Abstract
Erythropoietin (EPO) may be a beneficial tissue–protective cytokine. However, high doses of EPO are associate with adverse effects, including thrombosis, tumor growth, and hypertension. Carbamylated erythropoietin (CEPO) lacks both erythropoietic and vasoconstrictive actions. In this study, we compared the renoprotective, hemodynamic, and hematologic activities and survival effects of identical EPO and CEPO doses in rat models of clinically relevant AKI presentations, including ischemia-reperfusion–induced AKI superimposed on CKD (5000 U/kg EPO or CEPO; three subcutaneous injections) and ischemia-reperfusion–induced AKI in old versus young animals and male versus female animals (1000 U/kg EPO or CEPO; three subcutaneous injections). Compared with EPO therapy, CEPO therapy induced greater improvements in renal function and body weight in AKI on CKD animals, with smaller increases in hematocrit levels and similarly improved survival. Compared with EPO therapy in the other AKI groups, CEPO therapy induced greater improvements in protection and recovery of renal function and survival, with smaller increases in systolic BP and hematocrit levels. Overall, old or male animals had more severe loss in kidney function and higher mortality rates than young or female animals, respectively. Notably, mRNA and protein expression analyses confirmed the renal expression of the heterodimeric EPO receptor/CD131 complex, which is required for the tissue-protective effects of CEPO signaling. In conclusion, CEPO improves renal function, body and kidney weight, and survival in AKI models without raising hematocrit levels and BP as substantially as EPO. Thus, CEPO therapy may be superior to EPO in improving outcomes in common forms of clinical AKI.
Keywords: acute renal failure, chronic kidney disease, erythropoietin
AKI is a clinical problem with rising incidence over the last decade,1 and it is associated with high morbidity and mortality and represents an independent adverse prognostic variable in hospitalized patients.2 Even mild degrees of kidney dysfunction have a negative effect on outcome in various diseases.3 Temporary underperfusion of the kidney during different conditions followed by reperfusion, termed ischemia-reperfusion (I/R), represents the most common cause of AKI. I/R injury affects mainly the highly susceptible S3 segment of the corticomedullary junction, a nephron site where oxygen demand and delivery are just balanced under steady-state conditions and where a small decline in oxygen pressure can have serious consequences. Ischemic injury to the kidney triggers a cascade of events, including endothelial dysfunction,4 tubular cell damage and inflammation with consecutive loss of cell polarity,5 and apoptosis,6 together leading to a reduction of renal blood flow and GFR.
Erythropoietin (EPO), a 34-kD glycoprotein that is produced by fibroblast-like cells in the renal interstitium in response to hypoxia and anemia, was discovered by Carnot and Deflandre.7 Low–dose recombinant human EPO is indicated for the treatment of anemia in patients and can potentially slow the progression of CKD.8,9 EPO mainly acts on erythroid progenitor cells, but now, it has been recognized as having antiapoptotic, mitogenic, motogenic, and differentiation-inducing effects in several other cell types.10–12
We showed previously that authentic erythropoietin receptors (EPORs) are expressed on renal cells,13,14 as have others,15 and high-dose EPO has been shown to be renoprotective after either ischemic16 or cisplatin–induced renal injury17 in animal models. The affinity of the EPOR to EPO in kidney cells (Kd of approximately 1 nM)13 is much lower than normal concentrations of plasma EPO (1–10 pM), suggesting that high levels of EPO may be required to be renoprotective. However, the administration of high-dose EPO is associated with adverse effects, including renal fibrosis,18 hypertension,19 and erythrocytosis with subsequent hyperviscosity and thrombosis,20–23 thus potentially limiting the utility of EPO for renoprotection.
Carbamylated erythropoietin (CEPO) is an EPO derivative that lacks both erythropoietic and vasoconstrictive actions, because it does not activate the homodimeric EPOR but rather, signals through the heterodimeric EPO Common β–Receptor (CD131), also shared by GM-CSF, IL-3, and IL-5.24,25 Activation of this heteroreceptor, composed of EPOR and CD131, is cardio- and neuroprotective, in part mediated by anticoagulant and anti-inflammatory responses,26 and it ameliorates metabolic stress induced by hypoxia.27 In a rat model of I/R AKI, pretreatment with low-dose CEPO (100 U/kg) for 2 weeks was shown to be more effective than EPO at preserving and improving renal function, reducing apoptosis, increasing cellular proliferation, and increasing capillary formation without increasing hemoglobin concentrations like EPO did.28,29 High doses of CEPO (1000 U/kg) also prevented experimental cyclosporin nephrotoxicity30 and improved renal function in an unilateral ureteral obstruction model31 without increasing hemoglobin levels. Long–term CEPO treatment (1000 U/kg for 8 weeks) reduced fibrosis in a remnant kidney model without increasing hemoglobin levels.32
Although informative and important, the ability of animal models to predict outcomes in human clinical trials is limited.33 One reason may be that the animal models used do not adequately represent the comorbidity profiles of patients at risk for AKI and CKD. For example, the majority of animal research is carried out in young, healthy animals. In contrast, the patient populations that are most at risk for AKI are the elderly, who frequently have other comorbidities, such as underlying CKD, diabetes, congestive heart failure, and others.34–36 This discrepancy between preclinical and clinical outcomes has been observed in several major clinical trials, in which the renoprotective effects of IGF-1,37 Atrial Natriuretic Peptide,38 or EPO39 were compelling in preclinical studies, whereas they were absent in patients with AKI and common comorbidities. The objective of this study was to compare the renoprotective, hemodynamic, and hematologic activities and survival effects of CEPO and EPO in rats with various scenarios of experimental I/R AKI, including AKI superimposed on CKD, AKI in old rats (comorbidity models), and AKI in female and male rats. In these studies, CEPO improved renal function, body and kidney weights, and survival without raising the hematocrits and BPs, indicating that CEPO therapy may prove superior to EPO in improving outcomes in common forms of clinical AKI.
