Abstract
Colonic microbial metabolism substantially contributes to uremic solute production. p-Cresyl sulfate and indoxyl sulfate are the main representatives of solutes of microbial origin and also, protein-bound solutes, exhibiting high protein-binding affinity and dependence on tubular secretion. Phenylacetylglutamine is another microbial metabolite with high dependence on tubular secretion but low protein-binding affinity. The relevance of such solutes is unknown. Therefore, we prospectively followed 488 patients with CKD stages 1–5 and a measurement of serum phenylacetylglutamine by liquid chromatography-mass spectrometry. In a subgroup, we determined 24-hour urinary excretion as a surrogate of intestinal uptake as well as renal clearance of phenylacetylglutamine. We performed outcome analysis for mortality (51 events) and cardiovascular disease (75 events). Serum phenylacetylglutamine level correlated with 24-hour urinary excretion (rho=0.55; P<0.001) and clearance of phenylacetylglutamine (rho=−0.76; P<0.001). Phenylacetylglutamine clearance also correlated with eGFR (rho=0.84; P<0.001). Furthermore, serum phenylacetylglutamine level associated with mortality (hazard ratio per 1-SD increase, 1.77; 95% confidence interval, 1.22 to 2.57; P=0.003) and cardiovascular disease (hazard ratio, 1.79; 95% confidence interval, 1.32 to 2.41; P<0.001) after adjustment for age, sex, presence of diabetes mellitus, prior cardiovascular disease, and eGFR. Thus, serum phenylacetylglutamine level is elevated in patients with more advanced CKD and determined by intestinal uptake and renal clearance, and it is not fully accounted for by differences in eGFR. High serum phenylacetylglutamine level is a strong and independent risk factor for mortality and cardiovascular disease, suggesting the relevance of microbial metabolism and/or tubular dysfunction in CKD, irrespective of protein binding.
Keywords: chronic kidney disease, uremia, intestine, tubular epithelium
The colonic microbial metabolism is a key contributor to the syndrome of uremia.1,2 Vice versa, CKD along with renal dietary restrictions also have a substantial influence on microbial function.3 The most widely studied uremic retention solutes originating from colonic microbial metabolism are p-cresyl sulfate and indoxyl sulfate. p-Cresyl sulfate results from the combined actions of microbial fermentation of the amino acid tyrosine to p-cresol and endogenous sulfate conjugation. Likewise, indoxyl sulfate is the end product of microbial fermentation of the amino acid tryptophan to indole followed by endogenous oxidation and sulfate conjugation.2 Both p-cresyl sulfate and indoxyl sulfate have been repeatedly associated with mortality and cardiovascular disease in patients with CKD as supported by mechanistic studies.4–12
Phenylacetylglutamine (PAG) is another recently identified colonic microbial metabolite from amino acid fermentation. It results from glutamine conjugation of phenylacetic acid, which is almost exclusively derived from the microbial conversion of phenylalanine.1,13 Other than their microbial origin, p-cresyl sulfate, indoxyl sulfate, and PAG have in common that active tubular secretion and not glomerular filtration is the predominant pathway for their renal clearance.14–16 Renal clearance of PAG is highly efficient, approaching renal plasma flow in healthy individuals.16 As hypothesized by Sirich et al.,16 the potential toxic nature of these solutes in patients with CKD may be derived from their dependence on high tubular secretion, because evolution has provided the native kidney with the ability of highly efficient removal of toxic solutes.16 Currently, RRT cannot substitute for tubular function, making these solutes particularly prone to accumulation in patients on hemodialysis as illustrated by predialysis serum levels of PAG that are >100-fold higher than those of healthy volunteers.17
Although p-cresyl sulfate and indoxyl sulfate are considered representatives of the group of protein-bound solutes caused by high protein binding,18 PAG is much less protein bound, with a bound fraction of approximately 20%.16,19 In general, it is often stated that protein binding limits solute removal by the native kidney as well as RRT, thus promoting accumulation and possibly, toxicity.20 However, it can be assumed that only the free fraction of these solutes is biologically active and potentially harmful to the human body. Actually, as shown in the work by Sirich et al.,16 when considering free solute levels, protein binding enhances clearance by providing a readily accessible reservoir for efficient removal of the solute throughout its passage within the native kidney or dialyzer.16,17 Also, as the unbound solute fraction increases along with renal function decline, accumulation of the free solute fraction is even more pronounced, whereas increases in total solute concentrations are rather attenuated.17,20
To date, the bad reputation of p-cresyl sulfate and indoxyl sulfate has been mainly attributed to their protein binding characteristics,18 whereas the contribution of both tubular function and microbial metabolism may have been neglected. To test this hypothesis, we aimed to explore the relationship between serum PAG and adverse outcomes in patients with CKD not yet on dialysis. Furthermore, we investigated the differential contribution of renal function and intestinal uptake to serum levels of PAG in different stages of CKD.
