Mean LV end systolic diameter (SD), cm | 350 | 3.2 (0.8) | 3.1 (0.7) | 3.3 (0.8) |
| 12-Lead ECG characteristics | ||||
| Mean heart rate (SD), bpm | 306 | 71.4 (11.1) | 70.6 (10.6) | 72.1 (11.4) |
| Median heart rate variance (IQR), ms | 306 | 359 (122–1092) | 448 (149–1124) | 348 (119–1079) |
| Mean QTc (SD), ms | 306 | 484.6 (43.7) | 481.1 (46.8) | 487.3 (41.1) |
| Mean QRS duration (SD), ms | 358 | 100.4 (18.6) | 96.1 (14.4) | 103.6 (1.4) |
| Complete BBB, n (%) | 358 | 28 (8) | 6 (4) | 22 (11) |
| Mean spatial QRS-T angle (SD), ° | 358 | 90.4 (45.6) | 46.1 (18.9) | 123.0 (29.0) |
| SAECG characteristics | ||||
| Median filtered QRS duration (IQR), ms | 358 | 113 (106–124) | 111 (104.5–119.5) | 114 (107–128) |
| Median LAS40 (IQR) | 358 | 29 (20–37) | 31 (23–37) | 26 (19–38) |
| Median RMS40 (IQR) | 358 | 37.5 (23–26.6) | 37 (24.5–55) | 38 (23–60) |
| Median noise (IQR), μV | 358 | 0.3 (0.2–0.4) | 0.3 (0.2–0.3) | 0.3 (0.2–0.4) |
| Late potentials on SAECG, n (%) | 358 | 88 (25) | 35 (23) | 53 (26) |
BMI, body mass index; CHD, coronary heart disease; ACE, angiotensin-converting-enzyme; IQR, interquartile range; QTc, corrected QT interval; RMS40, root-mean-square voltage of the last 40 ms.
Closest to study clinic visit.
LVH defined as LV mass index >51 g/m2.7 in men and >47 g/m2.7 in women.
During 864.6 person-years of follow-up, there were 15 SCDs (incidence rate, 17.3; 95% confidence interval [95% CI], 10.5 to 28.8 per 1000 person-years), 35 cardiovascular deaths including SCD (incidence rate, 40.5; 95% CI, 29.1 to 56.4 per 1000 person-years), and 77 all-cause deaths (incidence rate, 89.1; 95% CI, 71.2 to 111.3 per 1000 person-years). The median time to death was 2.4 (interquartile range, 1.4–3.4) years. Among participants with QRS-T angle ≥75°, there were 27 cardiovascular deaths (incidence rate, 55.4; 95% CI, 38.0 to 80.8 per 1000 person-years) and 12 SCDs (incidence rate, 24.6; 95% CI, 14.0 to 43.4 per 1000 person-years); among participants with QRS-T angle <75°, there were eight cardiovascular deaths (incidence rate, 21.2; 95% CI, 10.6 to 42.4 per 1000 person-years) and three SCDs (incidence rate, 7.9; 95% CI, 2.6 to 24.6 per 1000 person-years).
Clinical characteristics of participants stratified by QRS-T angle are compared in Table 1. Overall, the mean left ventricular (LV) ejection fraction was normal. Participants with QRS-T angle ≥75° were more likely to have diabetes, LVH, bundle branch block (BBB), greater change in intradialytic weight, higher systolic and diastolic BPs, and wider unfiltered and filtered QRS. Late potentials were found in 88 (25%) participants who were predominantly older, white men with BBB as well as longer QRS duration (Supplemental Table 1). We also compared the characteristics by primary outcome status (Supplemental Table 2). Those who died were slightly older; had higher body mass index; had lower serum calcium, albumin, and creatinine levels; and had greater mean QRS-T angle.