Results
EPO or CEPO Treatment of Sprague–Dawley Rats with AKI Superimposed on CKD
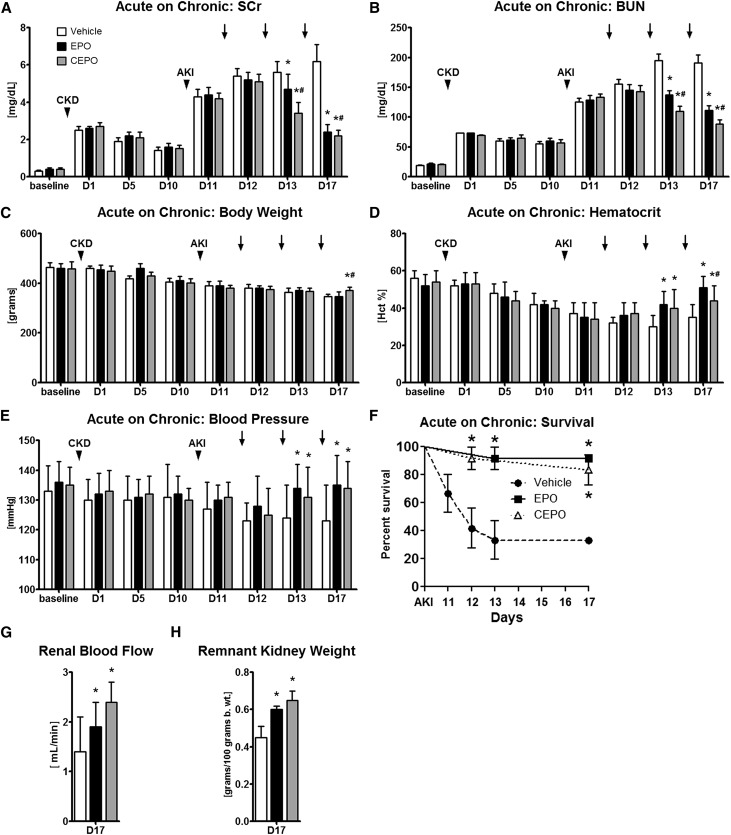
To study an animal model that represents a patient population in whom I/R AKI is superimposed on CKD, which is a particularly high–risk factor for AKI, total nephron numbers were reduced in male Sprague–Dawley rats (weight of 460±30 g; n=12 per group) by right nephrectomy and ligation of two to three branches of the left renal artery.40 This led to an increase in serum creatinine (SCr) levels by about sevenfold and their subsequent stabilization over the next 10 days at approximately fourfold above baseline (Figure 1A) and an initial increase in BUN of about 3.5-fold and stabilization after 10 days at 2.8-fold above baseline (Figure 1B). Animal weight steadily decreased for 10 days after nephron reduction surgery (Figure 1C), hematocrit also dropped (Figure 1D), and the systolic BP did not change significantly for 10 days after the CKD surgery (Figure 1E).
Figure 1.
CEPO is more effective than EPO in improving renal function after AKI superimposed on CKD. CKD was induced in male Sprague–Dawley rats by right nephrectomy and ligation of two to three branches of the left renal artery. This led to an increase in (A) SCr and (B) BUN and a decrease in (C) animal weight and (D) hematocrit (Hct). (E) Systolic BP did not change over the first 10 days. Ten days after selective renal artery branch ligation, AKI was induced by clamping of the remaining renal pedicle (35 minutes), and animals were treated 1 day later (vehicle, 5000 U/kg EPO sc, or 5000 U/kg CEPO sc) on days 11–13 after CKD. Both EPO and CEPO treatments led to lower (A) SCr and (B) BUN levels compared with vehicle-treated animals, with CEPO-treated animals showing (A and B) small but significantly greater improvements in kidney function than the EPO-treated groups and (C) better preserved body weights. (D) EPO treatment resulted in significantly higher Hct levels than in vehicle or CEPO-treated groups, whereas CEPO therapy resulted only in a moderate rise in Hct. (E) Systolic BP levels and (F) 7-day survival were higher in both CEPO and EPO groups compared with those in the vehicle-treated group. Compared with controls, both EPO- and CEPO-treated animals had (G) improved total renal blood flows on day 17 as well as (H) higher remnant kidney weights. Black bars indicate EPO treatment, gray bars indicate CEPO treatment, and white bars indicate vehicle treatment. Black arrowheads indicate CKD or AKI surgery as labeled. Black arrows indicate treatments on days 1–3. *P<0.05 compared with the vehicle control; #P<0.05 for CEPO compared with EPO.
On day 10 after CKD, the remaining renal pedicle was clamped for 35 minutes, inducing severe AKI with SCr levels at approximately 4.5 mg/dl (Figure 1A) and BUN levels at approximately 128 mg/dl (Figure 1B). It was then tested whether high doses of EPO or CEPO (5000 U/kg subcutaneously [sc]; n=12 per group) given 1 day after AKI on days 11–13 post-CKD were renoprotective. Both treatment groups were compared with vehicle-treated controls (n=12). Both EPO and CEPO treatments led to enhanced functional recovery of the ischemic kidney compared with that in vehicle-treated animals, with CEPO-treated animals showing small but significantly greater improvements in kidney function than that in EPO-treated groups (Figure 1, A and B) and better preserved body weights (Figure 1C). EPO treatment resulted in significantly higher hematocrit levels than those in vehicle or CEPO-treated groups, whereas CEPO therapy resulted only in a moderate rise in hematocrit (Figure 1D). Systolic BP levels (Figure 1E) and survival (Figure 1F) were higher in both CEPO and EPO groups compared with those in the vehicle-treated group.
Compared with controls, both EPO- and CEPO-treated animals had improved kidney blood flows on day 17 as well as higher remnant kidney weights (Figure 1, G and H).