Results
Inclusion and Baseline Characteristics
Between November of 2005 and September of 2006, 499 prevalent patients with CKD Kidney Disease Outcomes Quality Initiative stages 1–5 were included in the Leuven Mild-to-Moderate CKD Study. Measurements of serum PAG were available in a total of 488 patients (Supplemental Figure 1). Baseline characteristics of the study population according to tertiles of serum PAG are presented in Table 1. Glomerular disease was the most prevalent underlying renal disease (32.0%) followed by vascular disease (20.3%), congenital disease (including autosomal dominant polycystic disease; 13.9%), diabetic nephropathy (7.6%), and tubulointerstitial disease (7.0%).
Table 1.
Baseline characteristics of study population
| Variable | Overall, n=488 | PAG | P Value | ||
|---|---|---|---|---|---|
| Tertile 1, n=162 | Tertile 2, n=163 | Tertile 3, n=163 | |||
| Age, yr | 64 (50–74) | 51 (37–63) | 69 (57–75) | 72 (59–78) | <0.001 |
| Sex: men/women (%) | 270/218 (55.3/44.7) | 86/70 (53.1/46.9) | 92/71 (56.4/43.6) | 92/71 (56.4/43.6) | 0.54 |
| Prior CVD: yes/no (%) | 135/353 (27.7/72.3) | 23/139 (14.2/85.8) | 49/114 (30.1/69.9) | 63/100 (38.7/61.3) | <0.001 |
| Diabetes mellitus: yes/no (%) | 89/399 (18.2/81.8) | 15/147 (9.3/90.7) | 26/137 (16.0/84.0) | 48/115 (29.5/70.5) | <0.001 |
| Current smoker: yes/no (%) | 90/398 (18.4/81.6) | 37/125 (22.8/77.2) | 24/139 (14.7/85.3) | 29/134 (17.8/82.2) | 0.24 |
| Body mass index, kg/m2 | 25.69 (22.99–29.06) | 25.20 (22.49–29.02) | 26.06 (23.15–29.06) | 25.51 (23.04–29.05) | 0.63 |
| Systolic BP, mmHg | 135 (120–150) | 130 (120–150) | 139 (120–150) | 140 (122–160) | <0.01 |
| Diastolic BP, mmHg | 80 (70–85) | 80 (70–85) | 78 (70–84) | 80 (70–85) | 0.06 |
| Hemoglobin, g/dl | 13.3 (12.1–14.5) | 14.3 (13.1–15.4) | 13.3 (12.0–14.3) | 12.5 (11.4–13.5) | <0.001 |
| Albumin, g/L | 44.8 (42.4–46.9) | 45.8 (43.4–47.3) | 45.2 (43.3–47.2) | 43.7 (41.4–45.9) | <0.001 |
| C-reactive protein, mg/L | 2 (1–6) | 1 (1–5) | 2 (1–4) | 3 (1–7) | 0.004 |
| Cholesterol, mg/dl | 178 (152–205) | 184 (160–206) | 181 (156–207) | 165 (142–188) | <0.001 |
| LDL, mg/dl | 85 (66–111) | 91 (71–115) | 85 (68–114) | 78 (61–100) | 0.004 |
