Abstract
Accurate identification of risk factors for calcific uremic arteriolopathy (CUA) is necessary to develop preventive strategies for this morbid disease. We investigated whether baseline factors recorded at hemodialysis initiation would identify patients at risk for future CUA in a matched case-control study using data from a large dialysis organization. Hemodialysis patients with newly diagnosed CUA (n=1030) between January 1, 2010, and December 31, 2014, were matched by age, sex, and race in a 1:2 ratio to hemodialysis patients without CUA (n=2060). Mean ages for patients and controls were 54 and 55 years, respectively; 67% of participants were women and 49% were white. Median duration between hemodialysis initiation and subsequent CUA development was 925 days (interquartile range, 273–2185 days). In multivariable conditional logistic regression analyses, diabetes mellitus; higher body mass index; higher levels of serum calcium, phosphorous, and parathyroid hormone; and nutritional vitamin D, cinacalcet, and warfarin treatments were associated with increased odds of subsequent CUA development. Compared with patients with diabetes receiving no insulin injections, those receiving insulin injections had a dose-response increase in the odds of CUA involving lower abdomen and/or upper thigh areas (odds ratio, 1.49; 95% confidence interval, 1.03 to 2.51 for one or two injections per day; odds ratio, 1.88; 95% confidence interval, 1.30 to 3.43 for 3 injections per day; odds ratio, 3.74; 95% confidence interval, 2.28 to 6.25 for more than three injections per day), suggesting a dose-effect relationship between recurrent skin trauma and CUA risk. The presence of risk factors months to years before CUA development observed in this study will direct the design of preventive strategies and inform CUA pathobiology.
Keywords: hemodialysis, end stage kidney disease, clinical epidemiology, diabetes mellitus, obesity, vascular calcification
The lack of effective therapeutic interventions underscores a need to develop strategies to prevent calcific uremic arteriolopathy (CUA). Mortality rates for patients with CUA are extremely high and have remained over three times that for patients receiving hemodialysis without CUA. The reported prevalence of CUA is 4% in patients receiving chronic hemodialysis and recent reports suggest that incidence of CUA in patients receiving chronic hemodialysis is increasing.1–4 A first step in preventing CUA is to conduct a comprehensive ascertainment of risk factors for CUA. Previous studies evaluating CUA risk factors are limited by small sample size (number of patients with CUA ranging from five to 62)3–13 and have mostly focused on factors at or close to the diagnosis of CUA.3,5,8,10 The cross-sectional nature of these prior studies leaves uncertainty as to whether the outcome followed exposure or exposure resulted from the outcome.
We hypothesized that baseline factors recorded at the time of dialysis initiation would help to accurately profile patients at the highest risk for future CUA. Since CUA is typically seen in patients receiving chronic dialysis for >1 year,4,14,15 baseline factors recorded months to years prior to a CUA diagnosis can be the target of potential interventions to reduce the subsequent CUA risk. We tested this hypothesis by conducting a large nationally representative case-control study that compared characteristics at hemodialysis initiation between patients with CUA and matched patients receiving hemodialysis without CUA. Considering the previously described heterogeneity associated with anatomic distribution of CUA lesions (central lesions versus peripheral lesions),7,16 we also examined risk factors for central and peripheral CUA separately and investigated whether insulin injections as a source of skin trauma are associated with the risk of future CUA.
Results
Study Patient Characteristics
From chronic dialysis units affiliated with Fresenius Medical Care North America (FMCNA), we identified 1030 patients receiving hemodialysis with newly diagnosed CUA (cases) between January 1, 2010, and December 31, 2014. We then randomly selected contemporaneous patients receiving hemodialysis without CUA from the FMCNA database and matched them to cases in a 2:1 ratio by age (±5 years), sex, and race to serve as controls.
The median duration between hemodialysis initiation and CUA development was 925 days (interquartile range [IQR], 273–2185 days). Skin biopsy confirmation was reported for 55% and the remaining were diagnosed clinically. Matching produced equivalent age, sex, and race distributions for cases and controls (Table 1). Mean ages for cases and controls were 54 and 55 years, respectively. CUA was noted to have a predilection for women (67%) and white race (49%). Prevalence of diabetes mellitus was higher in cases compared with controls (61% versus 44%) and mean body mass index (BMI) for cases was 37 kg/m2 compared with 30 kg/m2 for controls. Serum phosphorus and parathyroid hormone (PTH) levels were higher at hemodialysis initiation among patients who eventually developed CUA, whereas serum calcium levels (both albumin corrected and albumin noncorrected) and 25-hydroxyvitamin D levels were similar. Nutritional vitamin D (9% versus 5%), cinacalcet (7% versus 2%), and phosphate binder (35% versus 31%) treatments were more prevalent at hemodialysis initiation among cases compared with controls, whereas activated vitamin D treatment use was less prevalent in cases compared with controls (32% versus 37%) and dialysate calcium levels (2.5±0.3 versus 2.5±0.2) were similar between cases and controls. Both calcium-containing (19% versus 17%) and noncalcium-containing phosphate binder treatments (16% versus 14%) were more prevalent at baseline among cases compared with controls; however, differences did not reach statistical significance (P values 0.06 and 0.07, respectively). Warfarin therapy at hemodialysis initiation was over three times more prevalent among cases compared with controls. At hemodialysis initiation, statin treatment had a higher prevalence among cases compared with controls (25% versus 22%), whereas erythropoiesis-stimulating agents had a lower prevalence in cases compared with controls (45% versus 56%). Among patients with diabetes, insulin use at hemodialysis initiation was more common in cases compared with controls (32% versus 27%; P=0.002).