EPO or CEPO Treatment of AKI in Young and Old Fischer (F344) Rats
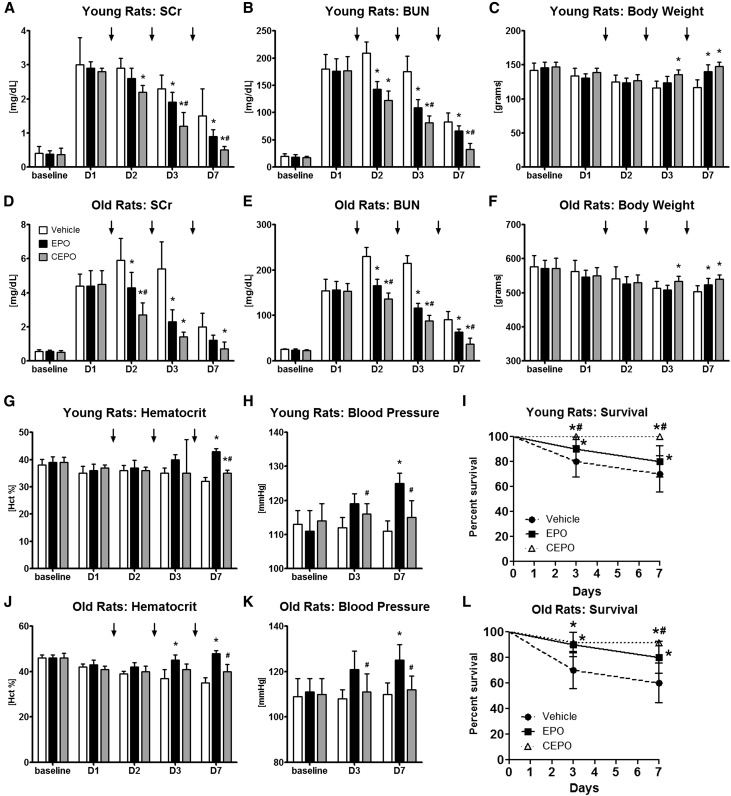
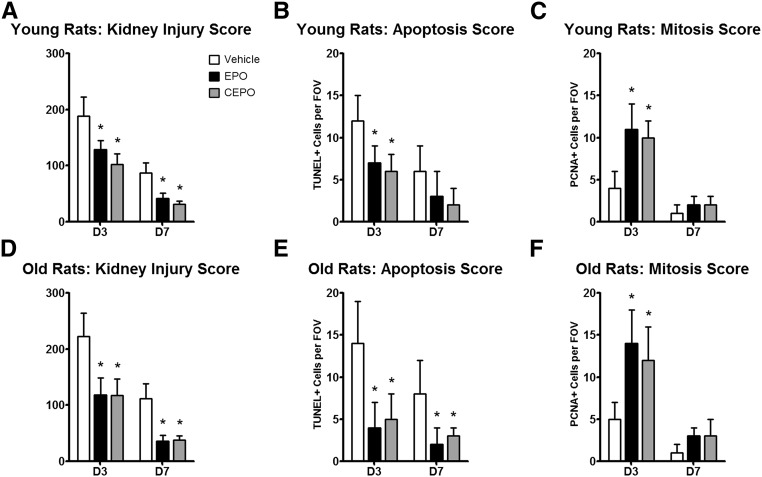
The objective in this group of experiments was to test whether equivalent doses of EPO (1000 U/kg sc; n=10) or CEPO (1000 U/kg sc; n=12) given on days 1–3 after the induction of I/R AKI in young (weight of 145±10 g; 10 weeks old) versus old (weight of 572±35 g; 80 weeks old) male F344 rats resulted in different outcomes between groups and compared with vehicle-treated controls (n=10). In young animals, I/R AKI (60 minutes clamping of both renal pedicles) induced moderate AKI with SCr values between 2.5 and 3.25 mg/dl on day 1 (Figure 2A) and an approximately ninefold increase in BUN (Figure 2B). CEPO treatment significantly improved recovery of renal function and improved recovery more effectively than EPO (Figure 2, A and B). On day 7, body weights of both treatment groups were significantly higher than those in controls (Figure 2C). EPO treatment resulted in significantly higher hematocrit and systolic BP levels compared with those in vehicle control and CEPO groups (Figure 2, G and H). Survival in CEPO–treated young rats was significantly improved compared with that in vehicle or EPO-treated animals (Figure 2I). Because older animals are more susceptible to ischemic injury, a reduced bilateral clamping time of 38 minutes was used to induce severe I/R AKI in old F344 male rats (weight of >550 g) with SCr values between 4 and 5 mg/dl on day 1 (Figure 2D). CEPO treatment given on days 1–3 after AKI significantly improved recovery of renal function compared with that of vehicle or EPO-treated animals (Figure 2, D and E). On day 7, body weights of both treatment groups were significantly higher than those in controls (Figure 2F). EPO treatment resulted in significantly higher hematocrit and systolic BP levels compared with those of the vehicle control or CEPO group (Figure 2, J and K). CEPO as well as EPO improved survival after AKI compared with that in vehicle-treated controls (Figure 2L). To further examine the effects of EPO and CEPO on renal tissue, histologic kidney sections were scored for injury, apoptosis (terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick–end labeling staining), and cellular proliferation (proliferating cell nuclear antigen+ cells). Both CEPO and EPO significantly reduced kidney injury on days 3 and 7 in young and old rats (n=5 from each group) and decreased apoptosis scores on day 3 in young and old rats and only in old rats at 7 days. On day 3, for both EPO- and CEPO-treated animals, cellular proliferation scores were significantly increased in both young and old animals compared with vehicle treatment (Figure 3).
Figure 2.
CEPO is superior to EPO for the treatment of AKI in either young or old F344 rats without raising the hematocrit (Hct) or BP. In young or old male F344 rats, AKI was induced with bilateral clamping of the renal pedicles (60 minutes for young rats and 38 minutes for old rats), and then, rats were treated (vehicle, 1000 U/kg EPO, or 1000 U/kg CEPO) at days 1–3 after AKI. In both (A and B) young and (D and E) old animals, I/R injury induced moderate AKI with elevated SCr and BUN levels. CEPO treatment reduced (A and D) SCr and (B and E) BUN levels significantly and more effectively than EPO. (C and F) On day 7, body weights of both treatment groups were significantly higher than those in controls. EPO treatment resulted in significantly higher (G and J) Hct and (H and K) systolic BP levels compared with vehicle control and CEPO groups. (I and L) Survival in CEPO-treated rats was significantly improved compared with that in vehicle or EPO-treated animals. Black bars indicate EPO treatment, gray bars indicate CEPO treatment, and white bars indicate vehicle treatment. Black arrows indicate treatments on days 1–3. *P<0.05 compared with the vehicle control; #P<0.05 for CEPO compared with EPO.
Figure 3.
