Abstract
The Hemodialysis (HEMO) Study showed that high-dose hemodialysis providing a single-pool Kt/Vurea of 1.71 provided no benefit over a standard treatment providing a single-pool Kt/Vurea of 1.32. Here, we assessed whether the high-dose treatment used lowered plasma levels of small uremic solutes other than urea. Measurements made ≥3 months after randomization in 1281 patients in the HEMO Study showed a range in the effect of high-dose treatment compared with that of standard treatment: from no reduction in the level of p-cresol sulfate or asymmetric dimethylarginine to significant reductions in the levels of trimethylamine oxide (−9%; 95% confidence interval [95% CI], −2% to −15%), indoxyl sulfate (−11%; 95% CI, −6% to −15%), and methylguanidine (−22%; 95% CI, −18% to −27%). Levels of three other small solutes also decreased slightly; the level of urea decreased 9%. All-cause mortality did not significantly relate to the level of any of the solutes measured. Modeling indicated that the intermittency of treatment along with the presence of nondialytic clearance and/or increased solute production accounted for the limited reduction in solute levels with the higher Kt/Vurea. In conclusion, failure to achieve greater reductions in solute levels may explain the failure of high Kt/Vurea treatment to improve outcomes in the HEMO Study. Furthermore, levels of the nonurea solutes varied widely among patients in the HEMO Study, and achieved Kt/Vurea accounted for very little of this variation. These results further suggest that an index only on the basis of urea does not provide a sufficient measure of dialysis adequacy.
Keywords: hemodialysis, uremia, hemodialysis adequacy
The great majority of patients on hemodialysis receive treatment three times per week. The efficacy of waste solute removal in these patients is assessed by measurement of Kt/Vurea. In the United States, treatment is considered adequate if single-pool Kt/Vurea (spKt/Vurea) is >1.2, and standards are similar in other countries.1 Results of the Hemodialysis (HEMO) Study played a large role in the adoption of these standards. Before the HEMO Study, observational studies had suggested that clinical benefit could be achieved by increasing spKt/Vurea as high as 1.7.2 However, the HEMO Study, a controlled trial carried out in prevalent patients, found no reduction in mortality in patients randomized to receive high-dose treatment with average Kt/Vurea of 1.71 compared with those receiving standard treatment with average Kt/Vurea of 1.32.3 The initial HEMO Study report showed that the higher Kt/Vurea prescription also had no discernable effect on secondary outcomes, which combined mortality with hospitalization for cardiac causes, hospitalization for infectious causes, and decline in the serum albumin. Subsequent analyses showed that the higher Kt/Vurea prescription also did not improve nutrition or health–related quality of life.4
A variety of explanations have been considered for the failure of high-dose treatment to provide benefit in the HEMO Study. One is that Kt/Vurea provides an imperfect index of the removal of toxic solutes that accumulate when the kidneys fail.5 High-dose treatment reduced predialysis urea levels by about 13% over the course of the HEMO Study. This study was performed to assess the effect of the higher Kt/Vurea on levels of small solutes, which behave differently than urea during dialysis. Another goal was to identify potential contributors to high levels of small solutes, which have been considered to have toxic effects.
Results
Measurements were made in 1281 patients for whom plasma samples were available during the first year after randomization in the HEMO Study. Characteristics of these patients as summarized in Table 1 did not differ notably from those of the 1846 patients in the parent study.3 Dialysis prescriptions are also summarized in Table 1. Higher Kt/Vurea in the high-dose group was achieved largely by increases in blood flow and treatment time, with lesser increases in dialysate flow and the dialyzer mass transfer area coefficient (KoA) for urea.
Table 1.
Subject characteristics and dialysis prescriptions at follow-up
| Characteristic | Intervention Group | P Value | |
|---|---|---|---|
| Standard Kt/Vurea, n=643 | High Kt/Vurea, n=638 | ||
| Characteristic | |||
| Age, yr | 58±14 | 58±14 | NS |
| Women, % | 57 | 57 | NS |
| Black race, % | 64 | 62 | NS |
| Diabetes, % | 46 | 45 | NS |
| On dialysis, yr | 3.8±4.1 | 3.8±4.2 | NS |
| Residual urea clearance >0, % | 27 | 29 | NS |
| Weight after dialysis, kg | 71±16 | 69±15 | 0.07 |
| Serum albumin, g/dl | 3.6±0.4 | 3.6±0.4 | NS |
| nPCR, g/kg per d | 1.00±0.26 | 1.07±0.31 | <0.001 |
| spKt/Vurea | 1.32±0.31 | 1.73±0.44 | <0.001 |
| Dialysis prescription | |||
| Treatment time, min | 193±62 | 221±63 | <0.001 |
| Blood flow rate, ml/min | 311±60 | 376±41 | <0.001 |
| Dialysate flow rate, ml/min | 639±134 | 708±116 | <0.001 |
| KoAurea, ml/min | 547±55 | 563±42 | <0.001 |
| Ultrafiltration, L | 2.9±1.3 | 3.0±1.3 | NS |
Values for blood flow rate are adjusted from the blood pump meter values as described by Depner et al.43 Values for KoAurea are estimated as described in Concise Methods. P values were estimated by t or rank sum test for continuous variables and proportion ratio test for categorical variables without correction for multiple comparisons (NS is P>0.05). Normality of continuous variables was tested using the Shapiro–Wilk test. The P values for continuous variables were similar, except for postdialysis weight P value was P=0.02 by t test and P=0.07 by rank sum test.