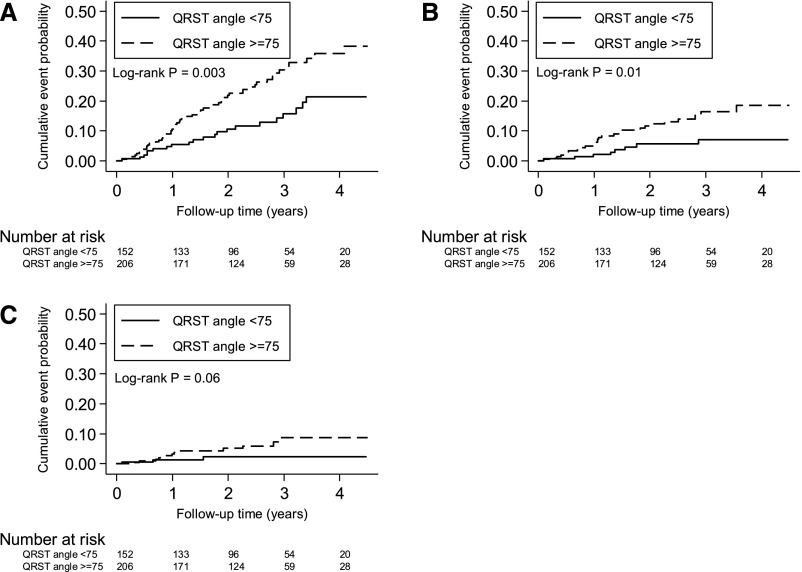
The unadjusted cumulative incidence of all-cause and cardiovascular mortality was higher in participants with abnormal QRS-T angle (Figure 1, A and B). The cumulative probability of SCD was higher in participants with abnormal QRS-T angle, but this was not statistically significant (Figure 1C). In the unadjusted Cox proportional hazards analysis (Table 2), each 10° increase in spatial QRS-T angle was associated with a 5% increased risk of all-cause death. Even after adjusting for demographic, clinical, and cardiovascular risk factors, every 10° increase in spatial QRS-T was consistently associated with a 5% increase in the risk of all-cause mortality. Higher QRS-T angle was also significantly associated with cardiovascular mortality. The association between continuous QRS-T angle and SCD was similar in direction and magnitude but was not statistically significant. When examined as a categorical variable, spatial QRS-T angle ≥75° was associated with a greater than twofold increase in the risk of all-cause mortality compared with spatial QRS-T angle <75° in both the unadjusted and fully adjusted models (Table 2). Spatial QRS-T angle ≥75° was also associated with a significantly increased risk of cardiovascular mortality and SCD. To confirm that there was a threshold effect, spatial QRS-T angle was divided into tertiles. Compared with the lowest tertile (<64.7°), both second (64.7°–111.5°; adjusted hazard ratio, 2.27; 95% CI, 0.90 to 5.68) and third tertiles (>115°; adjusted hazard ratio, 2.37; 95% CI, 0.93 to 6.09) had greater risk of cardiovascular mortality with similar magnitude of effect, but the associations were not statistically significant. The associations between QRS-T angle as a continuous variable and the outcomes were also consistent when deaths within 1 year after study enrollment were examined (Supplemental Table 3). Late potentials on SAECG were not associated with all-cause, cardiovascular, or sudden cardiac mortality in both unadjusted and adjusted Cox regression analyses (Supplemental Table 4).
Figure 1.
Mortality among participants on incident hemodialysis with QRS-T angle ≥75° or <75°. Cumulative (A) all–cause mortality, (B) cardiovascular mortality, and (C) sudden cardiac death.
Table 2.
Risk for all-cause mortality, cardiovascular mortality, and SCD associated with QRS-T angle among 358 patients on incident hemodialysis
| Model | Per 10° of Spatial QRS-T Angle | Spatial QRS-T Angle ≥75° | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| All-cause mortality | ||||
| Unadjusted | 1.05 (1.00 to 1.10) | 0.05a | 2.13 (1.28 to 3.53) | 0.003 |
| Model 1b | 1.05 (1.00 to 1.11) | 0.05a | 2.30 (1.38 to 3.84) | 0.001 |
| Model 2c | 1.05 (1.01 to 1.11) | 0.05a | 2.38 (1.41 to 4.04) | 0.001 |
| Cardiovascular mortality | ||||
| Unadjusted | 1.07 (1.00 to 1.16) | 0.06 | 2.73 (1.24 to 6.03) | 0.01 |
| Model 1b | 1.08 (1.00 to 1.16) | 0.05 | 2.84 (1.28 to 6.33) | 0.01 |
| Model 2c | 1.08 (1.00 to 1.17) | 0.04 | 2.99 (1.31 to 6.82) | 0.01 |
| SCD | ||||
| Unadjusted | 1.08 (0.97 to 1.21) | 0.18 | 3.42 (0.97 to 12.14) | 0.06 |
| Model 1b | 1.08 (0.96 to 1.20) | 0.20 | 3.39 (0.94 to 12.15) | 0.06 |
| Model 2c | 1.11 (0.98 to 1.25) | 0.10 | 4.52 (1.17 to 17.40) | 0.03 |
P<0.05.