EPO and CEPO reduce tissue injury and apoptosis and increase renal cell proliferation in young and old F344 rats after AKI. In young and old male F344 rats, both CEPO and EPO (A and D) significantly reduced kidney injury scores, (B and E) decreased terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick–end labeling+ (TUNEL+) cells, and (C and F) increased proliferating cell nuclear antigen+ (PCNA+) cells on day 3 after AKI. Black bars indicate EPO treatment, gray bars indicate CEPO treatment, and white bars indicate vehicle treatment. FOV, field of view. *P<0.05 compared with the vehicle control.
EPO or CEPO Treatment of AKI in Male and Female F344 Rats
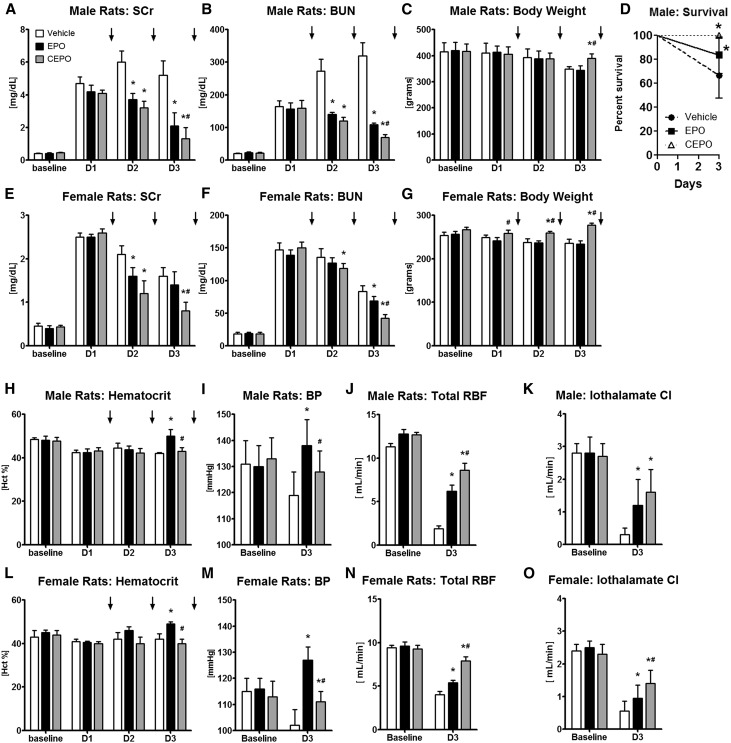
It is well known that males and females respond differently to renal injury, where women are often more protected than men.41,42 The objective in this group of experiments was to test whether CEPO (1000 U/kg sc; n=6) or EPO (1000 U/kg sc; n=6) treatment of AKI induced in adult male (weight of 420±35 g) or female (weight of 265±10 g) F344 rats (both 40 weeks old) alters the outcome of AKI compared with vehicle controls (n=6). In male rats, the severity of I/R AKI (35 minutes of bilateral renal pedicle clamping) was high on day 1, with SCr values between 4 and 5 mg/dl (Figure 4A), and BUN levels were elevated approximately 7.6-fold. EPO and CEPO treatment, given on days 1–3 in male rats after AKI, significantly improved recovery of renal function (Figure 4, A and B) compared with that in controls, and CEPO treatment resulted in better maintained body weight after I/R AKI (Figure 4C). Both CEPO and EPO significantly improved survival in male rats compared with controls (Figure 4D). In contrast, female rats developed only moderate I/R AKI after 50 minutes of bilateral pedicle clamping, with SCr levels rising to approximately 2.5 mg/dl SCr on day 1 (Figure 4E), and BUN levels increasing approximately 8.1-fold (Figure 4F).
Figure 4.
CEPO is more effective than EPO for the treatment of AKI in either male or female rats. In adult male and female F344 rats AKI was induced with bilateral clamping of the renal pedicles (35 minutes for male rats and 50 minutes for female rats), and then, rats were treated (vehicle, 1000 U/kg EPO sc, or 1000 U/kg CEPO sc) on days 1–3 after AKI. In both male and female rats, EPO or CEPO treatment given on days 1–3 after AKI significantly reduced (A and E) SCr and (B and F) BUN levels compared with vehicle controls, with additional benefit obtained with CEPO compared with EPO. (C and G) CEPO treatment resulted in better maintained body weight after I/R AKI. (D) Both CEPO and EPO significantly improved survival in male rats compared with that in controls. In both male and female rats, EPO treatment significantly elevated (H and L) hematocrit (Hct) and (I and M) systolic BP levels on day 3 compared with those in vehicle or CEPO groups. Both EPO and CEPO increased (J and N) renal blood flows (RBFs) and (K and O) iothalamate clearances (Cls) compared with those in vehicle-treated controls. (J and N) However, total RBFs in CEPO-treated animals were always higher than those in EPO-treated rats. Black bars indicate EPO treatment, gray bars indicate CEPO treatment, and white bars indicate vehicle treatment. Black arrows indicate treatments on days 1–3. *P<0.05 compared with the vehicle control; #P<0.05 for CEPO compared with EPO.
CEPO treatment improved recovery of renal function and was more effective than EPO in lowering SCr and BUN (Figure 4, E and F), and CEPO prevented weight loss after I/R AKI in female rats (Figure 4G). Also, survival on day 3 was 100% in all three treatment groups. In both male and female rats, EPO treatment significantly elevated hematocrit and systolic BP levels on day 3 compared with those in vehicle or CEPO groups (Figure 4, H, I, L, and M). To further characterize the effect of EPO and CEPO on renal function, renal blood flows and iothalamate clearances were measured. Both EPO and CEPO increased renal blood flow and iothalamate clearance compared with those in vehicle-treated controls on day 3 (Figure 4, J, K, N, and O). However, total renal blood flow in CEPO-treated animals was always higher than that in EPO-treated rats (Figure 4, J and N).