| HDL, mg/dl | 57 (47–72) | 60 (50–77) | 57 (47–70) | 56 (45–70) | 0.03 |
| Calcium, mg/dl | 9.6 (9.2–9.9) | 9.5 (9.2–9.9) | 9.6 (9.3–10.0) | 9.6 (9.2–9.9) | 0.57 |
| Phosphate, mg/dl | 3.3 (2.9–3.8) | 3.1 (2.7–3.5) | 3.2 (2.9–3.6) | 3.6 (3.2–4.2) | <0.001 |
| Parathormone, ng/L | 23.5 (12.5–51.4) | 14.1 (5.2–23.6) | 23.8 (14.3–47.7) | 46.0 (22.9–79.3) | <0.001 |
| Creatinine, mg/dl | 1.78 (1.26–2.43) | 1.15 (0.97–1.52) | 1.75 (1.42–2.13) | 2.51 (2.01–3.50) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 35 (23–56) | 66 (44–81) | 35 (27–45) | 22 (15–28) | <0.001 |
| Therapy with ACEI/ARB: yes/no (%) | 345/143 (70.7/29.3) | 113/49 (69.8/31.2) | 116/47 (71.2/28.8) | 116/47 (71.2/28.8) | 0.78 |
| Therapy with statins: yes/no (%) | 236/252 (48.4/51.6) | 59/103 (36.4/63.6) | 90/73 (55.2/44.8) | 87/76 (53.4/46.6) | 0.002 |
| Therapy with 25-OH vitamin D: yes/no (%) | 89/399 (18.2/81.8) | 15/147 (9.3/90.7) | 28/135 (17.2/82.8) | 46/117 (28.2/71.8) | <0.001 |
| Therapy with phosphate binder: yes/no (%) | 133/355 (27.3/72.7) | 23/139 (14.2/85.8) | 36/127 (22.1/77.9) | 74/89 (45.4/54.6) | <0.001 |
| PAG, μM | 6.21 (2.99–13.18) | 1.96 (1.06–2.97) | 6.20 (4.93–7.83) | 17.09 (13.14–27.51) | <0.001 |
Data are expressed as means (SDs) or medians (IQRs) as appropriate. Differences between tertiles were tested using Kruskal–Wallis or chi-squared test as appropriate. CVD, cardiovascular disease; ACEI, angiotensin–converting enzyme inhibitor; ARB, angiotensin receptor blocker; 25-OH vitamin D, 25-hydroxyvitamine D.
Determinants of Serum PAG
Serum PAG amounted to a median of 6.21 μM (interquartile range [IQR], 2.99–13.18). There was a strong inverse correlation between serum PAG and eGFR (Spearman rank correlation rho =−0.76; P<0.001). Other correlations between baseline characteristics and serum PAG are presented in Supplemental Table 1. In multivariate regression analysis, independent determinants of serum PAG were age (β=0.008; standardized β=0.12; P<0.001), body mass index (β=−0.01; standardized β=−0.06; P=0.04), hemoglobin (β=−0.05; standardized β=−0.08; P=0.03), albumin (β=−0.02; standardized β=−0.07; P=0.02), and eGFR (β=−1.26; standardized β=−0.66; P<0.001; model R2 =0.59) (Supplemental Table 2).