Pretreatment plasma solute levels in patients in the two treatment groups are summarized in Table 2. Pretreatment urea nitrogen values averaged 63±19 and 57±18 mg/dl in this study compared with the values of 62±14 and 54±11 mg/dl over the course of the parent study.3 The effect of the higher Kt/Vurea prescription on the levels of other solutes varied widely, with lesser reductions in the levels of most. The solutes measured included four organic anions—p-cresol sulfate (PCS), indoxyl sulfate (IS), hippurate (HIPP), and phenylacetylglutamine (PAG)—which have been found to rise to much higher levels relative to normal than urea in patients on hemodialysis.6,7 Of note, the average PCS level was no lower in the high-dose than the standard treatment group. In contrast, the IS level was lower by approximately the same proportion as the urea level. Numerically lesser reductions in the HIPP and PAG levels did not achieve conventional statistical significance. We also measured the levels of trimethylamine oxide (TMAO) and methylguanidine (MG), which have been found to accumulate to similarly high levels relative to normal in patients on dialysis, as well as asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). TMAO levels were lower by approximately the same proportion as urea levels in the high Kt/Vurea group, whereas MG levels were lower by a larger proportion. In contrast, the difference in Kt/Vurea had no effect on ADMA levels and only a slight effect on SDMA levels. Exclusion of patients with residual function did not affect these results, as shown in Supplemental Table 1. It should be noted that patients with residual urea clearance >1.5 ml/min per 35-L Watson volume were excluded from the HEMO Study so that effects of the differing dialysis prescriptions could be discerned more clearly. The differences in solute levels were also not affected by adjustment for age, sex, race, dialysis vintage, and plasma albumin concentration, as shown in Supplemental Table 2.
Table 2.
Plasma solute levels
| Solute | Effects of Standard and High–Dose Kt/Vurea Treatment on Solute Levels | Associations of All-Cause Mortality with Solute Levels | |||||
|---|---|---|---|---|---|---|---|
| Plasma Level Standard Kt/Vurea | Plasma Level High Kt/Vurea | Relative Difference (95% CI) | P Value | Modeled Relative Difference, % | Hazard Ratio (95% CI) | P Value | |
| Urea nitrogen, mg/dl | 63±19 | 57±18 | −9.4 (−12.5 to −6.3) | <0.001 | −10 | 1.06 (0.97 to 1.16) | 0.23 |
| PCS, mg/dl | 3.3±1.7 | 3.4±1.7 | 2.2 (−3.6 to 7.8) | 0.46 | −16 | 0.91 (0.83 to 1.00) | 0.04 |
| IS, mg/dl | 2.7±1.3 | 2.4±1.1 | −11.0 (−15.6 to −6.4) | <0.001 | −16 | 0.95 (0.87 to 1.06) | 0.40 |
| HIPP, mg/dl | 5.5±4.6 | 5.3±4.0 | −4.1 (−12.4 to 4.2) | 0.34 | −9 | 1.08 (0.98 to 1.18) | 0.11 |
| PAG, mg/dl | 4.6±3.0 | 4.3±2.6 | −6.8 (−13.3 to −0.2) | 0.04 | −8 | 1.03 (0.95 to 1.13) | 0.44 |
| TMAO, μM | 107±63 | 97±65 | −8.9 (−15.3 to −2.4) | <0.01 | −9 | 1.03 (0.94 to 1.12) | 0.54 |
| MG, μM | 8.7±4.7 | 6.7±3.8 | −22.5 (−27.3 to −17.7) | <0.001 | −14 | 1.02 (0.93 to 1.13) | 0.64 |
| ADMA, μM | 0.92±0.24 | 0.92±0.23 | 0.5 (−2.2 to 3.2) | 0.74 | −18 | 1.06 (0.98 to 1.15) | 0.16 |
| SDMA, μM | 4.4±1.4 | 4.2±1.3 | −4.0 (−7.4 to −0.7) | 0.02 | −16 | 1.07 (0.98 to 1.17) | 0.14 |
The relative differences for the two treatment groups are calculated as ((high Kt/Vurea group/standard Kt/Vurea group)−1)×100; 95% CIs and P values are calculated using bootstrapping with 2000 replicates. Negative values represent lower concentration in the high Kt/Vurea group. The observed differences in pretreatment solute levels are compared with those predicted by modeling as described in Concise Methods. Hazard ratios for all-cause mortality represent relative hazard of death per 1−SD higher solute concentration. Values were adjusted for age, sex, race, index of coexistent diseases score, diabetes, body mass index (categorized as <18, 18–24, or ≥25 kg/m2), systolic BP (categorized as <130, 130–159, or ≥160 mmHg), residual kidney function (any versus none), serum albumin, serum phosphate, and serum creatinine.
The observed differences in solute concentrations were compared with those modeled assuming that dialysis provided the only mechanism for solute removal and that solute production was the same in the two groups. The observed differences in urea, PAG, and TMAO were very close to the modeled values, and the observed differences in HIPP and IS were only slightly smaller. Modeling suggested, however, that, if solute production were constant and there was no nondialytic clearance, high-dose treatment would have resulted in lower plasma PCS and ADMA levels and higher MG levels than those observed. Because PCS and IS are largely protein bound and HIPP is partially protein bound, the free, unbound levels as well as the total levels of these solutes were also measured. The free solute levels followed the same pattern as total solute levels. Free fractions for PCS and IS in some HEMO samples, however, were greatly in excess of those seen in samples obtained locally, as summarized in Supplemental Table 3.