Model 1 includes the main exposure (either continuous QRS-T angle or dichotomized QRS-T angle), age, sex, and race.
Model 2 includes variables in model 1, β-blocker medication, coronary artery disease, and LV mass index.
The associations of spatial QRS-T angle with all outcomes were not significantly modified by BBB. Associations with the risk of all-cause and cardiovascular mortality and SCD were robust, even when BBB was excluded from the analysis (Supplemental Table 5). Similarly, when patients with QRS duration ≥120 ms were excluded, spatial QRS-T angle remained significantly associated with the risk of all-cause mortality, cardiovascular mortality, and SCD (Supplemental Table 6). Adjusting for each of these additional factors, such as QRS duration, serum electrolytes (calcium, potassium, and magnesium), dialysate electrolytes (calcium and potassium), intradialytic weight change, and albumin, in the main model did not attenuate the association of spatial QRS-T angle with mortality (Supplemental Table 7).
Other traditional ECG measures had modest association with mortality (Supplemental Table 8). Every 10-ms increase in QRS interval was associated with a lower risk of all-cause and cardiovascular mortality. Conversely, heart rate and LVH as detected by ECG were not associated with all-cause mortality, cardiovascular mortality, or SCD.
Discussion
In this prospective cohort study of participants on incident hemodialysis, spatial QRS-T angle, a measure of the electrophysiologic substrate, is an independent risk factor of all-cause mortality, cardiovascular mortality, and importantly, SCD. Spatial QRS-T angle characterizes abnormal ventricular conduction and global electrical heterogeneity, which ultimately predisposes to ventricular arrhythmias and SCD.9,12,13 The independent and robust association of QRS-T angle with cardiovascular mortality and SCD supports the hypothesis that arrhythmias are an important mechanism leading to death after hemodialysis initiation.5,14,15
This compelling association occurred in a young dialysis cohort with a low baseline prevalence of coronary artery disease and also, preserved LV function. This suggests that underlying defects in depolarization and secondary abnormalities in repolarization occur independently of aging, coronary, or LV disease and place patients on dialysis at risk for lethal arrhythmias. This ECG-derived metric, spatial QRS-T angle, may provide evidence of subclinical myocardial fibrosis independent of structural and functional abnormalities typically documented by ECG.16–18 Conversely, other electrophysiologic markers, such as ventricular late potentials by SAECG, did not associate with mortality; however, increased risk may be only evident in those with subepicardial lesions in the late activated regions of the myocardium at the very end of QRS.18
Spatial QRS-T angle is a well established measure of global electrical heterogeneity. In the general population, it is associated with risk for total mortality, cardiovascular death, and SCD in those with myocardial infarction and cardiomyopathy, whereas among patients on prevalent dialysis, it is associated with heart failure.9,12,19,20 We now extend the findings to an incident hemodialysis population with SCD. A previous report studied patients on hemodialysis with ECGs performed up to 18 months after dialysis initiation. Notwithstanding the potential survivor bias, the study showed similar magnitude and direction of risk for arrhythmic-related events.12 Unlike our study, this prior analysis could not account for clinical and cardiovascular characteristics known to significantly affect mortality in dialysis, especially LV function. Moreover, we were able to show, in those with preserved ejection fraction at baseline, that the QRS-T angle is independently associated with mortality and not just heart failure. The results also show a consistently higher risk of spatial QRS-T angle with SCD and cardiovascular and all-cause mortality, even after adjustment of strong risk factors for mortality in dialysis, such as albumin.