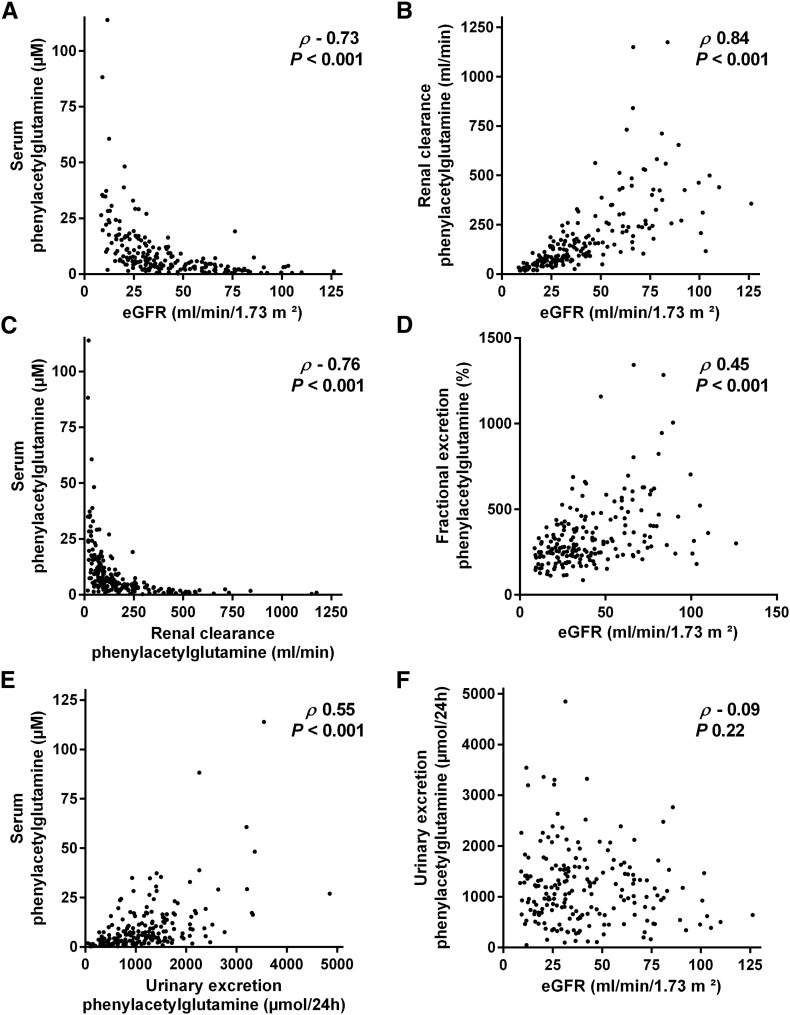
We also sampled 24-hour urinary collections in a subgroup of 200 patients to calculate renal clearance and 24-hour urinary excretion of PAG. Apart from a small, although significant difference in age (median =60 years old; IQR, 48–72 in the subgroup versus median =64 years old; IQR, 50–74 in the full cohort; P=0.02), there were no differences in baseline characteristics, including serum PAG, between the subgroup and the full cohort (data not shown). Renal clearance of PAG amounted to a median of 119 ml/min (IQR, 66–235), which was approximately 3.3-fold (IQR, 2.48–4.78) higher than eGFR. Although clearance of PAG was strongly correlated with eGFR (rho=0.84; P<0.001) (Figure 1B), correlation between serum PAG and clearance of PAG (rho=−0.76; P<0.001) was nominally higher than correlation between serum PAG and eGFR (rho=−0.73; P<0.001) (Figure 1, A and C) (Supplemental Figure 2). Correlation with serum PAG was also slightly lower when considering measured creatinine clearance instead of eGFR (rho=−0.69; P<0.001). Furthermore, we observed a significant and direct correlation between eGFR and fractional excretion of PAG (rho=0.45; P<0.001) (Figure 1D). When focusing on patients with eGFR<30 ml/min per 1.73 m2, we observed a moderate correlation between serum PAG and renal clearance of PAG (rho=−0.59; P<0.001) that was, again, nominally higher than the correlations between serum PAG and eGFR (rho=−0.48; P<0.001) and between serum PAG and measured creatinine clearance (rho=−0.43; P<0.001).
Figure 1.
Determinants of serum phenylacetylglutamine (PAG). Correlation between (A) serum PAG and eGFR, (B) renal clearance of PAG and eGFR, (C) serum PAG and renal clearance of PAG, (D) fractional excretion of PAG and eGFR, (E) serum PAG and urinary excretion of PAG, and (F) urinary excretion of PAG and eGFR (n=200).
In addition, we noted a significant correlation between serum PAG and 24-hour urinary excretion of PAG (rho=0.55; P<0.001), whereas there was no correlation between 24-hour urinary excretion of PAG and eGFR (rho=−0.09; P=0.22) (Figure 1, E and F). In linear regression, adding 24-hour urinary excretion of PAG to eGFR substantially improved model fit for serum PAG (R2=0.82) (Table 2).
Table 2.