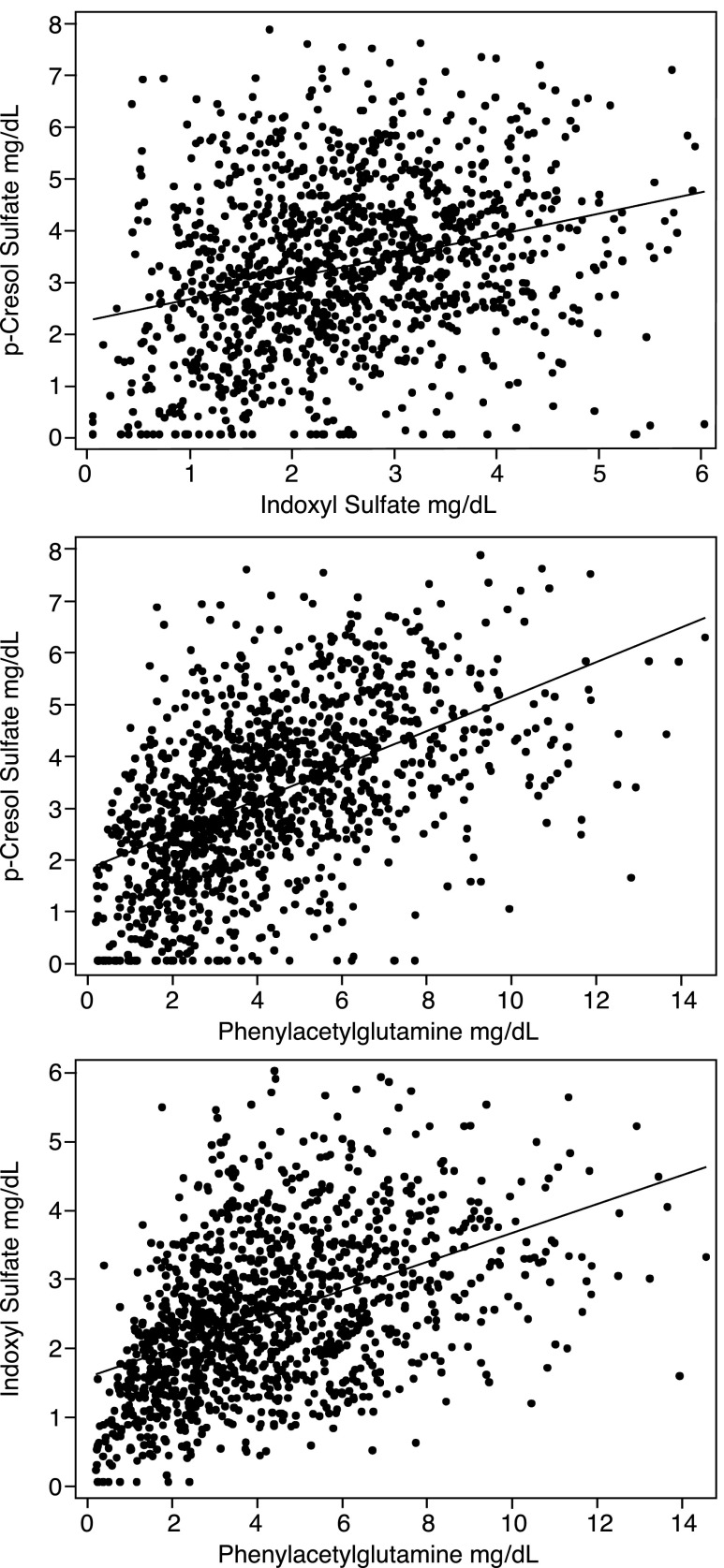
There was no clear association between all-cause mortality and solute levels, as further summarized in Table 2. Pairwise comparison revealed correlations between levels of the individual solutes, which were, in many patients, statistically significant but not strong, as summarized in Table 3 and depicted in Figure 1. Correlations between levels of the uremic solutes, demographic factors, and plasma albumin were generally weak, as summarized in Supplemental Table 4. With the exception of ADMA and SDMA, uremic solute levels were correlated with the normalized protein catabolic rate (nPCR), as summarized in Table 4 and depicted in Figure 2. These correlations, however, were again not strong, and adjustment for nPCR did not significantly alter the effect of difference between the treatment arms, as shown in Supplemental Table 5. With the exception of SDMA, solute levels did not tend to be higher in patients with lower body surface area, as further summarized in Table 4. As expected, high- compared with low-flux treatment had relatively little effect on solute levels, as shown in Supplemental Table 6.
Table 3.
Associations between pretreatment levels of individual solutes
| Solute | UN | PCS | IS | HIPP | PAG | TMAO | MG | ADMA | SDMA |
|---|---|---|---|---|---|---|---|---|---|
| UN | 1 | ||||||||
| PCS | 0.243a | 1 | |||||||
| IS | 0.317a | 0.256a | 1 | ||||||
| HIPP | 0.216a | 0.112a | 0.308a | 1 | |||||
| PAG | 0.307a | 0.542a | 0.451a | 0.344a | 1 | ||||
| TMAO | 0.225a | 0.130a | 0.214a | 0.145a | 0.303a | 1 | |||
| MG | 0.347a | 0.026 | 0.335a | 0.328a | 0.241a | 0.220a | 1 | ||
| ADMA | −0.057b | −0.059b | −0.024 | 0.050 | 0.060 | 0.096c | 0.133a | 1 | |
| SDMA | 0.130a | −0.033 | 0.158a | 0.092b | 0.083 | 0.073b | 0.331a | 0.419a | 1 |
Correlation coefficients between pretreatment solute levels after adjusting for the effect of Kt/Vurea on both solutes (partial correlation coefficients). Subjects with recorded urea reduction ratio >94% (n=17) or <5% (n=2) were excluded, because it was presumed that they represented error in sample collection or labeling. UN, urea nitrogen.
P<0.001.
P<0.05.
P<0.01.
Figure 1.
Correlations among plasma levels of three uremic solutes made by colon microbes from amino acids. Levels of these solutes and several other solutes varied widely among individual patients and were not strongly correlated with each other. The figure depicts solute levels without any adjustment. Correlation coefficients after adjustment for Kt/Vurea are summarized in Table 3. Values outside the 99th percentile were excluded.
Table 4.
Associations of pretreatment solute levels with nPCR and body surface area
| Solute | Association with nPCR | Association with BSA-Dubois | ||
|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | |
| Urea nitrogen | 0.90 (0.88 to 0.92) | <0.001 | 0.05 (−0.01 to 0.11) | 0.13 |
| PCS | 0.18 (0.12 to 0.23) | <0.001 | 0.07 (0.01 to 0.13) | 0.03 |
| IS | 0.24 (0.19 to 0.29) | <0.001 | 0.05 (−0.01 to 0.11) | 0.13 |
| HIPP | 0.16 (0.11 to 0.21) | <0.001 | 0.05 (−0.01 to 0.11) | 0.09 |
| PAG | 0.28 (0.22 to 0.33) | <0.001 | 0.01 (−0.05 to 0.07) | 0.77 |
| TMAO | 0.25 (0.20 to 0.31) | <0.001 | 0.04 (−0.02 to 0.10) | 0.21 |
| MG | 0.27 (0.22 to 0.32) | <0.001 | 0.10 (0.04 to 0.15) | 0.001 |
| ADMA | −0.07 (−0.12 to −0.01) | 0.02 | −0.04 (−0.10 to 0.02) | 0.23 |
| SDMA | 0.06 (<0.01 to 0.11) | 0.04 | −0.14 (−0.20 to −0.08) | <0.001 |
Values were first adjusted for age, sex, race, diabetes, vintage, albumin, and Kt/Vurea group. Solutes, nPCR, and BSA-Dubois are natural log transformed followed by standardization (mean =0 and SD=1); β-coefficient represents change in log–standardized solute concentration per 1−U (=1−SD) increase in log-standardized nPCR or BSA-Dubois. BSA-Dubois, body surface area by the Dubois formula.