Renal Cells Express CD131
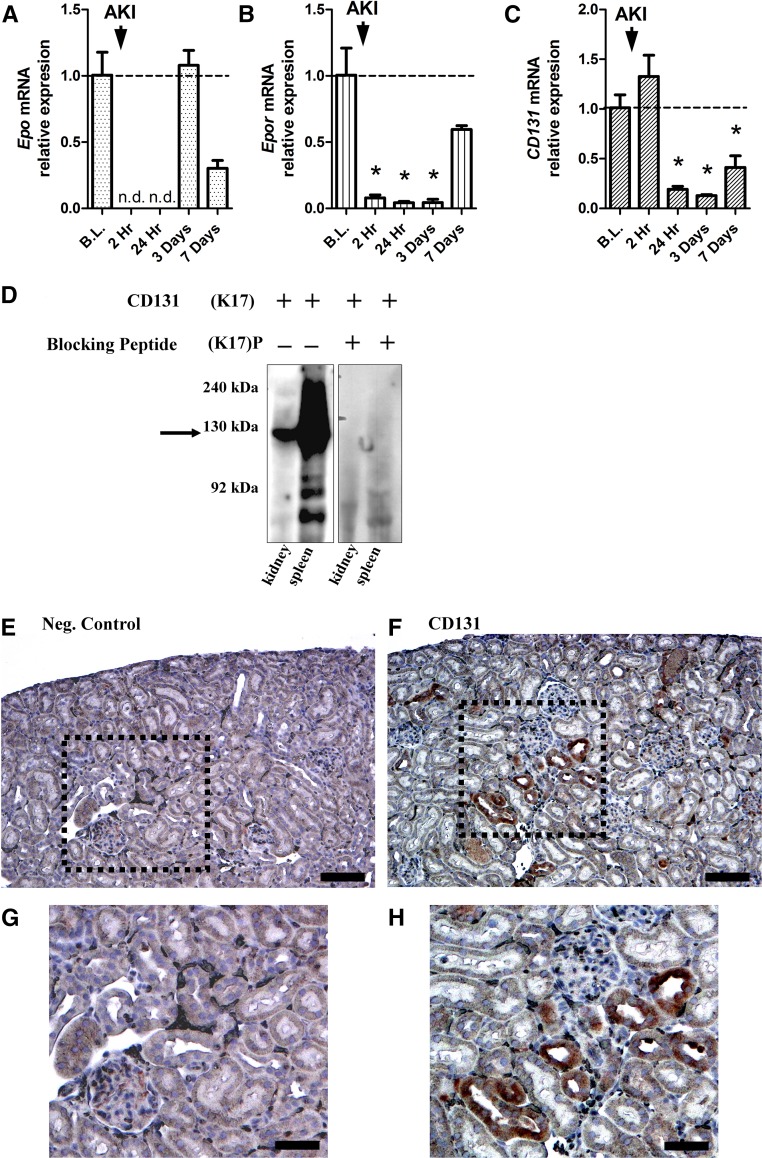
CEPO has been shown to mediate tissue protection through an EPO and CD131 heterodimeric receptor.26 Although the EPOR has been shown to be expressed in the kidney,13,43 there is currently limited information on CD131 expression. We, therefore, assessed CD131 expression in the kidney by determining RNA and protein levels. To study the time course of Epo, Epor, and CD131 gene expression, kidney tissues from the cortex of female F344 rats were analyzed at various time points after AKI (55 minutes of bilateral clamping) by quantitative RT-PCR. Epo mRNA was detectable in baseline controls and dropped below detectable levels at 2 and 24 hours after AKI.
Epo mRNA levels then returned to baseline levels at 3 and 7 days post-AKI (Figure 5A). Epor mRNA levels fell below baseline at 2 and 24 hours and 3 days after AKI, but then, they returned toward baseline levels by 7 days after AKI (Figure 5B). CD131 mRNA expression was below baseline levels at 24 hours and 3 days and rose slightly (not significantly) at 7 days after AKI (Figure 5C). Expression of CD131 at the protein level was confirmed by Western blot in spleen tissue (positive control) and kidney tissue with a band at approximately 130 kD that was no longer detected when the blot was incubated with sc-678 P (Santa Cruz Biotechnology, Santa Cruz, CA), a specific blocking peptide for CD131 (Figure 5D). Specific immunohistochemical staining showed expression of CD131 mainly in the renal cortex in proximal tubular cells (Figure 5, F and H) that was not observed in the primary antibody omitted control (Figure 5, E and G). On the basis of this result and other published results,26,44 CD131 and EPOR expression in the kidney provides a plausible signaling system for the effectiveness of CEPO in enhancing renal recovery from AKI.
Figure 5.
CD131 is expressed in the kidney. (A) In adult female Fisher rats, quantitative RT-PCR analysis of renal tissue at several time points after AKI showed that Epo mRNA levels were detectable in baseline (B.L.) controls (dashed line), dropped below detectable levels after AKI, and returned to baseline levels at 3 and 7 days post-AKI. (B) Epor mRNA levels fell below baseline levels at 2 and 24 hours and 3 days after AKI, and then, they returned toward baseline levels on day 7. (C) CD131 mRNA levels decreased and remained below baseline levels at 24 hours and 3 days, with a small but insignificant increase 7 days after AKI. *P<0.05 compared with the baseline control. (D) A Western blot for CD131 showed high expression in kidney and spleen tissues but not in the presence of a CD131–specific blocking peptide. CD131 has a molecular mass of approximately 130 kD (black arrow). Immunohistochemistry of kidney tissue showing (E) a negative control (Neg. Control) and (F) staining with CD131. (G) A magnified view of the area in the black box from E shows the negative control. (H) A magnified view of the area in the black box from F shows intensive CD131 staining of proximal tubular cells. n.d., not detectable. Scale bars, 100 μm in E and F; 200 μm in G and H.
Discussion
Recombinant EPO derivatives are clinically used for the treatment of anemia in patients with CKD.8 Promising preclinical research suggested that high-dose EPO would be protective against a variety of tissue injuries, including neurologic, cardiac, and renal injuries.11,12 However, subsequent clinical trials found that high-dose EPO can lead to severe adverse effects, such as hypertension,45 thromboembolic events, and increased risk of death.23
CEPO is an EPO derivative that has been shown to be tissue protective for the brain, heart, and other tissues after various forms of injury; however, CEPO does not activate the EPOR, and it does not increase hematocrit or BP levels.27,46–48 We show in this study using different commonly encountered clinical scenarios of AKI (AKI superimposed on CKD, the elderly, and male versus female) that both EPO and CEPO are renoprotective after I/R AKI, enhance both recovery of renal function and tissue repair, and improve animal survival. CEPO in contrast to EPO did not have significant effects on systolic BPs and hematocrits. Therefore, this distinct therapeutic profile of CEPO is likely to be advantageous in certain clinical settings.