Table 1.
Comparison of baseline characteristics at hemodialysis initiation between patients who subsequently developed CUA (cases) and age-, sex-, and race-matched patients who did not develop CUA (controls)
| Characteristic | Cases (n=1030) | Controls (n=2060) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, yr | 54±15 | 55±15 | Not applicable |
| Sex, female % | 67 | 67 | Not applicable |
| Race | Not applicable | ||
| White % | 49 | 49 | |
| Black % | 28 | 28 | |
| Other % | 23 | 23 | |
| Comorbidities and vital signs | |||
| Diabetes mellitus, % | 61 | 44 | <0.001 |
| Obesity, % | 65 | 42 | <0.001 |
| Weight, kg | 101.2±29.3 | 82.0±25.5 | <0.001 |
| BMI, kg/m2 | 36.7±10.2 | 30.3±8.5 | <0.001 |
| Systolic BP, mmHg | 150±31 | 148±27 | 0.04 |
| Diastolic BP, mmHg | 78±18 | 78±17 | 0.66 |
| Mineral bone parameters and therapies | |||
| Serum calcium (albumin corrected), mg/dl | 9.1±0.8 | 9.0±0.8 | 0.04 |
| Serum phosphorus, mg/dl | 4.9±2.3 | 4.6±2.0 | 0.001 |
| Serum PTH, pg/ml | 379 (184, 651) | 250 (100, 471) | <0.001 |
| Serum ALP, U/L | 116.6±87.5 | 106.8±74.3 | 0.002 |
| Serum 25-hydroxyvitamin D, ng/ml | 19.4±10.1 | 16.7±11.2 | 0.07 |
| Dialysate calcium, mmol/L | 2.5±0.3 | 2.5±0.2 | 0.20 |
| Nutritional vitamin D treatment, % | 9 | 5 | <0.001 |
| Activated vitamin D treatment, % | 32 | 37 | 0.02 |
| Cinacalcet treatment, % | 7 | 2 | <0.001 |
| Phosphate-binding agent treatment, % | 35 | 31 | 0.01 |
| Other laboratory parameters and medications | |||
| Serum albumin, g/dl | 3.5±0.5 | 3.5±0.6 | 0.49 |
| Hemoglobin, g/dl | 10.2±1.5 | 10.4±1.5 | 0.01 |
| Serum bicarbonate, mEq/L | 22.5±3.9 | 22.5±4.0 | 0.61 |
| sPKtV | 1.5±0.4 | 1.6±0.4 | <0.001 |
| Warfarin treatment, % | 14 | 4 | <0.001 |
| Statin treatment, % | 25 | 22 | 0.04 |
| ESA treatment, % | 45 | 56 | <0.001 |
| ACEi/ARB treatment, % | 15 | 13 | 0.22 |
Mean ± SD is reported for all continuous variables except for serum PTH where IQR is reported. ALP, alkaline phosphatase; sPKtV, single pool KtV; ESA, erythropoiesis-stimulating agent; ACEi/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker.
Risk Factors for Future CUA Development at the Time of Hemodialysis Initiation
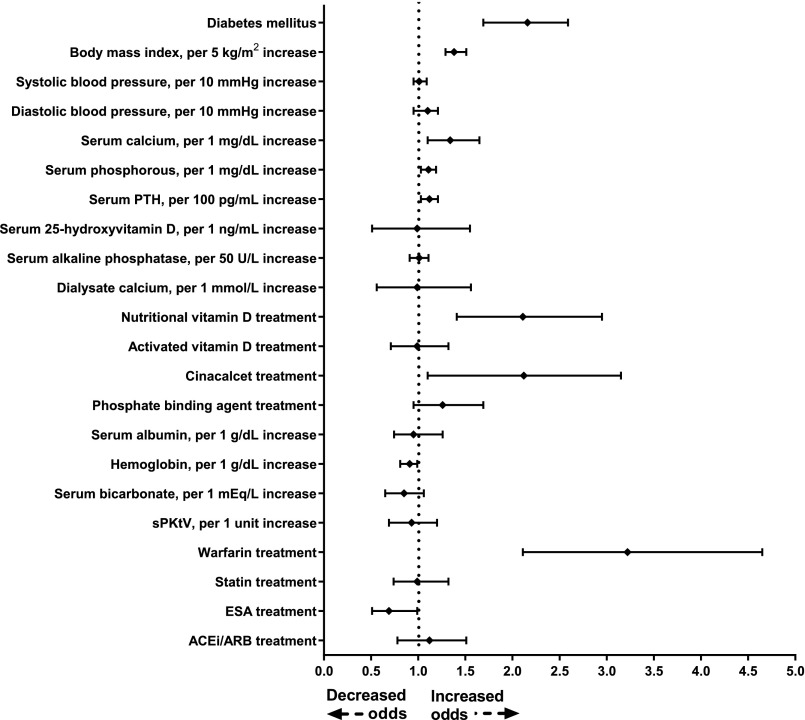
In multivariable conditional logistic regression analyses adjusted for all covariates listed in Table 1, diabetes mellitus (odds ratio [OR], 2.16; 95% confidence interval [95% CI], 1.69 to 2.59), higher BMI (for every 5 kg/m2 increase: OR, 1.38; 95% CI, 1.29 to 1.51), higher albumin-corrected serum calcium (for every 1 mg/dl increase: OR, 1.33; 95% CI, 1.12 to 1.61), higher serum phosphorous (for every 1 mg/dl increase: OR, 1.11; 95% CI, 1.03 to 1.19), higher PTH (for every 100 pg/ml increase: OR, 1.12; 95% CI, 1.03 to 1.21), nutritional vitamin D use (OR, 2.11; 95% CI, 1.41 to 2.95), cinacalcet use (OR, 2.12; 95% CI, 1.10 to 3.15), and warfarin use (OR, 3.22; 95% CI, 2.11 to 4.65) at hemodialysis initiation were associated with increased odds of future CUA development, whereas higher hemoglobin (for every 1 g/dl increase: OR, 0.81; 95% CI, 0.91 to 0.99) and use of erythropoiesis-stimulating agents (OR, 0.69; 95% CI, 0.51 to 0.99) were associated with reduced odds (Figure 1). Phosphate binder treatments (both calcium-containing and noncalcium-containing agents) did not demonstrate significant association with future CUA development.