Interestingly, in our study, the best threshold of QRS-T angle is lower than previously used in other studies but similar to that in the Multi-Ethnic Study of Atherosclerosis, which yielded a similar graded risk above the 95th percentile of the QRS-T angle.9,17 In a general community population, both QRS-T angle >105° and CKD were associated with increased risk of 1-year mortality by 1.5–2 times, indicating a possible interaction with kidney disease and common activated pathways leading to subclinical endocardial and kidney interstitial fibrosis.16 The specific thresholds for spatial QRS-T angle in a normal healthy population across ethnicities and all ages have not been derived; thus, clinically relevant cut points cannot be provided yet for routine clinical screening. Prior studies used ranges from 40° to >100°, depending on the population and method of derivation. The largest study population of >40,000 individuals free of cardiac disease and with age >55 years suggested that spatial QRS-T angles from 0° to 50° are normal, that spatial QRS-T angles from 50° to 100° are borderline, and that spatial QRS-T angles >100° are abnormal.9,21 Similarly, we examined QRS-T angle in tertiles given the limited power and showed a threshold effect around 75°, suggesting that there is a greater risk of mortality in individuals with QRS-T angle ≥75°.
This study did not find an association of late potentials on SAECG with mortality. Several prior studies reported intradialytic differences in SAECG, such as worsening in low-amplitude signals (<40 mV) in the terminal portion (LAS40) and prolongation of filtered QRS after dialysis.22,23 In our study, late potentials were found in 24% of participants, similar to previous reports of smaller populations.23 Although ventricular late potentials have a strong mechanistic association with ventricular arrhythmias, the SAECG method used may not detect areas of slow conduction occurring early on within the QRS complex.24,25 In addition, the delayed LV epicardial activation caused by interventricular conduction abnormality (which could manifest by wide QRS-T angle) can mask late potentials by SAECG.24 Because abnormal SAECG (late potentials on SAECG) characterizes the presence of a subepicardial scar, the lack of association between late potentials on SAECG and SCD suggests that persons with ESRD may have other arrhythmogenic properties of the myocardium that predispose them to SCD. Even after exclusion of participants with BBB and QRS≥120 ms, however, late potentials remained nonsignificant. The association of late potentials with myocardial fibrosis perhaps indicates late–stage cardiac disease, which was not evident in our incident dialysis population.
Consistent with previous reports, LVH was frequently observed in this study at initiation of dialysis.26 After adjusting for LV mass, there was no attenuation in risk of spatial QRS-T angle and mortality. This suggests that LV structural changes, such as increased LV mass, may be uncoupled from depolarization activity.27 Currently, an ECG is suggested at the time of dialysis by Kidney Disease: Improving Global Outcomes, but it may be warranted to include ECG metrics, such as the spatial QRS-T angle derived from a routine 12–lead ECG.28 Other ECG metrics derived from a 12-lead ECG have also been shown to be predictive of cardiovascular mortality in those with CKD before starting hemodialysis; however, we report no significant associations of the standard ECG parameters with mortality, and the magnitude of effect is not as great as with spatial QRS-T angle.29 The role of ECG monitoring has not been a central focus in the clinical management of patients on dialysis, and therefore, the inclusion of the spatial QRS-T angle and its effect on clinical decision making need to be considered.
There are several limitations to this study. Not all participants completed the cardiovascular study visit, because they were hospitalized, had events, or were too overwhelmed with initiating dialysis. Although the statistical power of the study was sufficient to determine independent association or tested exposure variables, it was not sufficient for many subgroup analyses. We did observe that there was a borderline association of ECG LVH and QRS duration with SCD with similar magnitude and direction as in past studies, but it was not statistically significant, most likely because of limited sample size. Independent validation of our findings is also required in other incident hemodialysis populations. Despite the limitations, important strengths need to be acknowledged. This is a large prospective cohort study of patients on incident hemodialysis with cardiovascular and clinical characterization and a large proportion of blacks. The cohort also has comprehensive baseline cardiovascular phenotyping, including a detailed assessment of electrophysiologic substrate. Importantly, we were able to investigate the cause of death by adjudication and address potential confounding by important factors, such as dialysis–related treatment parameters.
A straightforward measure of the electrophysiologic substrate using a 12-lead ECG, the spatial QRS-T angle, is associated with an increased risk of SCD within a few years after starting hemodialysis and independent of traditional, dialysis–related, and clinical risk factors. Future studies are needed to determine if interventions can mitigate this risk. Spatial QRS-T angle could be included in a future risk scores to guide management of patients on hemodialysis.