The expression of functional EPOR in nonhematopoietic tissues is currently controversial, and additional research will be required to determine the contribution of the EPOR to tissue protection from EPO or CEPO. Other laboratories have reported that there is no significant expression of functional EPOR on nonhematopoietic tissues, including cancer cell lines49 and endothelial, cardiac, neuronal,50 and renal cells.51 This is in contrast to numerous other reports showing that functional EPORs are expressed in the kidney and that renal tissue responds to EPO treatment.13,43,52 The organ-protective effects of CEPO are achieved independently of activation of the classic EPOR homodimer but rather, are through stimulation of CD131, a constant component of GM-CSF, IL-3, and IL-5 receptors.24,26,44 We show here that CD131 (Common β-Receptor) is expressed in the kidney. Time course gene expression studies indicate that Epo, Epor, and CD131 gene expressions are downregulated after AKI but return to varying degrees by day 7. There is also functional evidence for the role of CD131 in EPO therapy, because knockout of CD131 abolishes the tissue-protective properties of EPO in the nervous system, the heart,26 and a mouse sepsis model.44 On the basis of our observations, we conclude and agree with other investigators28,29,53 that CEPO might present a preferable therapeutic for the treatment of AKI with significantly fewer side effects than high-dose EPO.
Concise Methods
CEPO and EPO
Recombinant human EPO was from Dragon Pharmaceuticals (Vancouver, BC, Canada). CEPO was provided by Warren Pharmaceuticals (Ossining, NY) and previously characterized.24 One unit erythropoietic activity corresponds to approximately 8 ng EPO. In these studies, nonhematopoietic CEPO was given in equivalent doses. EPO and CEPO have approximately equal molecular masses of 34 kD and were dissolved in sterile 0.9% saline before sc injection.
In Vivo Experimental Protocols
All procedures involving animals were approved by the Institutional Animal Care and Use Committees of the University of Utah and the Veterans Affairs Medical Center. Animals were housed at a constant temperature and humidity, with a 12:12-hour light-dark cycle, and they had unrestricted access to a standard diet and tap water.
AKI Superimposed on CKD
Male Sprague–Dawley rats (weight of 460±30 g; n=12 per group; 20 weeks old) were anesthetized with isoflurane. A sterile abdominal incision was made, and kidneys were exposed. The right kidney was nephrectomized, and two to three left renal artery branches were ligated to induce infarction of approximately 75%–85% of the left kidney, which was confirmed by visual inspection. The abdomen was closed, and the animal was allowed to recover. SCr and BUN were measured on days 1, 5, and 10 after renal mass reduction to assess renal functions. AKI was induced on day 10 after renal mass reduction (CKD baseline). For superimposed AKI, animals were reanesthetized, an abdominal incision was made, the left kidney was exposed, and the left renal pedicle was clamped for 35 minutes to induce I/R injury of the kidney. After visually confirmed reflow postclamp removal, the abdominal incision was closed, and the animals were allowed to recover. Blood from the tail vein was collected on day 1 and the following days to assess renal function (SCr and BUN). Treatment (vehicle: n=12; EPO: 5000 U/kg, n=12; or CEPO: 5000 U/kg, n=12) in all of the groups was sc injected on days 1–3 post-I/R AKI. Animals were followed for 7 days and euthanized on day 7 after induction of AKI superimposed on CKD.
AKI in Young Versus Old Rats
Young male F344 rats (weight of 145±10 g; 10 weeks old) and old male F344 rats (weight of 572±35 g; 80 weeks old; Charles River Laboratories, Wilmington, MA) were anesthetized with isoflurane, a sterile abdominal incision was made, kidneys were exposed, and both renal pedicles were clamped for 60 minutes in young rats and 38 minutes in old rats to induce I/R AKI. Treatment (vehicle controls: young group, n=10; old group, n=10; EPO: 1000 U/kg sc for 3 days; young group, n=10; old group, n=10; or CEPO: 1000 U/kg sc for 3 days; young group, n=12; old group, n=12) was given on days 1–3 after I/R AKI. One half of the animals in each group were euthanized on day 3 (for renal injury, apoptosis, and mitosis scoring), and the other one half of the animals in each group were euthanized on day 7 after induction of AKI.
AKI in Male Versus Female Rats
AKI was induced in adult male (weight of 417±32 g; 40 weeks old; 35 minutes of clamping of both renal pedicles) or female (weight of 259±7 g; 40 weeks old; 50 minutes of clamping) F344 rats as described above, and treatment (vehicle: n=6; EPO: 1000 U/kg sc for 3 days, n=6; or CEPO: 1000 U/kg sc for 3 days, n=6) was started on day 1 for 3 days.
Renal Function Studies
SCr, BUN, and hematocrit were determined by routine methods. Systolic BP levels, total renal blood flows by the Laser Doppler technique, and 125I-iothalamte clearances were measured as described.25,54 The kidney injury score, apoptosis, and mitosis scores were determined as previously reported.55,56
Real–Time RT-PCR
AKI was induced in adult female (55 minutes of bilateral renal pedicle clamping) F344 rats, and renal cortex tissue was harvested at 2 and 24 hours and 3 and 7 days post-AKI (n=3 per time point). RNA for real-time PCR was extracted with an RNeasy Kit (Qiagen, Germantown, MD) and included the DNase digestion step to eliminate contaminating DNA. Reverse transcription was performed using Moloney Murine Leukemia Virus Reverse Transcription (Invitrogen, Carlsbad, CA) for 60 minutes at 42°C. Real-time PCR with relative quantification of target gene copy numbers in relation to β-actin transcripts was carried out using the following primers: β-actin: forward: cactgtgttggcatagaggtc; reverse: agagggaaatcgtgcgtgaca; Epo (NM_017001): forward: agtcgcgttctggagaggta; reverse: aggatggcttctgagagcag; Epor (NM_017002): forward: tgagtgtgtcctgagcaacc; reverse: ccagcacagtcagcaacagt; and IL-3 receptor β–subunit (CD131, NM_133555): forward: gggaggacagcaagacagag; reverse: tgaggaagaccaggatgagg. The Smart-Cycler System (Cepheid, Sunnyvale, CA) was used to monitor real–time PCR amplification and determine cycle threshold (Ct) values using SYBR Green I (Invitrogen). Relative gene expression is presented as 2−ΔΔCt normalized to β-actin and baseline control samples.