Figure 1.
Multivariable conditional logistic regression model showing ORs of future CUA development by patient characteristics at hemodialysis initiation. Filled diamond denotes point estimate of ORs and error bars represent 95% CI. ACEi/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; ESA, erythropoiesis-stimulating agents; sPKtV, single pool KtV.
In multivariable analyses adjusted for covariates identified by stepwise selection, the same covariates were independently associated with increased odds of CUA development.
Sensitivity Analyses
We conducted conditional logistic regression analyses and examined the association between cinacalcet and nutritional vitamin D use and CUA development across the severity categories for each mineral metabolism marker. Overall the associations were consistent; however, sample size introduced limitations on statistical significance for some associations (Supplemental Table 1). To overcome this sample size limitation, we identified all the remaining patients (n=259,212), in addition to 1030 CUA cases and 2060 controls, who were receiving dialysis care at the FMCNA during the contemporaneous study period. In this large cohort (total n=262,302, mean age =62 years [±15 years], 57% men, 62% white, and 32% black), 5246 patients (2%) were treated with cinacalcet within the first 90 days of hemodialysis initiation and 12,286 patients (5%) were treated with nutritional vitamin D within the first 90 days of hemodialysis initiation. In logistic regression analyses, increased risks for CUA were noted with cinacalcet and nutritional vitamin D across all the mineral metabolism severities (Supplemental Table 2). In multivariable-adjusted analyses adjusted for mineral metabolism markers, use of cinacalcet (OR, 2.49; 95% CI, 1.79 to 3.46) and nutritional vitamin D (OR, 2.35; 95% CI, 1.67 to 3.34) were associated with increased CUA risk.
We also examined whether association of cinacalcet with CUA varies across cinacalcet dosing by categorizing cinacalcet dose as ≤60 mg/d and >60 mg/d. In univariate analyses, ≤60 mg/d cinacalcet dose (OR, 4.21; 95% CI, 2.80 to 6.33) and >60 mg/d cinacalcet dose (OR, 3.10; 95% CI, 1.52 to 6.33) were associated with increased odds of CUA. In multivariable analyses adjusted for all covariates listed in Table 1, low-dose cinacalcet was associated with statistically significant increased odds of CUA (OR, 2.15; 95% CI, 1.11 to 3.92). High-dose cinacalcet was also associated with increased odds of CUA; however, sample size likely introduced limitations to statistical significance (OR, 1.98; 95% CI, 0.91 to 5.29).
The relationship between various risk factors and CUA development was not materially different in sensitivity analyses restricted to patients with CUA with reported diagnostic skin biopsy confirmation (n=567) and their corresponding matching controls (n=1134) (Supplemental Table 3).
Central CUA
Data on CUA lesion(s) location were available for 69% of cases (n=710). Patients with lesions involving chest, trunk, buttocks, genitals, lower extremities proximal to knee joints, and upper extremities proximal to elbow joints were classified as having a central distribution, whereas patients with lesions restricted to lower extremities at and distal to knee joints, upper extremities at and distal to elbow joints, and head were classified as having a peripheral distribution. Most patients with CUA had lesions in central distribution (n=582). When compared with CUA patients with lesions limited to peripheral distribution, patients with CUA with central distribution were younger and had a higher prevalence of obesity and warfarin use (Table 2). In multivariable analyses restricted to patients with central CUA and their corresponding controls and adjusted for all covariates listed in Table 2, the following independent risk factors for central CUA were identified: diabetes mellitus (OR, 3.07; 95% CI, 1.69 to 6.01), BMI (for every 5 kg/m2 increase: OR, 1.52; 95% CI, 1.15 to 1.82), albumin-corrected serum calcium (for every 1 mg/dl increase: OR, 1.31; 95% CI, 1.07 to 2.23), warfarin use (OR, 3.98; 95% CI, 2.21 to 10.21), and insulin use (OR, 1.79; 95% CI, 1.04 to 2.98). In multivariable analyses adjusted for covariates identified by stepwise selection, the results were similar. The relationship between these risk factors and central CUA development was not materially different in sensitivity analyses restricted to patients with central CUA with reported diagnostic skin biopsy confirmation (n=378) and their corresponding matching controls (n=756).