Figure 2.
Correlations of plasma levels of two uremic solutes made from amino acids with nPCR. Levels of these solutes and several other solutes increased significantly with increasing protein catabolic rates, but the correlations were not strong, as summarized in Table 4.
Discussion
Kt/Vurea became our standard measure of dialysis adequacy on the basis of the 1985 analysis by Gotch and Sargent8 of the National Cooperative Dialysis Study (NCDS). Gotch and Sargent8 found that Kt/Vurea predicted treatment failure better than BUN and that outcomes were better with Kt/Vurea of ≥0.9 than with lower values. Whether increasing Kt/Vurea in the range >0.9 improved outcomes could not be determined because of the small size of the NCDS. Subsequent observational studies suggested, however, that more dialysis was better, and average Kt/Vurea values gradually increased.
The HEMO Study, conducted between 1995 and 2000, tested whether the move toward higher Kt/Vurea values was justified.3 In the HEMO Study, 1846 patients were randomized to receive standard or high-dose treatment, providing spKt/Vurea values averaging 1.32 and 1.71, respectively. The study used a 2×2 design and also, compared low- and high-flux treatment, providing β2-microglobulin clearances averaging 3 and 34 ml/min. Although a Kt/Vurea value of 1.32 was called standard, the participants’ pre–enrollment Kt/Vurea values averaged 1.6, reflecting the general impression that a higher Kt/Vurea was beneficial. There was widespread disappointment when high-dose treatment failed to improve outcomes.
Several explanations for the failure of the HEMO Study’s high-dose treatment to improve outcomes have been considered.9–11 One is that increasing Kt/Vurea does not sufficiently reduce the plasma levels of toxic solutes. This study assessed the effect of the HEMO Study’s 30% difference in Kt/Vurea on a variety of small solutes. We found that, on average, the HEMO Study’s high-dose treatment reduced the levels of these solutes even less than it reduced the level of urea.
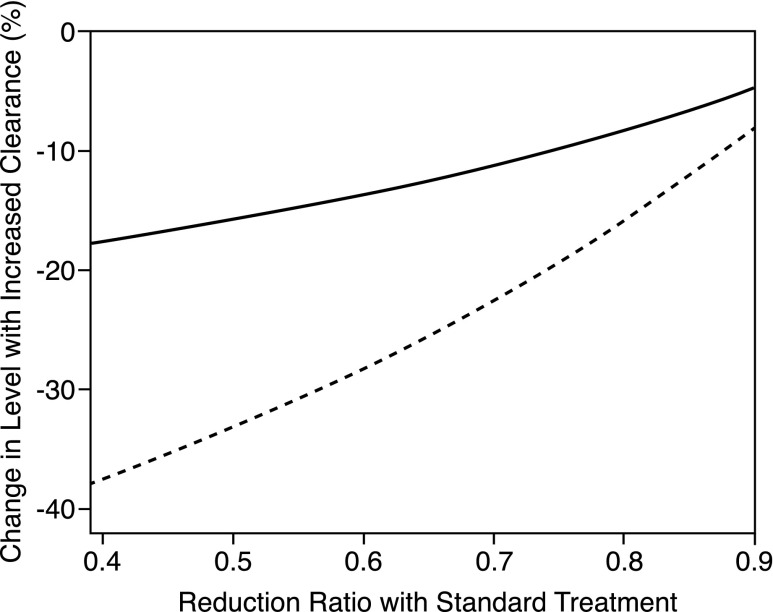
A major factor limiting the extent to which increasing Kt/Vurea can reduce solute levels is the intermittency of treatment. If a solute’s plasma concentration is reduced to a low level at the end of a conventional dialysis session, more aggressive treatment cannot remove much more of that solute unless treatment time is greatly extended. Thus, as illustrated in Figure 3, the solute reduction ratio limits our ability to control solute levels with thrice weekly dialysis. The urea reduction ratio averaged 66% in patients in the HEMO Study receiving standard treatment. The predicted effect of a 30% increase in time-averaged clearance is, therefore, to reduce pretreatment urea levels by only about 10%, close to the result observed in the HEMO Study. Reduction ratios for other solutes were not measured in the HEMO Study, but reduction ratios for HIPP, PAG, and TMAO have been found to be close to or higher than that of urea, as summarized in Supplemental Table 7. The intermittency of treatment can, thus, explain why the HEMO Study’s high Kt/Vurea prescription achieved only small reductions in levels of these solutes. With thrice weekly treatment, even larger increases in clearance and/or time would not greatly reduce their levels, as illustrated in Figure 3 and Supplemental Figure 1.
Figure 3.
Intermittency limits our ability to reduce solute levels by increasing dialytic clearance and/or time. The figure shows the predicted effects on average pretreatment plasma solutes levels of increasing the dialytic clearance to increase Kt/V by 30% (solid line) and 100% (dashed line) plotted as a function of the reduction ratio achieved by standard thrice weekly treatment. Even a very large increase in clearance can effect only a modest reduction in the plasma level when the reduction ratio achieved by standard therapy is high. Values are modeled for a treatment time of 200 minutes and assume no nondialytic clearance and solute distribution in a single compartment. The effect of increasing time rather than clearance shows little difference, whereas the effect on time-averaged levels is slightly larger than the effect on pretreatment levels, as illustrated in Supplemental Figure 1.