Western Blots
Proteins were extracted from female Sprague–Dawley kidney or spleen tissues using the BCA Protein Assay Kit (Pierce, Rockford, IL), electrophoretically resolved with NuPAGE, and transferred to a nitrocellulose membrane (Invitrogen). The nitrocellulose membrane was blocked with skim milk and probed with anti–IL-3/IL-5/granulocyte macrophage colony-stimulating factor receptorβ rabbit polyclonal antibody (K17; sc-678) for CD131 (Santa Cruz Biotechnology). The blocking peptide was also from Santa Cruz Biotechnology (sc-678 P). Secondary antibody incubation (goat anti–rabbit horseradish peroxidase; Pierce) with or without the blocking peptide was followed by autoradiography.
CD131 Immunohistochemistry
Paraffin sections of normal female Sprague–Dawley kidneys were deparaffinized with xylene and rehydrated in an alcohol series and water. After incubation with a peroxidase-blocking reagent, slides were labeled with a 1:100 dilution of a primary anti–IL-3/IL-5/granulocyte macrophage colony-stimulating factor receptorβ rabbit polyclonal antibody (K17) for CD131 (Santa Cruz Biotechnology) for 60 minutes and stained with the EnVision System (DAKO, Carpentaria, CA). For the negative control, the primary antibody was omitted before EnVision (DAKO) staining.
Statistical Analyses
Data are expressed as means±SEMs. Primary data collection used Excel (Microsoft, Redmond, WA), and statistical analyses were carried out using Prism (GraphPad Software Inc., San Diego, CA). ANOVA or t tests were used to assess differences between data means as appropriate. A P value of <0.05 was considered significant.
Disclosures
None.
Acknowledgments
We thank Dr. Tianxin Yang (University of Utah) for providing the use of the Laser Doppler equipment to measure renal blood flow.
Carbamylated erythropoietin was provided by the Kenneth S. Warren Institute and Warren Pharmaceuticals (Ossining, NY). This work was funded by the Veterans Affairs Medical Center, an American Heart Association grant in aid, the National Kidney Foundation of Utah and Idaho (Provo, UT), and the Dialysis Research Foundation (Ogden, UT).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hsu C-Y, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS: Community-based incidence of acute renal failure. Kidney Int 72: 208–212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ricci Z, Cruz D, Ronco C: The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int 73: 538–546, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Molitoris BA, Sutton TA: Endothelial injury and dysfunction: Role in the extension phase of acute renal failure. Kidney Int 66: 496–499, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Molitoris BA, Falk SA, Dahl RH: Ischemia-induced loss of epithelial polarity. Role of the tight junction. J Clin Invest 84: 1334–1339, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagher PC: Apoptosis in ischemic renal injury: Roles of GTP depletion and p53. Kidney Int 66: 506–509, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Jelkmann W: Erythropoietin after a century of research: Younger than ever. Eur J Haematol 78: 183–205, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Hörl WH: Anaemia management and mortality risk in chronic kidney disease. Nat Rev Nephrol 9: 291–301, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Rossert J, Froissart M, Jacquot C: Anemia management and chronic renal failure progression. Kidney Int Suppl 99: S76–S81, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Sasaki R, Masuda S, Nagao M: Pleiotropic functions and tissue-specific expression of erythropoietin. News Physiol Sci 16: 110–113, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Brines M, Cerami A: Discovering erythropoietin’s extra-hematopoietic functions: Biology and clinical promise. Kidney Int 70: 246–250, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Arcasoy MO: The non-haematopoietic biological effects of erythropoietin. Br J Haematol 141: 14–31, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Westenfelder C, Biddle DL, Baranowski RL: Human, rat, and mouse kidney cells express functional erythropoietin receptors. Kidney Int 55: 808–820, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Westenfelder C: Unexpected renal actions of erythropoietin. Exp Nephrol 10: 294–298, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Echigoya MH, Obikane K, Nakashima T, Sasaki S: Glomerular localization of erythropoietin receptor mRNA and protein in neonatal and mature mouse kidney. Nephron, Exp Nephrol 100: e21–e29, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Yang CW, Li C, Jung JY, Shin SJ, Choi BS, Lim SW, Sun BK, Kim YS, Kim J, Chang YS, Bang BK: Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J 17: 1754–1755, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Vaziri ND, Zhou XJ, Liao SY: Erythropoietin enhances recovery from cisplatin-induced acute renal failure. Am J Physiol 266: F360–F366, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Gobe GC, Bennett NC, West M, Colditz P, Brown L, Vesey DA, Johnson DW: Increased progression to kidney fibrosis after erythropoietin is used as a treatment for acute kidney injury. Am J Physiol Renal Physiol 306: F681–F692, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Vaziri ND: Cardiovascular effects of erythropoietin and anemia correction. Curr Opin Nephrol Hypertens 10: 633–637, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Henke M, Laszig R, Rübe C, Schäfer U, Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, Frommhold H: Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet 362: 1255–1260, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Smith KJ, Bleyer AJ, Little WC, Sane DC: The cardiovascular effects of erythropoietin. Cardiovasc Res 59: 538–548, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jähnig P, Herrmann M, Knauth M, Bähr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C EPO Stroke Trial Group : Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 40: e647–e656, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Robertson CS, Hannay HJ, Yamal J-M, Gopinath S, Goodman JC, Tilley BC, Baldwin A, Rivera Lara L, Saucedo-Crespo H, Ahmed O, Sadasivan S, Ponce L, Cruz-Navarro J, Shahin H, Aisiku IP, Doshi P, Valadka A, Neipert L, Waguspack JM, Rubin ML, Benoit JS, Swank P Epo Severe TBI Trial Investigators : Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA 312: 36–47, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie Q-W, Coleman T, Cerami A, Brines M: Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 305: 239–242, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Coleman TR, Westenfelder C, Tögel FE, Yang Y, Hu Z, Swenson L, Leuvenink HGD, Ploeg RJ, d’Uscio LV, Katusic ZS, Ghezzi P, Zanetti A, Kaushansky K, Fox NE, Cerami A, Brines M: Cytoprotective doses of erythropoietin or carbamylated erythropoietin have markedly different procoagulant and vasoactive activities. Proc Natl Acad Sci U S A 103: 