Table 2.
Comparison of baseline characteristics at hemodialysis initiation between patients who subsequently developed central CUA and those who developed peripheral CUA
| Characteristic | Central CUA (n=582) | Peripheral CUA (n=128) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, yr | 53±14 | 56±15 | 0.02 |
| Sex, female % | 73 | 61 | 0.02 |
| Race | 0.26 | ||
| White, % | 57 | 49 | |
| Black, % | 26 | 32 | |
| Other, % | 17 | 19 | |
| Comorbidities and vital signs | |||
| Diabetes mellitus, % | 62 | 55 | 0.15 |
| Obesity, % | 70 | 54 | 0.002 |
| Weight, kg | 103.9±27.1 | 94.7±28.4 | 0.003 |
| BMI, kg/m2 | 37.7±9.9 | 33.8±9.2 | <0.001 |
| Systolic BP, mmHg | 149±30 | 153±35 | 0.29 |
| Diastolic BP, mmHg | 77±17 | 81±23 | 0.08 |
| Mineral bone parameters and therapies | |||
| Serum calcium (albumin corrected), mg/dl | 9.1±0.8 | 9.0±0.8 | 0.47 |
| Serum phosphorus, mg/dl | 4.8±2.2 | 4.8±2.3 | 0.92 |
| Serum PTH, pg/ml | 366 (180, 638) | 403 (219, 659) | 0.96 |
| Serum ALP, U/L | 116.6±83.5 | 123.2±109.4 | 0.54 |
| Serum 25-hydroxyvitamin D, ng/ml | 18.7±11.3 | 20.1±11.2 | 0.25 |
| Dialysate calcium, mmol/L | 2.5±0.3 | 2.5±0.3 | 0.55 |
| Nutritional vitamin D treatment, % | 8 | 8 | 0.96 |
| Activated vitamin D treatment, % | 36 | 43 | 0.14 |
| Cinacalcet treatment, % | 6 | 5 | 0.63 |
| Phosphate-binding agent treatment, % | 37 | 40 | 0.63 |
| Other laboratory parameters and medications | |||
| Serum albumin, g/dl | 3.5±0.6 | 3.6±0.6 | 0.16 |
| Hemoglobin, g/dl | 10.4±1.8 | 10.3±1.6 | 0.65 |
| Serum bicarbonate, mEq/L | 22.0±4.0 | 22.1±4.2 | 0.94 |
| sPKtV | 1.5±0.5 | 1.4±0.4 | 0.51 |
| Warfarin treatment, % | 18 | 8 | 0.01 |
| Statin treatment, % | 26 | 21 | 0.34 |
| Erythropoiesis-stimulating agent treatment, % | 51 | 48 | 0.57 |
| Angiotensin pathway blocker treatment, % | 17 | 14 | 0.38 |
| Insulin treatment, % | 22 | 14 | 0.06 |
Mean ± SD is reported for all continuous variables except for serum PTH where IQR is reported. ALP, alkaline phosphatase; sPKtV, single pool KtV.
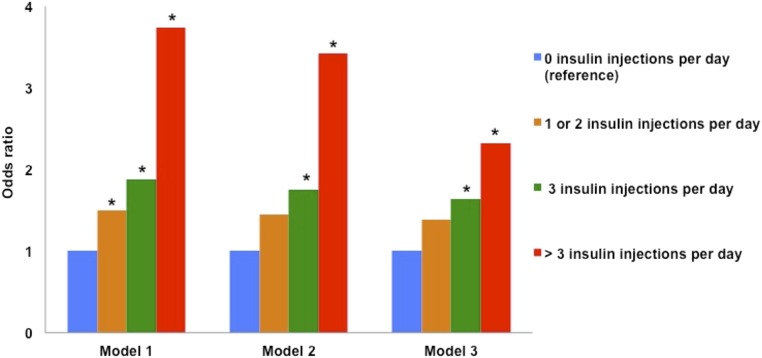
To further examine whether recurrent skin trauma induced by insulin injections poses a risk for CUA, we conducted analyses restricted to patients with diabetes and CUA lesions involving usual sites for insulin administration such as lower abdomen and/or upper thigh areas (n=348) and controls with diabetes mellitus (n=906). Among patients with diabetes and CUA lesions involving lower abdomen and/or upper thigh areas, 54% were not receiving insulin therapy at hemodialysis initiation, 16% were receiving one or two, 12% were receiving three, and 18% were receiving more than three insulin injections per day. Among control patients with diabetes, 73% were not receiving any insulin therapy at hemodialysis initiation, 16% were receiving one or two, 6% were receiving three, and 5% were receiving more than three insulin injections per day. Compared with patients with diabetes receiving no insulin injections, those receiving one or two (OR, 1.49; 95% CI, 1.03 to 2.51), those receiving three (OR, 1.88; 95% CI, 1.30 to 3.43), and those receiving more than three (OR, 3.74; 95% CI, 2.28 to 6.25) insulin injections per day had a dose-response increase in the odds of CUA lesions (Figure 2). In models adjusted for demographics and for covariates with statistical significance in univariate analyses (including BMI), effects were attenuated but remained significant for those receiving three and more than three insulin injections per day. We observed a significant trend between the odds of CUA and increasing insulin injection frequency across all the tertiles of BMI, suggesting that the association between CUA and insulin injections is likely more reflective of skin trauma than larger body size (Supplemental Table 4).