Regression analysis: Associations of serum PAG (Ln) with 24-hour urinary excretion and eGFR (n=200)
| Variable | Unit | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|
| β | P Value | R2 | β | Standardized β | P Value | R2 | ||
| 24-h Urinary excretion (Ln) | Micromoles per 24 h | 0.92 | <0.001 | 0.36 | 0.85 | 0.55 | <0.001 | 0.82 |
| eGFR (Ln) | Milliliters per minute per 1.73 m2 | −1.33 | <0.001 | 0.52 | −1.25 | −0.68 | <0.001 | 0.82 |
Ln, natural logarithmic transformation.
Furthermore, we calculated 24-hour protein intake and studied its relationship with serum PAG and 24-hour urinary excretion of PAG. Although there was no significant correlation between 24-hour protein intake and serum PAG (rho=−0.07; P=0.32), we noted a significant correlation between 24-hour protein intake and 24-hour urinary excretion of PAG (rho=0.35; P<0.001). In multivariate regression analysis, including demographic and biochemical parameters, 24-hour protein intake was also significantly associated with serum PAG (β=0.96; standardized β=0.24; P<0.001).
Serum PAG and Overall Mortality
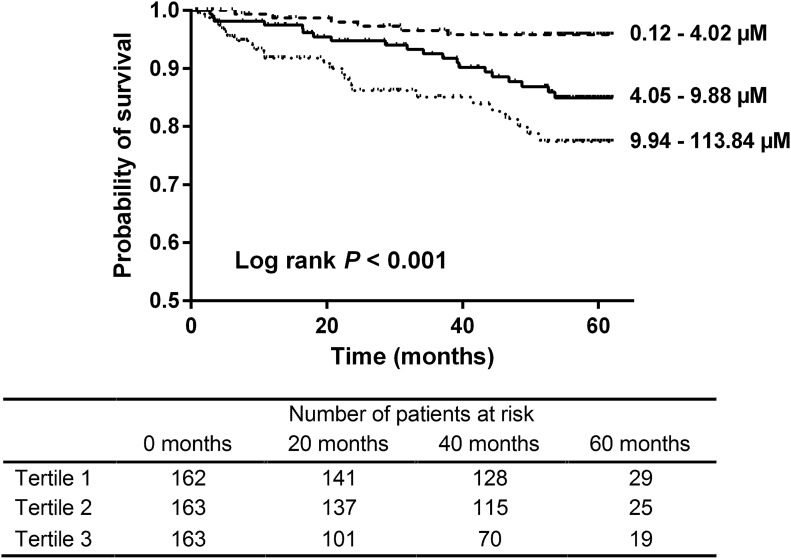
During follow-up, we observed a total of 51 deaths (Supplemental Table 3), with significantly more deaths among patients with serum PAG in higher tertiles (tertiles 1–3: 6, 20, and 25 events, respectively; log rank P<0.001) (Figure 2). In univariate Cox proportional hazard analysis, serum PAG was also significantly associated with overall mortality (hazard ratio [HR] per 1-SD increase, 2.17; 95% confidence interval [95% CI], 1.57 to 2.99; P<0.001) (Table 3). This association remained significant after adjustment for age, sex, presence of diabetes mellitus, prior cardiovascular disease, and eGFR (full-model HR, 1.77; 95% CI, 1.22 to 2.57; P=0.003).
Figure 2.
Phenylacetylglutamine (PAG) and overall mortality. Kaplan–Meier survival curve. Tertiles of serum PAG. Tertiles 1–3: 6, 20, and 25 events, respectively. Log rank test P<0.001.
Table 3.
Cox proportional hazard survival analysis (n=51 events)
| Variable | HR per 1-SD Increase (95% CI) | P Value |
|---|---|---|
| Unadjusted: PAG (Ln) | 2.17 (1.57 to 2.99) | <0.001 |
| Full model with adjustment for age, sex, diabetes mellitus, prior cardiovascular disease, and eGFR (Ln) | 1.77 (1.22 to 2.57) | 0.003 |
Ln, natural logarithmic transformation.