5965–5970, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie Q-W, Smart J, Su-Rick C-J, Pobre E, Diaz D, Gomez D, Hand C, Coleman T, Cerami A: Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A 101: 14907–14912, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fantacci M, Bianciardi P, Caretti A, Coleman TR, Cerami A, Brines M, Samaja M: Carbamylated erythropoietin ameliorates the metabolic stress induced in vivo by severe chronic hypoxia. Proc Natl Acad Sci U S A 103: 17531–17536, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura R, Isaka Y, Ichimaru N, Takahara S, Okuyama A: Carbamylated erythropoietin protects the kidneys from ischemia-reperfusion injury without stimulating erythropoiesis. Biochem Biophys Res Commun 353: 786–792, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Imamura R, Okumi M, Isaka Y, Ichimaru N, Moriyama T, Imai E, Nonomura N, Takahara S, Okuyama A: Carbamylated erythropoietin improves angiogenesis and protects the kidneys from ischemia-reperfusion injury. Cell Transplant 17: 135–141, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Abe T, Isaka Y, Imamura R, Kakuta Y, Okumi M, Yazawa K, Ichimaru N, Tsuda H, Nonomura N, Takahara S, Okuyama A: Carbamylated erythropoietin ameliorates cyclosporine nephropathy without stimulating erythropoiesis. Cell Transplant 21: 571–580, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Kitamura H, Isaka Y, Takabatake Y, Imamura R, Suzuki C, Takahara S, Imai E: Nonerythropoietic derivative of erythropoietin protects against tubulointerstitial injury in a unilateral ureteral obstruction model. Nephrol Dial Transplant 23: 1521–1528, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Imamura R, Isaka Y, Sandoval RM, Ichimaru N, Abe T, Okumi M, Yazawa K, Kitamura H, Kaimori J, Nonomura N, Rakugi H, Molitoris BA, Takahara S: A nonerythropoietic derivative of erythropoietin inhibits tubulointerstitial fibrosis in remnant kidney. Clin Exp Nephrol 16: 852–862, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR: Can animal models of disease reliably inform human studies? PLoS Med 7: e1000245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL: Epidemiology and outcomes of acute renal failure in hospitalized patients: A national survey. Clin J Am Soc Nephrol 1: 43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ: Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Anderson S, Eldadah B, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kimmel PL, Molitoris BA, Murthy M, O’Hare AM, Schmader KE, High KP: Acute kidney injury in older adults. J Am Soc Nephrol 22: 28–38, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Hirschberg R, Kopple J, Lipsett P, Benjamin E, Minei J, Albertson T, Munger M, Metzler M, Zaloga G, Murray M, Lowry S, Conger J, McKeown W, O’shea M, Baughman R, Wood K, Haupt M, Kaiser R, Simms H, Warnock D, Summer W, Hintz R, Myers B, Haenftling K, Capra W, Pike M, Guler HP: Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int 55: 2423–2432, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Allgren RL, Marbury TC, Rahman SN, Weisberg LS, Fenves AZ, Lafayette RA, Sweet RM, Genter FC, Kurnik BR, Conger JD, Sayegh MH Auriculin Anaritide Acute Renal Failure Study Group : Anaritide in acute tubular necrosis. N Engl J Med 336: 828–834, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Endre ZH, Walker RJ, Pickering JW, Shaw GM, Frampton CM, Henderson SJ, Hutchison R, Mehrtens JE, Robinson JM, Schollum JBW, Westhuyzen J, Celi LA, McGinley RJ, Campbell IJ, George PM: Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int 77: 1020–1030, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K: Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol 16: 2557–2564, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR: Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res 67: 594–603, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV: Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 279: 52282–52292, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Hu M-C, Shi M, Cho HJ, Zhang J, Pavlenco A, Liu S, Sidhu S, Huang LJ-S, Moe OW: The erythropoietin receptor is a downstream effector of Klotho-induced cytoprotection. Kidney Int 84: 468–481, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coldewey SM, Khan AI, Kapoor A, Collino M, Rogazzo M, Brines M, Cerami A, Hall P, Sheaff M, Kieswich JE, Yaqoob MM, Patel NS, Thiemermann C: Erythropoietin attenuates acute kidney dysfunction in murine experimental sepsis by activation of the β-common receptor. Kidney Int 84: 482–490, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Perazella MA: Nonhematologic complications of erythropoietin therapy. Semin Dial 19: 279–284, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, Ghezzi P: Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res 952: 128–134, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Grasso G, Sfacteria A, Erbayraktar S, Passalacqua M, Meli F, Gokmen N, Yilmaz O, La Torre D, Buemi M, Iacopino DG, Coleman T, Cerami A, Brines M, Tomasello F: Amelioration of spinal cord compressive injury by pharmacological preconditioning with erythropoietin and a nonerythropoietic erythropoietin derivative. J Neurosurg Spine 4: 310–318, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A: Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A 97: 10526–10531, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laugsch M, Metzen E, Svensson T, Depping R, Jelkmann W: Lack of functional erythropoietin receptors of cancer cell lines. Int J Cancer 122: 1005–1011, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Sinclair AM, Coxon A, McCaffery I, Kaufman S, Paweletz K, Liu L, Busse L, Swift S, Elliott S, Begley CG: Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood 115: 4264–4272, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Elliott S, Busse L, Swift S, McCaffery I, Rossi J, Kassner P, Begley CG: Lack of expression and function of erythropoietin receptors in the kidney. Nephrol Dial Transplant 27: 2733–2745, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Hand CC, Brines M: Promises and pitfalls in erythopoietin-mediated tissue protection: Are nonerythropoietic derivatives a way forward? J Investig Med 59: 1073–1082, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brines M, Cerami A: The receptor that tames the innate immune response. Mol Med 18: 486–496, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westenfelder C, Arevalo GJ, Crawford PW, Zerwer P, Baranowski RL, Birch FM, Earnest WR, Hamburger RK, Coleman RD, Kurtzman NA: Renal tubular function in glycerol-induced acute renal failure. Kidney Int 18: 432–444, 1980 [DOI] [PubMed] [Google Scholar]
- 55.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C: Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Isaac J, Tögel FE, Westenfelder C: Extent of glomerular tubularization is an indicator of the severity of experimental acute kidney injury in mice. Nephron, Exp Nephrol 105: e33–e40, 2007 [DOI] [PubMed] [Google Scholar]