Figure 2.
ORs of future CUA involving lower abdomen and/or upper thigh areas by number of insulin injections per day at hemodialysis initiation. Model 1 is an unadjusted model; model 2 is adjusted for age, race, and sex; model 3 is adjusted for covariates independently associated with increased risk of CUA involving lower abdomen and/or upper thigh areas in patients with diabetes mellitus. *P<0.05 (significant difference from reference values; zero insulin injections per day).
Peripheral CUA
In multivariable analyses restricted to patients with peripheral CUA (n=128) and their corresponding controls and adjusted for all covariates listed in Table 2, the following independent risk factors for peripheral CUA were identified: diabetes mellitus (OR, 2.51; 95% CI, 1.35 to 4.21), BMI (for every 5 kg/m2 increase: OR, 1.24; 95% CI, 1.10 to 1.66), and activated vitamin D treatment (OR, 1.98; 95% CI, 1.09 to 3.65). In multivariable analyses adjusted for covariates identified by stepwise selection, the same covariates were independently associated with increased odds of peripheral CUA development. In sensitivity analyses restricted to patients with peripheral CUA with reported diagnostic skin biopsy (n=53) and their corresponding matching controls (n=106), the relationship was similar in direction but not significant, due to limited sample sizes.
CUA Incidence Rate and Mortality
In the entire cohort of FMCNA patients receiving dialysis between January 1, 2010, and December 31, 2014, a mean CUA incidence rate was 3.49 per 1000 patient-years (95% CI, 3.30 to 3.72). We noted a significantly increased CUA incidence rate in warfarin users of 6.24 per 1000 patient-years (95% CI, 5.87 to 6.67) compared with 3.41 per 1000 patient-years (95% CI, 3.27 to 3.75) in warfarin nonusers (P<0.001). Mean incidence rate of CUA involving lower abdomen and/or upper thigh areas progressively increased with increasing frequency of insulin injections: 2.27 per 1000 patient-years (95% CI, 2.09 to 2.57) for those not receiving any insulin; 2.71 per 1000 patient-years (95% CI, 2.41 to 2.85), 3.01 per 1000 patient-years (95% CI, 2.73 to 3.20), and 3.35 per 1000 patient-years (95% CI, 2.98 to 5.34) for those receiving one or two, three, and more than three insulin injections per day, respectively (P for trend <0.001).
Mortality rates were 27% at 6 months after CUA diagnosis and 45% at 12 months after CUA diagnosis. Median time to death was 151 days (IQR, 107–368 days) from CUA diagnosis.
Discussion
For a complex disease such as CUA, multiple risk factors are expected. Aligned with this possibility, we now report significant associations of baseline diabetes mellitus, obesity, mineral bone disease, warfarin, and insulin injections with future development of CUA in a large, nationally representative, matched case-control study. Diabetes mellitus and obesity were associated with both central and peripheral CUA, whereas insulin injections and warfarin were specifically associated with central CUA. The majority of patients with CUA were white women.
Prior studies designed to investigate risk factors for CUA suffered from sample size limitations3–5,7–13; the largest published study to date included 62 patients with CUA.3 Limited sample size of prior studies restricted multivariable adjustments and separate analyses for central versus peripheral CUA.3,5 Furthermore, most prior studies evaluated risk factors at the time of or close to the CUA diagnosis,3–5,8,9,11,12 a major impediment to uncovering potential causality. A classic demonstration of this is the almost universal positive association between lower serum albumin and CUA3,5,11; however, when measured months to years prior to CUA, low serum albumin was not a significant risk factor in this study. In contrast, factors likely unchanged by dialysis such as diabetes status, obesity, and of course white race and female sex, were reported previously and in our study as well. Since the current study examined risk factors at the time of hemodialysis initiation, months to years prior to the CUA diagnosis, we provide potential insights into the CUA pathobiology and suggest potentially modifiable factors to prevent and treat CUA.
We believe that the results of this study further strengthen the observations reported in previous cross-sectional studies regarding associations between increased serum calcium, serum phosphorous, and PTH levels and CUA risk, and hint that previous cross-sectional studies that did not observe similar positive associations could be either biased due to lack of lag time between exposure and outcome or levels of these parameters could be different once CUA develops, due to deposition of minerals in skin tissue and/or due to treatments introduced to treat mineral bone disease following CUA development. Although median PTH levels at hemodialysis initiation were higher for CUA cases compared with controls in our study, they were still within the Kidney Disease Improving Global Outcomes study recommended range of within two to nine times the upper range of normal for the majority of CUA cases, raising questions regarding what should be a target PTH for CUA prevention and how this could be achieved.17 Novel observations from our large study regarding differential associations between therapeutic agents targeted at mineral and bone metabolism abnormalities (cinacalcet and nutritional vitamin D associated with increased odds of subsequent CUA, whereas such association was not observed with activated vitamin D treatment in the primary analysis) demonstrate the significance of evaluating risk factors months to years prior to CUA development, when treatment decisions are not confounded by the existent CUA. These findings will need careful assessment in other prospective registries.