Concise Methods
Study Population and Data Collection
The PACE Study is a prospective cohort study of patients on incident hemodialysis in the Baltimore metropolitan area in Maryland. The study protocol was approved by the Johns Hopkins Institutional Review Board. All participants provided written informed consent. Details of the study protocol have been described previously.11 Briefly, ESRD patients were eligible for enrollment if they (1) were 18 years of age or older, (2) had started hemodialysis within 6 months of enrollment, and (3) were English speakers. Exclusion criteria were (1) diagnosed active cancer other than nonmelanoma skin cancer, (2) implanted pacemaker or implantable cardioverter defibrillator, and (3) pregnancy or nursing. In addition, in this analysis, we excluded study participants without available baseline SAECG. Participants underwent a baseline cardiovascular study visit on a nondialysis day within 6 months of initiation of hemodialysis. Detailed medical history, family history, and dialysis laboratories were collected as previously described.11 Average levels of serum albumin, creatinine, calcium, magnesium, and potassium were calculated using values from routine dialysis laboratory blood work during 90 days before the study clinic visit. Intradialytic weight change was calculated as the difference between postdialysis weight and predialysis weight and averaged using values during 90 days before the study visit. Prevalent coronary artery disease, hypertension, and diabetes were determined by physician abstraction from medical records and self-report questionnaires. Antihypertensive medications were assessed at each study visit. Dialysate calcium and potassium values closest to the study visit were recorded.
Echocardiographic examination was performed using a Vivid 7 Cardiac Ultrasound Machine (GE Healthcare, Waukesha, WI). Conventional measurements included LV end diastolic and end systolic diameters and LV ejection fraction by the biplane Simpson method.30 LV mass was estimated as recommended by the American Society of Echocardiography.30 LV mass index normalized by body surface area and height2.7 was calculated. Body surface area was calculated with the Mosteller equation using reported estimated dry weight. Echocardiographic LVH was defined as LV mass index >51 g/m2.7 in men and >47 g/m2.7 in women.31,32
SAECG Recording
SAECG (MAC 5000 with Hi-Res Module; GE Healthcare) signal was recorded using standard x, y, and z orthogonal leads placement at rest with 1000-Hz sampling rate, band pass filtering at 40–250 Hz, and average of 350 QRS complexes as an averaging target. The recorded SAECG signal was analyzed by two different approaches. First, SAECG analysis of late potentials was performed by a commercial Hi-Res SAECG Module (GE Healthcare). Second, the raw unfiltered averaged xyz ECG signal was exported via the ECG Research Workstation, HiRes Module (GE Healthcare), and mean spatial QRS-T angle was measured by the software developed in MATLAB (MathWorks, Inc., Natick, MA).
SAECG Analyses of Late Potentials
The following SAECG characteristics of the filtered QRS were evaluated: (1) total filtered QRS duration, (2) LAS40, and (3) root-mean-square voltage of the last 40 ms.33 Ventricular late potentials were considered positive when two or more of the following criteria were fulfilled: (1) filtered QRS duration>114 ms, (2) LAS40>38 ms, and (3) root-mean-square voltage of the last 40 ms <20 μV.
Measurement of Spatial QRS-T Angle
The unfiltered averaged xyz ECG signal (sampling rate of 1000 Hz; amplitude resolution of 1 μV) was used for the measurement of spatial QRS-T angle. In this study, spatial QRS-T angle was measured as an angle between spatial mean QRS vector and spatial peak T vector (Figure 1). The mean (but not peak) value of the spatial QRS vector over time during the QRS loop was used to characterize the spatial QRS vector as previously described.9 Peak spatial T vector was defined as a vector between the origin point of QRS and T loops and the farthest point in the T loop.34 We chose to use peak (but not mean) T vector, because the end of the T wave (and T loop) on the raw SAECG signal was truncated in patients with very long cardiac cycles. We chose to use mean (but not peak) QRS vector, because QRS loops in the study participants were asymmetric. Spatial QRS-T angle was calculated using the definition of the normalized inner product of spatial mean QRS vector and spatial peak T vector. Accuracy of an automated placement of fiducial points was reviewed by two investigators (E.G. and M.K.).