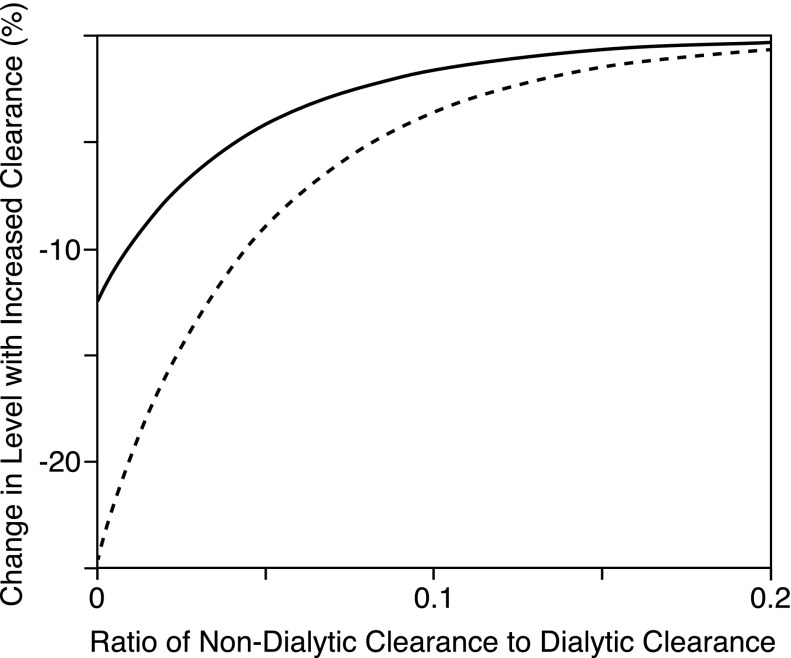
Intermittency cannot explain the failure of high-dose treatment to reduce levels of solutes, like ADMA and PCS, which have lower reduction ratios than urea. Two other factors may limit our ability to reduce plasma levels of such solutes. The first is a nonrenal clearance mechanism that continues to operate when the kidneys fail. The second is a change in solute production. Measurement of labeled solute clearances would be required to distinguish these possibilities. We suspect, however, that the presence of nonrenal clearance explains the failure of the HEMO Study’s high Kt/Vurea prescription to reduce ADMA levels. ADMA may be cleared outside the kidney by more than one mechanism.12–14 Also, the nonrenal clearance of ADMA in humans may be a considerable portion of that provided by hemodialysis.12,13 This nonrenal clearance could account for the observation that plasma ADMA levels rise to only about twice the normal level in patients with advanced renal insufficiency who are not yet on dialysis as well as the finding that more aggressive dialysis did not lower ADMA levels in patients in the HEMO Study.13,15 The presence of nonrenal clearance may also account for the less than predicted reduction of SDMA levels in the HEMO Study’s high Kt/Vurea group. Nonrenal clearance of SDMA, although difficult to quantify, has been shown.13,16 As illustrated in Figure 4, a nonrenal clearance equal to only a fraction of the dialytic clearance will limit our ability to reduce plasma solute levels by increasing the intensity of dialysis.
Figure 4.
The presence of a nondialytic clearance limits our ability to reduce solute levels by increasing dialytic clearance and/or time. The figure shows the predicted effects on pretreatment plasma solutes levels of increasing the dialytic clearance to increase Kt/V by 30% (solid line) and 100% (dashed line) plotted as a function of the ratio of the nondialytic clearance to the dialytic clearance. A nondialytic clearance, which is only a small fraction of the dialytic clearance, operating continually blunts the effect of increasing the dialytic clearance. Values are modeled for a treatment time of 200 minutes and an initial Kt/V of 1.3. The effect of increasing time rather than clearance shows little difference, and the effect on time-averaged levels is slightly larger than the effect on pretreatment levels, as illustrated in Supplemental Figure 2.
For PCS, we suspect that the failure of the HEMO Study’s high Kt/Vurea prescription to lower plasma levels reflected a change in solute production rather than the presence of nonrenal clearance. Previous studies suggest that plasma PCS does not rise to high levels in patients on peritoneal dialysis, because low dialytic clearance is associated with low PCS production.17 Increased PCS production could similarly explain our finding that PCS levels were no lower in patients on high-dose treatment than in patients on standard treatment in the HEMO Study. If the production of PCS was the same in the two groups, we could otherwise explain the similarity of their plasma PCS levels only by the presence of a nonrenal PCS clearance greater than the time–averaged dialytic clearance. Also, the finding in the work by Poesen et al.18 that urinary PCS excretion remains stable as eGFR declines in CKD is not consistent with a nonrenal PCS clearance of this magnitude.
Among the solutes that we studied, only MG exhibited a greater than predicted reduction in plasma levels with high Kt/Vurea treatment. Like the failure of high Kt/Vurea treatment to reduce PCS levels, this discrepancy can be explained by a change in solute production. MG is made, at least in part, from creatinine, and the combination of increased production and reduced clearance causes plasma MG levels to rise out of proportion to the decline in GFR in kidney disease.19–21 We presume that the HEMO Study’s high Kt/Vurea treatment reduced creatinine levels and therefore, reduced MG production, so that plasma MG levels fell more than was predicted assuming equal production in the high and low Kt/Vurea groups.
Higher Kt/Vurea values in the HEMO Study were achieved largely by higher blood flow and longer treatment time, as summarized in Table 1. Theoretically, increases in blood flow and treatment time can have differing effects on solute levels. We did not, however, detect separate effects of blood flow and treatment time on solute levels, as summarized in Supplemental Table 8. This is not surprising given that adjustments in these parameters were not randomized and that the values used varied over a limited range. Maximizing dialyzer size and dialysate flow, which might have increased protein–bound solute clearances in the HEMO Study’s high-dose group, was not attempted.22
A notable finding of this study was that achieved Kt/Vurea accounted for remarkably little of the variation in nonurea solute levels. This finding, as depicted in Figure 5, is in accord with prior cross–sectional analyses.23 Also, we believe it should encourage additional review of the concept that dialysis adequacy can be assessed by an index only on the basis of urea.5,24 With the exception of ADMA and SDMA, levels of the solutes that we measured varied more widely than those of urea. Despite the availability of a large number of measurements, however, we were unable to distinguish factors that accounted for much of this variation. Levels of all of the solutes, except ADMA and SDMA, were correlated, albeit not strongly, with nPCR. The association of PCS, IS, and PAG with nPCR can be explained by colon microbial production of these solutes from amino acids, which escape digestion in the small intestine. Also, the association of TMAO with nPCR may likewise be explained by colon microbial metabolism of nutrients containing a trimethylamine moiety that are found in protein–rich animal foods.25 We also observed correlations among levels of many individual solutes. However, even among the solutes known to be made by colon microbes from amino acids, the strength of these correlations was modest, which has previously been reported for PCS and IS.26 Underlying differences in the production of microbially derived compounds may reflect differences in the composition of the microbiome.27–29 We found that PCS levels were higher in patients with diabetes as previously reported.30 This difference and others in solute levels among patients with diabetes could likewise be related to differences in diet and/or microbial metabolism.