Our study findings support the ongoing and future translational investigations focused on metabolic syndrome, adipose tissue biology, mineral bone disease, skin trauma, and vitamin K metabolism in the pathogenesis of CUA.1,18,19 Insulin-related skin trauma is particularly interesting and has been previously speculated in case reports.20,21 Dermal arteriolar endothelial damage caused due to immune reactions to insulin triggering a procoagulant state or adverse anabolic effects of insulin on local dermal architecture are potential underlying mechanisms; however, these actions may not be unique to insulin as various other skin traumas, such as chronic resting of elbows on thighs and application of ice packs, have also been reported to trigger CUA.20 Our confirmation of association between warfarin therapy (vitamin K antagonist) and CUA will inform risk-benefit discussion of warfarin use in patients receiving hemodialysis, especially as novel oral anticoagulants become available for this population. This confirmation also stimulates examination of other potential causes of vitamin K deficiency (e.g., suboptimal intake, antibiotic exposure, etc.) for CUA. Patients receiving dialysis have a high prevalence of vitamin K deficiency, even in the absence of warfarin therapy,22,23 and future research is needed to determine if correction of vitamin K deficiency prevents CUA. Ideally, only a randomized controlled trial can conclusively address the efficacy of preventive approaches; however, limitations of rare disease research and ethical concerns need to be taken into account and creative trial designs will be needed.24–26
While our study had several strengths, we cannot ignore certain limitations. Residual confounding cannot be excluded and causality cannot be inferred. Although the increased CUA risks among patients using cinacalcet and nutritional vitamin D were consistent across the severity spectrum of mineral and bone metabolism markers, the observational nature of our study cannot rule out reverse causality. This is especially relevant because in the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial, relative CUA risk reduction of 69%–75% in the cinacalcet arm compared with the placebo arm was noted,27,28 and in the Study to Evaluate Cinacalcet Plus Low Dose Vitamin D on Vascular Calcification in Subjects With Chronic Kidney Disease Receiving Hemodialysis (ADVANCE) trial there was a trend toward attenuation in the progression of aortic and valvular calcifications with cinacalcet treatment.29 In our case-control analyses restricted to patients with moderate to severe hyperparathyroidism to resemble the EVOLVE trial, cinacalcet use did not have a statistically significant association with CUA risk (Supplemental Table 1) but sample size was limited; and indeed, a statistically significant association across all the categories of PTH levels was observed when we analyzed data from the entire contemporaneous FMCNA cohort (Supplemental Table 2). Similarly, causality to skin trauma cannot be attributed in an observational study. We relied on the treating clinicians to report CUA cases introducing a potential misclassification bias. There is a potential for survivorship bias; however, this would tend to bias the results toward the null. Due to uncertainty regarding the precise time of CUA development, we did not conduct repeated measures analyses to examine risk factors at 3 months or 6 months prior to CUA; however, we are certain that none of the cases had CUA at the time of hemodialysis initiation, and the earliest CUA diagnosis in terms of dialysis initiation timing was at 194 days.
In conclusion, risk factors for CUA are present months to years prior to CUA development in patients receiving hemodialysis. CUA has a predilection for white, female, diabetic, and obese patients and, especially in these patients, research and clinical attention should focus on avoiding additional CUA triggers such as vitamin K antagonism or deficiency, skin trauma, and mineral bone abnormalities.
Concise Methods
The study protocol was approved and granted a waiver of informed consent by the New England Institutional Review Board.
Study Patients
Data were derived from the FMCNA database that prospectively captures data elements for the purposes of health care delivery and epidemiologic studies. Multiple prior high-quality publications have resulted from the FMCNA database used in this study.30,31 Approximately 35% of the United States adult outpatient dialysis population is represented in the FMCNA database. The Clinical Quality Group and Data Entry Error Reduction Task Force at FMCNA mandates protocols and audits incoming information to ensure data are accurately documented for clinical care and Medicare billing. A single provider (Spectra Laboratories, Rockleigh, NJ) performs all laboratory testing and the results are directly downloaded into the database. Patient prescriptions for oral and home medications are charted in the FMCNA database on standardized medication tables. Registered nurses at the dialysis facilities transcribe each drug name with its dose, route, start date, and stop date, as labeled on the medication bottle, into the database forms. Injectable medications administered at dialysis are recorded for each patient on a per treatment basis.
CUA cases have been prospectively tracked at the FMCNA since January 2010 by requesting clinicians to report every new CUA case to FMCNA and to submit relevant information in a questionnaire that records information on how the CUA diagnosis was made (biopsy confirmation versus clinical diagnosis) and characteristics of CUA skin lesions. New CUA cases diagnosed between January 1, 2010, and December 31, 2014 in adult (aged >18 years) patients receiving hemodialysis at one of the FMCNA facilities were eligible for inclusion in this matched case-control study. After excluding patients with CUA receiving peritoneal dialysis (n=107) and patients for whom the ascertainment of CUA diagnosis could not be done due to nonavailability of the questionnaire to be filled out by a treating clinician (n=152), we identified 1030 CUA cases to be included in this study. In all these cases, the ascertainment of CUA diagnosis was made by a manual review (by S.U.N.) of questionnaires filled out by treating clinicians. Controls (hemodialysis patients without the diagnosis of CUA receiving dialysis care at one of the FMCNA facilities during the contemporaneous time period) were randomly selected and matched to cases on age (±5 years), sex, and race in 2:1 ratio. The final sample size was 1030 cases and 2060 controls. In addition to these CUA cases and controls, we also identified all the remaining patients (n=259,212) who were receiving dialysis care at the FMCNA during the contemporaneous study period, to conduct sensitivity analyses and to compute CUA incidence rate.