Definition of Complete BBB
Orthogonal xyz ECGs and Dower–derived 12–lead ECGs were reviewed by two investigators (L.G.T. and A.O.), who evaluated presence of complete left or right BBB according to the established criteria.35
Primary Outcome
The PACE Study participants were followed prospectively with yearly clinic visits. In addition, the research coordinator would conduct phone interviews every 6 months and visit the dialysis units frequently to check with patients and clinic staff.11 Mortality was confirmed by chart review, death certificate, or Centers for Medicare & Medicaid Services (CMS) Death Notification Form 2746. All records from family and dialysis unit interviews; hospital, emergency room, and ambulance records; death certificates; and the CMS-2746 Death Notification forms were reviewed by two trained physician abstractors, and the final determination of cause of death was conducted by the chair of the end point committee or delegate. The chair decided any discordant reviews after committee discussion. We defined SCD as a sudden collapse presumed to be caused by an arrhythmia occurring out of the hospital or in the emergency room witnessed or unwitnessed since the last dialysis treatment. Cardiovascular mortality was defined as the composite of SCD and death caused by arrhythmias, cardiac arrest, ischemic cardiovascular disease, and ischemic cerebrovascular disease either in or outside the hospital.
Statistical Analyses
Continuous normally distributed variables were expressed as means±SDs and compared using t tests. Categorical variables were expressed as frequency (%) and compared by Pearson chi–squared tests. Receiver operating characteristic curves were generated for spatial QRS-T angle. Sensitivity and specificity of each value of spatial QRS-T angle were evaluated. The optimal cut point value of spatial QRS-T angle was determined by several methods: (1) the method by Youden,36 which maximizes the sum of sensitivity and specificity; (2) the method by Liu,37 which maximizes the product of sensitivity and specificity; and (3) the nearest to (0,1) method, which finds the cut point on the receiver operating characteristic curve with perfect sensitivity and specificity. The cut point was bootstrapped (500 replications) to estimate 95% CIs. All three methods (the methods by Youden36 and Liu37 and the nearest to [0,1] method) identified a single optimal threshold value for the spatial QRS-T angle at 74.4° (Supplemental Figure 1), with a bootstrapped 95% CI from 62.6° to 85.8°. Sensitivity at the identified optimal threshold was satisfactory (73%), whereas specificity was low (47%). We rounded to the nearest integer value and dichotomized the exposure into those with abnormal wide spatial QRS-T angle (≥75°) and those with normal spatial QRS-T angle (<75°). Past studies have used other QRS-T angle thresholds on the basis of different methods.12,19,38–40 Additionally, we examined the association of QRS-T angle in tertiles with cardiovascular mortality.
Unadjusted Kaplan–Meier survival analysis was used to compare time to event probabilities in patients with spatial QRS-T angle above the threshold. The log rank statistic was computed to test the equality of survival distributions.
Multivariable Cox proportional hazards regression analyses were performed for all evaluated predictors. A forward model–building approach involving variable selection on the basis of changes in effect size, known associations with mortality in patients on dialysis, and significant P values was used to build the final multivariable model. Cox regression analyses were stratified by left or right BBB. In addition, sensitivity analyses were conducted excluding participants with interventricular conduction delay (QRS≥120 ms) and BBB. Finally, we tested for an interaction between QRS-T angle and BBB using the likelihood ratio test.41,42
Proportional hazards assumption was tested on the basis of Schoenfield residuals for each Cox model. To avoid overfitting, several Cox models were generated with adjustment of demographic, cardiovascular, dialysis-related, and inflammatory factors.5 We checked for nonlinear association between mean spatial QRS-T angle as a continuous variable and all-cause mortality using restricted cubic splines. All missing data were imputed using the multiple imputation by chained equations method.43 All statistical analyses were performed in Stata 14.1 (StataCorp., College Station, TX). A two–tailed P value <0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We dedicate the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) Study to our friend and colleague W.H.L.K. We thank the PACE Study staff and participants and the nephrologists and staff of the DaVita and MedStar dialysis units in the Baltimore area. We also thank Dr. Joel Xue (GE Healthcare, Waukesha, WI) for providing GE ECG Research Utility software for analysis.
The PACE Study was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK072367 (to R.S.P.). This study was partially supported by National Heart, Lung, and Blood Institute grant R01HL118277 (to L.G.T.). This study was partially supported by Boston Scientific as an investigator-initiated research project (L.G.T.).