Figure 5.
Pretreatment plasma solute levels plotted against measured Kt/Vurea in individual patients. Patients receiving standard treatment are represented by blue circles, and those receiving high-dose treatment are represented by red circles. Differences in Kt/Vurea accounted for very little of the wide variation in solute levels.
There was a trend in the HEMO Study for women to benefit from high-dose treatment.31,32 This finding could be explained by the assumption that waste solute production is more nearly proportional to body surface area than body weight. Women, in whom body surface area is, on average, greater relative to volume than in men, might then be expected to have higher solute levels with treatment providing any given Kt/Vurea.32–34 We did not, however, detect a sex effect on solute levels in this study. Also, with the exception of SDMA, levels of the solutes that we studied were not inversely related to body surface area.
We believe that these findings can help to explain the failure of a high Kt/Vurea to provide clinical benefit. ADMA and PCS are two of the solutes that have most frequently been associated with cardiovascular disease and mortality in patients on dialysis.13,30,35–37 The failure of high Kt/Vurea treatment to reduce levels of these solutes is, thus, particularly interesting. We think that the failure of a high Kt/Vurea to achieve larger reductions in the levels of other solutes may also have clinical relevance. With the exception of ADMA and SDMA, levels of these solutes are elevated out of proportion to that of urea in patients on dialysis. Similar elevations in concentration have also been reported for many other uremic solutes.6,38 Also, if such solutes are toxic, it is perhaps not surprising that slight reductions in levels that are so far above normal should fail to have a notable salutary effect.
A limitation of this study is that levels of only eight uremic solutes were assessed. PCS, IS, TMAO, and ADMA were studied, because they have been associated with ill effects, and others were included to extend the variety of kinetic behaviors examined.7,13,36,39 We think it likely, however, that the HEMO Study’s increase in Kt/Vurea caused similarly small reductions in the levels of many other solutes. Other limitations should be acknowledged. Associations among solute levels and clinical parameters may be obscured by variation in solute levels over time, which has not been assessed for the solutes that we measured. Measurement error in stored samples may also have impaired our ability to detect correlations. Remeasurement of urea values in a subset of samples as described in Concise Methods provided only a limited index of the magnitude of such error. We used a single-compartment model to compare observed differences in solute levels with those that might have been achieved if there was no nondialytic clearance and if solute production was the same in the two groups. Assuming multiple compartments, however, would not notably alter the magnitude of predicted differences between the groups, as further described in Concise Methods.
In conclusion, we found that the HEMO Study’s large increase in Kt/Vurea failed to greatly reduce the levels of a variety of low molecular weight uremic solutes. Failure to achieve greater reduction in solute levels can be accounted for by not only the intermittency of treatment but also, increases in solute production and/or the presence of nondialytic clearance. Much longer and/or more frequent treatment than was prescribed in the HEMO Study would be required to achieve greater reductions in solute levels using conventional dialysis techniques. Better knowledge of solute toxicity is required to refine our index of treatment adequacy. Such knowledge could allow us to lower the levels of toxic solutes by increasing their clearance or reducing their production without large increases in weekly treatment time.
Concise Methods
HEMO Study Samples
Stored samples of pretreatment plasma obtained during the first year after randomization were available for 1281 subject through the National Institutes of Health repository. The days of the dialysis cycle and the intervals after randomization at which samples were collected are summarized in Supplemental Table 9.
Assay Methods
Concentrations of PCS, IS, HIPP, and PAG were assayed by stable isotope dilution liquid chromatography/mass spectrometry (MS)/MS using a modification of a previously described method.40 Total plasma concentrations were measured after deproteination with methanol. Initial measurements were made after another 10-fold dilution: samples in which total HIPP and PAG fell below the assay range were reassayed at a lesser dilution. Recoveries averaged 100%±5% for PCS, 107%±6% for IS, 98%±13% for HIPP, and 96%±8% for PAG. The coefficients of variation for quality control (QC) samples run with each assay were 3% for PCS, 9% for IS, 6% for HIPP, and 6% for PAG. Free plasma concentrations of PCS, IS, and HIPP were measured in ultrafiltrate prepared using Nanosep 30K Omega Separators (Pall, Ann Arbor, MI) and analyzed at a fivefold dilution. Assays were the same as for total solute determinations, except that indoxyl-3a,4,5,6,7,7a-13C6-sulfate was used as the internal standard for IS. Recoveries averaged 99%±7% for PCS, 107%±6% for IS, 112%±6% for HIPP, and 106%±7% for PAG. The coefficients of variation for QC samples run with each assay were 6% for PCS, 5% for IS, 5% for HIPP, and 5% for PAG.
Concentrations of TMAO, ADMA, SDMA, and MG were also determined by liquid chromatography/MS/MS. TMAO-d9 (Cambridge Isotope Laboratories, Andover, MA), ADMA-d7 (Cambridge Isotope Laboratories), SDMA-d6 (Toronto Research Chemicals, North York, ON, Canada), and MG-d3 (Toronto Research Chemicals) were used as internal standards. Plasma was deproteinized by mixture with an internal standard solution and methanol (2:1:20 vol:vol:vol). Five microliters each sample supernatant was injected in a Shimadzu Prominence LC-20A System (Shimadzu, Tokyo, Japan), and analytes were separated on a silica column (150×2.1 mm2; 3-μm Luna silica; Phenomenex, Torrance, CA) at room temperature. The mobile phase was 90% methanol containing 10 mM ammonium formate and 0.2% (vol/vol) formic acid at a flow rate of 0.2 ml/min. MS was performed on an API 4000 Triple Quadrupole Mass Spectrometer (AB Sciex, Framingham, MA) with electrospray ionization in the positive mode. Ion transitions used for quantitation were mass to charge (m/z) 76→58 for TMAO, m/z 203→70 for ADMA, m/z 203→70 for SDMA, and m/z 74→ 57 for MG, with corresponding transitions for the internal standards. Recoveries averaged 113%±6% for TMAO, 102%±8% for ADMA, 115%±6% for SDMA, and 107%±5% for MG. The coefficients of variation for QC samples run with each assay were 5% for TMAO, 1% for ADMA, 5% for SDMA, and 4% for MG. Stored samples from a local study of dialysis kinetics by Sirich et al.7 were reanalyzed to estimate values for the KoA and the volume of distribution (Vd) for these solutes. When measured values fell to <80% of the lowest standard, a value halfway between zero and the low end of the standard curve was imputed for all solutes. Values for the free fraction were not calculated when values for total or free concentrations were imputed. To provide some index of the error caused by solute measurement in stored samples, urea values were remeasured in the local clinical laboratory in a subset of 68 samples, which were chosen randomly, except that volume was insufficient in five of the 73 samples originally selected. Results, as depicted in Supplemental Figure 3, showed overall good agreement with the original values reported in the HEMO Study, except that one of the stored pretreatment samples appeared to have been a mislabeled post–treatment sample on the basis of comparison with values in the HEMO Study database.