Study Data
Data on demographics, comorbidities, dialysis treatments, medications, and laboratory variables were obtained from the FMCNA database described above. These data were obtained within the first 90 days of hemodialysis initiation. Medication exposure was defined as any use of that medication within the first 90 days of hemodialysis initiation. For patients who had multiple laboratory measurements (including for mineral bone parameters) within the first 90 days of hemodialysis initiation, mean levels were computed for analyses. The median numbers of mineral metabolism parameter values available for computation during the first 90 days of hemodialysis initiation were as follows: serum calcium, 5 (IQR, 3–5); phosphorous, 5 (IQR, 3–5); PTH, 3 (IQR, 2–4); and 25-hydroxyvitamin D, 1 (IQR, 1–2). Serum calcium levels were corrected for albumin using the following formula: albumin-corrected serum calcium = measured serum calcium + 0.8 (4 − measured serum albumin).32 Data on CUA lesions and method of CUA diagnosis were obtained from a review of the questionnaires provided by treating clinicians.
Statistical Analyses
Frequency (for categorical variables), mean and SD values (for normally distributed variables), and median and IQR (for non-normally distributed variables) were reported. Categorical variables were compared between cases and controls using a χ2 test. Continuous variables were compared using independent samples t test or Mann–Whitney U test. Comparisons were also made between patients with central CUA and peripheral CUA and between patients with diabetes and CUA lesions involving lower abdomen and/or upper thigh areas and controls with diabetes mellitus.
To test the association between risk factors and CUA, conditional logistic regression models were applied to compute OR and 95% CI. Any confounding effect from age, sex, and race was handled via the matching procedure and all the other covariates listed in Table 1 were included in primary multivariable-adjusted analyses. We also conducted secondary multivariable-adjusted analyses adjusted for covariates identified by stepwise selection and sensitivity analyses restricted to patients with CUA with reported diagnostic biopsy confirmation. Separate multivariable conditional logistic regression analyses (adjusted for all covariates listed in Table 2, adjusted for covariates identified by stepwise selection, and restricted to patients with CUA with reported diagnostic biopsy confirmation) were also conducted to identify independent risk factors for central and peripheral CUA. We examined the association between insulin injections and CUA involving usual sites of insulin administration in patients with diabetes by constructing unadjusted and adjusted logistic regression models for three categories of insulin frequency (one or two, three, and more than three injections per day), with zero injections per day as a referent. We conducted conditional logistic regression analyses to examine the risk of CUA for cinacalcet users and nutritional vitamin D users across tertiles of mineral metabolism markers in the case/control population and also conducted logistic regression analyses on data from the entire FMCNA cohort during the contemporaneous time period.
We calculated the CUA incidence rate (mean, 95% CI) per 1000 patient-years in the FMCNA cohort by assuming it followed a Poisson distribution and applying the following equation: CUA incidence rate = number of new CUA cases/sum of person follow-up time at risk.
The Cochran–Armitage test was conducted to examine the underlying trend of CUA incidence rate across patient groups by their frequency of insulin injections.
All analyses were performed using the SAS program (version 9.4) (SAS Institute Inc., Cary, NC). Statistical significance was set as P<0.05.
Disclosures
S.U.N. reports receiving lecture honoraria from Sanofi-Aventis (Bridgewater, NJ) and is a prior recipient of Nephrology Fellowship Award from Sanofi-Aventis (Bridgewater, NJ). R.I.T. is a consultant to Fresenius Medical Care North America (Waltham, MA) and Celgene (Summit, NJ), and has received a research grant from Abbott Laboratories (Chicago, IL). Drs. Hymes, Maddux, and Chan are employees of the Fresenius Medical Care North America (Waltham, MA).
Supplementary Material
Acknowledgments
The authors would like to thank the employees of Fresenius Medical Care North America for their contributions to calciphylaxis research.
This work was conducted with the support of a KL2/Catalyst Medical Research Investigator Training award to S.U.N. (an appointed KL2 award) from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources [NCRP] and the National Center for Advancing Translational Sciences, he National Institutes of Health (NIH) Award KL2 TR001100). S.U.N. is also supported by National Kidney Foundation’s Young Investigator Award, Fund for Medical Discovery Award from Massachusetts General Hospital’s Executive Committee on Research (R00000000007190), American Heart Association’s NCRP Summer 2014 Mentored Clinical and Population Research Award (15CRP22900008), and by American Heart Association’s NCRP Winter 2015 Fellow-to-Faculty Transition Award (15FTF25980003). R.I.T. is supported by NIH grants DK094872 and DK094486.
Preliminary findings of this manuscript were presented as a poster abstract at the American Society of Nephrology Kidney Week 2015 on November 7th, 2015 in San Diego, CA.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the NIH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Calcific Uremic Arteriolopathy Revisited,” on pages 3233–3235.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015091065/-/DCSupplemental.