For comparison, we also explored the relationship between overall mortality and serum levels of free p-cresyl sulfate. As can be derived from Table 4, serum free p-cresyl sulfate was significantly associated with overall mortality in univariate analysis, whereas there was no independent association after adjustment for the same covariates.
Table 4.
Cox proportional hazard analysis for PAG and free p-cresyl sulfate
| Model | PAG (Ln) | Free p-Cresyl Sulfate (Ln) | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Mortality | ||||
| Unadjusted | 2.17 (1.57 to 2.99) | <0.001 | 2.23 (1.57 to 3.15) | <0.001 |
| Full model | 1.77 (1.22 to 2.57) | 0.003 | 1.30 (0.81 to 2.07) | 0.28 |
| Cardiovascular disease | ||||
| Unadjusted | 2.21 (1.70 to 2.87) | <0.001 | 2.25 (1.69 to 2.98) | <0.001 |
| Full model | 1.79 (1.32 to 2.41) | <0.001 | 1.58 (1.15 to 2.17) | 0.005 |
Full model with adjustment for age, sex, diabetes mellitus, prior cardiovascular disease, and eGFR (Ln). HR is presented per 1-SD increase (95% CI). Ln, natural logarithmic transformation.
Serum PAG and Cardiovascular Disease
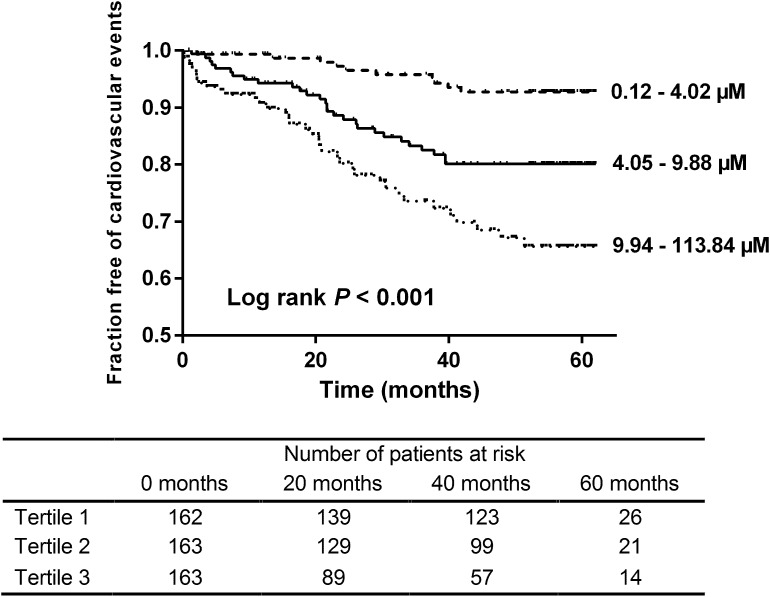
Next, we explored the relationship between serum PAG and cardiovascular disease, observing 75 cardiovascular events during follow-up (Supplemental Table 4). The number of events was significantly higher among patients with PAG in higher tertiles of serum PAG (tertiles 1–3: 10, 28, and 37 events, respectively; log rank P<0.001) (Figure 3). Serum PAG was also significantly associated with cardiovascular disease in univariate Cox proportional hazard analysis (HR, 2.21; 95% CI, 1.70 to 2.87; P<0.001) (Table 5). In addition, the relationship between serum PAG and cardiovascular events during follow-up remained significant after adjustment for age, sex, presence of diabetes mellitus, prior cardiovascular disease, and eGFR (full-model HR, 1.79; 95% CI, 1.32 to 2.41; P<0.001).
Figure 3.
Phenylacetylglutamine (PAG) and cardiovascular disease. Kaplan–Meier curve of time to first cardiovascular event. Tertiles of serum PAG. Tertiles 1–3: 10, 28, and 37 events, respectively. Log rank test P<0.001.
Table 5.
Cox proportional hazard analysis of time to first cardiovascular event (n=75 events)
| Variable | HR per 1-SD Increase (95% CI) | P Value |
|---|---|---|
| Unadjusted: PAG (Ln) | 2.21 (1.70 to 2.87) | <0.001 |
| Full model with adjustment for age, sex, diabetes mellitus, prior cardiovascular disease, and eGFR (Ln) | 1.79 (1.32 to 2.41) | <0.001 |
Ln, natural logarithmic transformation.