Some of the data in this article were presented at the American Health Association Scientific Sessions held November 7–11, 2015 in Orlando, FL.
Footnotes
Deceased.
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015080916/-/DCSupplemental.
References
- 1.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 3.US Renal Data System: USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013, pp 263–270 [Google Scholar]
- 4.Shamseddin MK, Parfrey PS: Sudden cardiac death in chronic kidney disease: Epidemiology and prevention. Nat Rev Nephrol 7: 145–154, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, Powe NR, Coresh J, Klag MJ: The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 74: 1335–1342, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Ritz E, Wanner C: The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol 3: 920–929, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Weir MR: Debate from the 2012 ASH Annual Scientific Sessions: Should blood pressure be reduced in hemodialysis patients? Con position. J Am Soc Hypertens 6: 443–447, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Breithardt G, Cain ME, el-Sherif N, Flowers N, Hombach V, Janse M, Simson MB, Steinbeck G: Standards for analysis of ventricular late potentials using high resolution or signal-averaged electrocardiography. A statement by a Task Force Committee between the European Society of Cardiology, the American Heart Association and the American College of Cardiology. Eur Heart J 12: 473–480, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Oehler A, Feldman T, Henrikson CA, Tereshchenko LG: QRS-T angle: A review. Ann Noninvasive Electrocardiol 19: 534–542, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens SM, Reinier K, Chugh SS: Increased left ventricular mass as a predictor of sudden cardiac death: Is it time to put it to the test? Circ Arrhythm Electrophysiol 6: 212–217, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parekh RS, Meoni LA, Jaar BG, Sozio SM, Shafi T, Tomaselli GF, Lima JA, Tereshchenko LG, Estrella MM, Kao WH: Rationale and design for the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) study. BMC Nephrol 16: 63, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bie M, Koopman MG, Gaasbeek A, Dekker FW, Maan AC, Swenne CA, Scherptong RW, van Dessel PF, Wilde AA, Schalij MJ, Rabelink TJ, Jukema JW: Incremental prognostic value of an abnormal baseline spatial QRS-T angle in chronic dialysis patients. Europace 15: 290–296, 2013 [DOI] [PubMed]
- 13.Borleffs C, Scherptong RW, Man SC, van Welsenes GH, Bax JJ, van Erven L, Swenne CA, Schalij MJ: Predicting ventricular arrhythmias in patients with ischemic heart disease: Clinical application of the ECG-derived QRS-T angle. Circ Arrhythm Electrophysiol 2: 548–554, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT Jr.: Controlling the epidemic of cardiovascular disease in chronic renal disease: What do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis 32: 853–906, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE: Sudden cardiac death in end-stage renal disease patients: A 5-year prospective analysis. Hypertension 56: 210–216, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Strauss DG, Mewton N, Verrier RL, Nearing BD, Marchlinski FE, Killian T, Moxley J, Tereshchenko LG, Wu KC, Winslow R, Cox C, Spooner PM, Lima JA: Screening entire health system ECG databases to identify patients at increased risk of death. Circ Arrhythm Electrophysiol 6: 1156–1162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh JA 3rd, Soliman EZ, Ilkhanoff L, Ning H, Liu K, Nazarian S, Lloyd-Jones DM: Prognostic value of frontal QRS-T angle in patients without clinical evidence of cardiovascular disease (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 112: 1880–1884, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberger JJ, Subačius H, Patel T, Cunnane R, Kadish AH: Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol 63: 1879–1889, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Kors JA, Kardys I, van der Meer IM, van Herpen G, Hofman A, van der Kuip DA, Witteman JC: Spatial QRS-T angle as a risk indicator of cardiac death in an elderly population. J Electrocardiol 36[Suppl]: 113–114, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Pavri BB, Hillis MB, Subacius H, Brumberg GE, Schaechter A, Levine JH, Kadish A; Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators : Prognostic value and temporal behavior of the planar QRS-T angle in patients with nonischemic cardiomyopathy. Circulation 117: 3181–3186, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki T, Froelicher VF, Myers J, Chun S, Wang P: Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm 2: 73–78, 2005 [DOI] [PubMed]
- 22.Ichikawa H, Nagake Y, Makino H: Signal averaged electrocardiography (SAECG) in patients on hemodialysis. J Med 28: 229–243, 1997 [PubMed] [Google Scholar]