References
- 1.Hayden MR, Kolb LG, Khanna R: Calciphylaxis and the cardiometabolic syndrome. J Cardiometab Syndr 1: 76–79, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Nigwekar SU, Solid CA, Ankers E, Malhotra R, Eggert W, Turchin A, Thadhani RI, Herzog CA: Quantifying a rare disease in administrative data: the example of calciphylaxis. J Gen Intern Med 29[Suppl 3]: S724–S731, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigwekar SU, Bhan I, Turchin A, Skentzos SC, Hajhosseiny R, Steele D, Nazarian RM, Wenger J, Parikh S, Karumanchi A, Thadhani R: Statin use and calcific uremic arteriolopathy: a matched case-control study. Am J Nephrol 37: 325–332, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelis M, Wong LL, Myers SA, Wong LM: Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery 122: 1083–1089, discussion 1089–1090, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, Sato Y Japanese Calciphylaxis Study Group : A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant 27: 1580–1584, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Nigwekar SU, Kroshinsky D, Nazarian RM, Goverman J, Malhotra R, Jackson VA, Kamdar MM, Steele DJ, Thadhani RI: Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 66: 133–146, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazhar AR, Johnson RJ, Gillen D, Stivelman JC, Ryan MJ, Davis CL, Stehman-Breen CO: Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int 60: 324–332, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR: Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 56: 569–579, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Zacharias JM, Fontaine B, Fine A: Calcium use increases risk of calciphylaxis: a case-control study. Perit Dial Int 19: 248–52, 1999 [PubMed]
- 10.Fine A, Zacharias J: Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int 61: 2210–2217, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed S, O’Neill KD, Hood AF, Evan AP, Moe SM: Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis 37: 1267–1276, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Bleyer AJ, Choi M, Igwemezie B, de la Torre E, White WL: A case control study of proximal calciphylaxis. Am J Kidney Dis 32: 376–383, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Galloway PA, El-Damanawi R, Bardsley V, Pritchard NR, Fry AC, Ojha SK, Hiemstra TF: Vitamin K antagonists predispose to calciphylaxis in patients with end-stage renal disease. Nephron 129: 197–201, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Sprague SM: Painful skin ulcers in a hemodialysis patient. Clin J Am Soc Nephrol 9: 166–173, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigwekar SU, Brunelli SM, Meade D, Wang W, Hymes J, Lacson E Jr: Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol 8: 1162–1170, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandenburg VM, Cozzolino M, Ketteler M: Calciphylaxis: a still unmet challenge. J Nephrol 24: 142–148, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Moe SM, Drüeke TB, Block GA, Cannata-Andía JB, Elder GJ, Fukagawa M, Jorgetti V, Ketteler M, Langman CB, Levin A, MacLeod AM, McCann L, McCullough PA, Ott SM, Wang AY, Weisinger JR, Wheeler DC, Persson R, Earley A, Moorthi R, Uhlig K: Kidney Disease: Improving Global Outcomes CKDMBDWG: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Chen NX, O’Neill K, Akl NK, Moe SM: Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun 449: 151–156, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Brandenburg VM, Schurgers LJ, Kaesler N, Püsche K, van Gorp RH, Leftheriotis G, Reinartz S, Koos R, Krüger T: Prevention of vasculopathy by vitamin K supplementation: can we turn fiction into fact? Atherosclerosis 240: 10–16, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Ruggian JC, Maesaka JK, Fishbane S: Proximal calciphylaxis in four insulin-requiring diabetic hemodialysis patients. Am J Kidney Dis 28: 409–414, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Handa SP, Sohi PS: Proximal calciphylaxis in four insulin-requiring diabetic hemodialysis patients. Am J Kidney Dis 29: 812, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Pilkey RM, Morton AR, Boffa MB, Noordhof C, Day AG, Su Y, Miller LM, Koschinsky ML, Booth SL: Subclinical vitamin K deficiency in hemodialysis patients. Am J Kidney Dis 49: 432–439, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Schlieper G, Westenfeld R, Krüger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, Djuric Z, Damjanovic T, Ketteler M, Vermeer C, Dimkovic N, Floege J, Schurgers LJ: Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol 22: 387–395, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ketteler M, Biggar PH: Evolving calciphylaxis--what randomized, controlled trials can contribute to the capture of rare diseases. Clin J Am Soc Nephrol 10: 726–728, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandenburg VM, Cozzolino M, Mazzaferro S: Calcific uremic arteriolopathy: a call for action. Semin Nephrol 34: 641–647, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Kovesdy CP, Kalantar-Zadeh K: Observational studies versus randomized controlled trials: avenues to causal inference in nephrology. Adv Chronic Kidney Dis 19: 11–18, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Floege J, Kubo Y, Floege A, Chertow GM, Parfrey PS: The Effect of Cinacalcet on Calcific Uremic Arteriolopathy Events in Patients Receiving Hemodialysis: The EVOLVE Trial. Clin J Am Soc Nephrol 10: 800–807, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS EVOLVE Trial Investigators : Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367: 2482–2494, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, Moustafa M, Goodman WG, Lopez N, Downey G, Dehmel B, Floege J ADVANCE Study Group : The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 26: 1327–1339, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Chan KE, Newton-Cheh C, Gusella JF, Maddux FW: Heritability of Risk for Sudden Cardiac Arrest in ESRD. J Am Soc Nephrol 26: 2815–2820, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW: Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation 131: 972–979, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne RB, Little AJ, Williams RB, Milner JR: Interpretation of serum calcium in patients with abnormal serum proteins. BMJ 4: 643–646, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.