Modeling
The predicted effect of high-dose versus standard treatment on nonurea solute levels was modeled on the basis of standard analyses of solute kinetics during dialysis treatment.41 An MATLAB (R2014; Mathworks, Natick, MA) routine generated solute concentration profiles and pretreatment values on each of the three treatment days from values for treatment time, dialytic clearance, Vd, and an assumed constant generation rate. The modeled pretreatment values were appropriately weighted for the days in the cycle on which the repository values had been collected. Values for dialytic clearances of the bound solutes were calculated from values for blood flow, dialysate flow, hematocrit, and KoA using a previously described model.42 Input values in these calculations were the average values of blood flow, dialysate flow, and treatment time provided in Table 1 along with hematocrit and estimated values for KoA, the median observed free fraction, and Vd for each solute. Average KoAurea values were obtained from values calculated for the dialyzer types used in the HEMO Study on the basis of crossdialyzer urea clearance measurements made at 4 months as described by Depner et al.43 For this analysis, KoAurea values were adjusted to yield values that would be obtained if blood water flow and not total blood flow was entered into the clearance equation by Michaels.44 KoA values for PCS, HIPP, and IS were then calculated as a fraction of KoAurea on the basis of previous in vitro studies.22 Values for the clearances of the unbound solutes were estimated using the ratio of their clearances to urea clearances observed in patients studied locally by Sirich et al.7
Modeling was initially performed using a single compartment with volume estimated by dividing the amount of solute removed by the difference in pretreatment and post–treatment solute concentrations in patients studied locally (Supplemental Table 7) and assuming that Vd had the same proportion to body weight in patients in the HEMO Study. Additional analysis was performed to assess the effect of assuming solute distribution in two compartments as described by Eloot et al.21 Use of values for compartment volumes and intercompartmental clearance for MG adapted from the work by Eloot et al.21 resulted in only a small change in the predicted reduction in average pretreatment MG level in the high-dose compared with the standard Kt/Vurea group as shown in Supplemental Table 10. Intercompartmental clearance values along with multicompartment volumes for the other solutes have not been published. We found, however, that assumption of various compartment volumes and intercompartmental clearances also had little effect on the predicted reduction in average pretreatment levels for PCS (Supplemental Table 10) and other solutes (not shown). Urea was modeled using a standard two–compartment model.41,42
Statistical Analyses
Values for spKt/Vurea were used in describing group means and relating solute levels to Kt/Vurea throughout. The relative difference between high and standard Kt/Vurea was calculated as ((high Kt/Vurea group/standard Kt/Vurea group)−1)×100. Negative values imply lower concentration in the high Kt/Vurea group; 95% CIs for the differences were calculated using bootstrapping with 2000 replicates. Correlation between solute pairs, each adjusted for Kt/Vurea, was calculated using partial correlation coefficients. To relate changes in solute concentration with changes in predictors (age, sex, race, diabetes, vintage, albumin, nPCR, and body surface area), the solutes (and selected continuous predictors) were standardized (mean =0 and SD=1) after natural log transformation. This modeling strategy equalizes the variance, allowing the magnitude of β-coefficients to be compared across solutes. The association between solutes and predictors was also analyzed visually by scatterplots of solutes versus predictors. Baseline characteristics of the study participants were compared across the dialysis dose groups using proportion ratio tests for categorical variables and t or rank sum tests for continuous variables. Variable distributions for continuous variables were assessed visually by histograms and Shapiro–Wilk tests.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was part of the Retained Organic Solutes and Clinical Outcomes in Hemodialysis Study supported by National Institutes of Health (NIH) grant R01DK80123. T.L.S. was supported by a career development award of the Research Service of the Veterans Administration, and T.S. was supported by NIH grant K23DK083514.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “HEMO Revisited: Why Kt/Vurea Only Tells Part of the Story,” on pages 3235–3237.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015091035/-/DCSupplemental.