- 23.Morales MA, Gremigni C, Dattolo P, Piacenti M, Cerrai T, Fazi A, Pelosi G, Vergassola R, Maggiore Q: Signal-averaged ECG abnormalities in haemodialysis patients. Role of dialysis. Nephrol Dial Transplant 13: 668–673, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Simson MB, Untereker WJ, Spielman SR, Horowitz LN, Marcus NH, Falcone RA, Harken AH, Josephson ME: Relation between late potentials on the body surface and directly recorded fragmented electrograms in patients with ventricular tachycardia. Am J Cardiol 51: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 25.Josephson ME, Simson MB, Harken AH, Horowitz LN, Falcone RA: The incidence and clinical significance of epicardial late potentials in patients with recurrent sustained ventricular tachycardia and coronary artery disease. Circulation 66: 1199–1204, 1982 [DOI] [PubMed] [Google Scholar]
- 26.Charytan D: Is left ventricular hypertrophy a modifiable risk factor in end-stage renal disease. Curr Opin Nephrol Hypertens 23: 578–585, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacharova L, Estes EH, Bang LE, Hill JA, Macfarlane PW, Rowlandson I, Schillaci G: Second statement of the working group on electrocardiographic diagnosis of left ventricular hypertrophy. J Electrocardiol 44: 568–570, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Workgroup KD; K/DOQI Workgroup : K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45[Suppl 3]: S1–S153, 2005 [PubMed] [Google Scholar]
- 29.Deo R, Shou H, Soliman EZ, Yang W, Arkin JM, Zhang X, Townsend RR, Go AS, Shlipak MG, Feldman HI: Electrocardiographic measures and prediction of cardiovascular and noncardiovascular death in CKD. J Am Soc Nephrol 27: 559–569, 2016 [DOI] [PMC free article] [PubMed]
- 30.Lang R, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography : Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Cuspidi C, Meani S, Negri F, Giudici V, Valerio C, Sala C, Zanchetti A, Mancia G: Indexation of left ventricular mass to body surface area and height to allometric power of 2.7: Is the difference limited to obese hypertensives? J Hum Hypertens 23: 728–734, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Foppa M, Duncan BB, Rohde LE: Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc Ultrasound 3: 17, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, Maron BJ, Page RL, Passman RS, Siscovick D, Stevenson WG, Zipes DP; American Heart Association; American College of Cardiology Foundation; Heart Rhythm Society : American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific Statement on Noninvasive Risk Stratification Techniques for Identifying Patients at Risk for Sudden Cardiac Death. A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. J Am Coll Cardiol 52: 1179–1199, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Sur S, Han L, Tereshchenko LG: Comparison of sum absolute QRST integral, and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PLoS One 8: e57175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society; Endorsed by the International Society for Computerized Electrocardiology : AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part III: Intraventricular conduction disturbances: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. J Am Coll Cardiol 53: 976–981, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Youden WJ: Index for rating diagnostic tests. Cancer 3: 32–35, 1950 [DOI] [PubMed] [Google Scholar]
- 37.Liu X: Classification accuracy and cut point selection. Stat Med 31: 2676–2686, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A: Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: The Women’s Health Initiative. Circulation 113: 473–480, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Whang W, Shimbo D, Levitan EB, Newman JD, Rautaharju PM, Davidson KW, Muntner P: Relations between QRS|T angle, cardiac risk factors, and mortality in the third National Health and Nutrition Examination Survey (NHANES III). Am J Cardiol 109: 981–987, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang ZM, Prineas RJ, Case D, Soliman EZ, Rautaharju PM; ARIC Research Group : Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the atherosclerosis risk in communities study). Am J Cardiol 100: 844–849, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, Bailey JJ, Childers R, Gorgels A, Josephson M, Kors JA, Macfarlane P, Mason JW, Pahlm O, Rautaharju PM, Surawicz B, van Herpen G, Wagner GS, Wellens H; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society : AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: Endorsed by the International Society for Computerized Electrocardiology. Circulation 119: e251–e261, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Anonymous: Signal-averaged electrocardiography. J Am Coll Cardiol 27: 238–249, 1996 [PubMed] [Google Scholar]
- 43.White IR, Royston P, Wood AM: Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30: 377–399, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.