References
- 1.Hemodialysis Adequacy 2006 Work Group : Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48[Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Port FK, Ashby VB, Dhingra RK, Roys EC, Wolfe RA: Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol 13: 1061–1066, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Rocco MV, Dwyer JT, Larive B, Greene T, Cockram DB, Chumlea WC, Kusek JW, Leung J, Burrowes JD, McLeroy SL, Poole D, Uhlin L HEMO Study Group : The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2321–2334, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Meyer TW, Sirich TL, Hostetter TH: Dialysis cannot be dosed. Semin Dial 24: 471–479, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A European Uremic Toxin Work Group : Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sirich TL, Funk BA, Plummer NS, Hostetter TH, Meyer TW: Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol 25: 615–622, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotch FA, Sargent JA: A mechanistic analysis of the National Cooperative Dialysis Study (NCDS). Kidney Int 28: 526–534, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Himmelfarb J: The HEMO study—where do we go from here? Curr Opin Nephrol Hypertens 12: 587–591, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Levin N, Greenwood R: Reflections on the HEMO study: The American viewpoint. Nephrol Dial Transplant 18: 1059–1060, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Locatelli F: Dose of dialysis, convection and haemodialysis patients outcome--what the HEMO study doesn’t tell us: The European viewpoint. Nephrol Dial Transplant 18: 1061–1065, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Ronden RA, Houben AJ, Teerlink T, Bakker JA, Bierau J, Stehouwer CD, De Leeuw PW, Kroon AA: Reduced renal plasma clearance does not explain increased plasma asymmetric dimethylarginine in hypertensive subjects with mild to moderate renal insufficiency. Am J Physiol Renal Physiol 303: F149–F156, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Schepers E, Speer T, Bode-Böger SM, Fliser D, Kielstein JT: Dimethylarginines ADMA and SDMA: The real water-soluble small toxins? Semin Nephrol 34: 97–105, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Rodionov RN, Martens-Lobenhoffer J, Brilloff S, Burdin DV, Jarzebska N, Demyanov AV, Hohenstein B, Weiss N, Bode-Böger SM: Acetylation of asymmetric and symmetric dimethylarginine: An undercharacterized pathway of metabolism of endogenous methylarginines. Nephrol Dial Transplant 31: 57–63, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Schepers E, Barreto DV, Liabeuf S, Glorieux G, Eloot S, Barreto FC, Massy Z, Vanholder R European Uremic Toxin Work Group (EUTox) : Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clin J Am Soc Nephrol 6: 2374–2383, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siroen MP, van der Sijp JR, Teerlink T, van Schaik C, Nijveldt RJ, van Leeuwen PA: The human liver clears both asymmetric and symmetric dimethylarginine. Hepatology 41: 559–565, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Pham NM, Recht NS, Hostetter TH, Meyer TW: Removal of the protein-bound solutes indican and p-cresol sulfate by peritoneal dialysis. Clin J Am Soc Nephrol 3: 85–90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poesen R, Viaene L, Verbeke K, Claes K, Bammens B, Sprangers B, Naesens M, Vanrenterghem Y, Kuypers D, Evenepoel P, Meijers B: Renal clearance and intestinal generation of p-cresyl sulfate and indoxyl sulfate in CKD. Clin J Am Soc Nephrol 8: 1508–1514, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonella M, Barsotti G, Lupetti S, Giovannetti S: Factors affecting the metabolic production of methylguanidine. Clin Sci Mol Med 48: 341–347, 1975 [DOI] [PubMed] [Google Scholar]
- 20.Marescau B, Nagels G, Possemiers I, De Broe ME, Becaus I, Billiouw JM, Lornoy W, De Deyn PP: Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism 46: 1024–1031, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Eloot S, Torremans A, De Smet R, Marescau B, De Wachter D, De Deyn PP, Lameire N, Verdonck P, Vanholder R: Kinetic behavior of urea is different from that of other water-soluble compounds: The case of the guanidino compounds. Kidney Int 67: 1566–1575, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Luo FJ, Patel KP, Marquez IO, Plummer NS, Hostetter TH, Meyer TW: Effect of increasing dialyzer mass transfer area coefficient and dialysate flow on clearance of protein-bound solutes: A pilot crossover trial. Am J Kidney Dis 53: 1042–1049, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eloot S, Van Biesen W, Glorieux G, Neirynck N, Dhondt A, Vanholder R: Does the adequacy parameter Kt/V(urea) reflect uremic toxin concentrations in hemodialysis patients? PLoS One 8: e76838, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanholder R, Glorieux G, Eloot S: Once upon a time in dialysis: The last days of Kt/V? Kidney Int 88: 460–465, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Hazen SL, Brown JM: Eggs as a dietary source for gut microbial production of trimethylamine-N-oxide. Am J Clin Nutr 100: 741–743, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijers BK, De Loor H, Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P: p-Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin J Am Soc Nephrol 4: 1932–1938, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer TW, Hostetter TH: Uremic solutes from colon microbes. Kidney Int 81: 949–954, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Vaziri ND, Zhao YY, Pahl MV: Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment [published online ahead of print April 16, 2015]. Nephrol Dial Transplant doi: 10.1093/ndt/gfv095 [DOI] [PubMed]
- 29.Donia MS, Fischbach MA: Human microbiota. Small molecules from the human microbiota. Science 349: 1254766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P: Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73: 1174–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Depner T, Daugirdas J, Greene T, Allon M, Beck G, Chumlea C, Delmez J, Gotch F, Kusek J, Levin N, Macon E, Milford E, Owen W, Star R, Toto R, Eknoyan G Hemodialysis Study Group : Dialysis dose and the effect of gender and body size on outcome in the HEMO Study. Kidney Int 65: 1386–1394, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Daugirdas JT, Depner TA, Greene T, Kuhlmann MK, Levin NW, Chertow GM, Rocco MV: Surface-area-normalized Kt/V: A method of rescaling dialysis dose to body surface area-implications for different-size patients by gender. Semin Dial 21: 415–421, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar SR, Kuhlmann MK, Kotanko P, Zhu F, Heymsfield SB, Wang J, Meisels IS, Gotch FA, Kaysen GA, Levin NW: Metabolic consequences of body size and body composition in hemodialysis patients. Kidney Int 70: 1832–1839, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Spalding EM, Chandna SM, Davenport A, Farrington K: Kt/V underestimates the hemodialysis dose in women and small men. Kidney Int 74: 348–355, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Tripepi G, Mattace Raso F, Sijbrands E, Seck MS, Maas R, Boger R, Witteman J, Rapisarda F, Malatino L, Mallamaci F, Zoccali C: Inflammation and asymmetric dimethylarginine for predicting death and cardiovascular events in ESRD patients. Clin J Am Soc Nephrol 6: 1714–1721, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G: The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J Am Soc Nephrol 25: 1897–1907, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CJ, Wu V, Wu PC, Wu CJ: Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One 10: e0132589, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL: Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116: 448–455, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW: Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 84: 585–590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Depner TA: Prescribing Hemodialysis: A Guide to Urea Modeling, Norwall, MA, Kluwer Academic Publishers, 1991 [Google Scholar]
- 42.Walther JL, Bartlett DW, Chew W, Robertson CR, Hostetter TH, Meyer TW: Downloadable computer models for renal replacement therapy. Kidney Int 69: 1056–1063, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Depner TA, Greene T, Daugirdas JT, Cheung AK, Gotch FA, Leypoldt JK: Dialyzer performance in the HEMO Study: In vivo K0A and true blood flow determined from a model of cross-dialyzer urea extraction. ASAIO J 50: 85–93, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Michaels AS: Operating parameters and performance criteria for hemodialyzers and other membrane-separation devices. Trans Am Soc Artif Intern Organs 12: 387–392, 1966 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.