As can be derived from Table 4, associations between cardiovascular events and serum solute levels were comparable for PAG and free p-cresyl sulfate.
Discussion
In this study, we explored the behavior of PAG, a recently identified uremic retention solute originating from microbial metabolism of the amino acid phenylalanine in patients with mild to moderate CKD. The key findings are as follows: (1) serum levels of PAG are higher in patients with more advanced CKD; (2) interindividual variability in serum PAG can be explained by differences in renal clearance, even when adjusted for eGFR, as well as by differences in 24-hour urinary excretion as a marker of intestinal uptake; and (3) high serum PAG is a strong and independent risk factor for overall mortality and cardiovascular disease, pointing to the relevance of microbial metabolism and tubular secretion, irrespective of protein binding.
In recent years, there has been increasing interest in the colonic microbial metabolism as a contributor to uremic retention solutes accumulating in CKD, with p-cresyl sulfate and indoxyl sulfate as the main representatives of this group of solutes.1,2 Both solutes have been repeatedly associated to adverse outcomes in patients with renal dysfunction.4–8,21 This has been mainly attributed to their protein binding characteristics,18 especially in patients on RRT and when potentially wrongfully, focusing on removal of total serum levels.22 In patients not yet on dialysis, these protein-bound solutes depend on highly efficient tubular secretion for their renal clearance.14–16 It remains unknown whether other solutes with a colonic microbial origin and substantial tubular secretion but without high protein binding may also be of relevance in patients with CKD. Therefore, we measured serum levels of PAG in 488 patients with CKD not yet on dialysis and studied their determinants as well as their relationship with adverse outcomes.
Serum levels of PAG were associated with age, body mass index, hemoglobin, albumin, and renal function (model R2 =0.59). In the subgroup of patients with availability of 24-hour urinary collection, higher daily protein intake was another factor related to higher serum levels of PAG. Diminished renal function measured by eGFR (Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]) was the strongest determinant of higher levels of serum PAG, although considerable interindividual variability could still be observed. In a subgroup of patients with 24-hour urinary collection, we also measured renal clearance of PAG. Median renal PAG clearance was approximately 3.3-fold higher than eGFR, pointing to highly efficient tubular secretion and confirming previous findings in healthy volunteers.16 From this, it can be estimated that tubular secretory removal of PAG outweighs removal by glomerular filtration by more than twofold. Overall, renal clearance of PAG also declined slightly more than eGFR, suggesting saturation of tubular transporters at higher stages of CKD. Although in general, clearance of PAG was strongly correlated with eGFR, correlation between serum PAG and clearance of PAG was nominally higher than correlation between serum PAG and eGFR. Thus, although eGFR can be considered a reasonable estimate of renal clearance of PAG, disagreement between eGFR and renal PAG clearance may contribute to the observed variability of individual serum levels of PAG that is still present after adjusting for eGFR. Additional research will be necessary to explore tubular transporter mechanisms that are involved in renal clearance of PAG. We also measured 24-hour urinary excretion of PAG, which assuming negligible nonrenal clearance of PAG, functions as an indirect estimate of 24-hour intestinal uptake. We observed substantial interindividual variability of intestinal uptake of PAG, albeit without a clear influence of renal function, thus also contributing to the wide dispersion of individual serum levels of PAG as already shown for p-cresyl sulfate and indoxyl sulfate.23
In patients with mild to moderate CKD, serum PAG was strongly related to overall mortality and cardiovascular disease, even when adjusted for age, sex, presence of diabetes mellitus, prior cardiovascular disease, and eGFR. This finding confirms and extends a previous observation in patients on hemodialysis showing a relationship between serum PAG and cardiovascular disease.24 In addition, the association between serum solute levels and adverse outcomes was as least as strong for PAG as for free p-cresyl sulfate, highlighting the relevance of solutes with a microbial origin and highly efficient tubular secretion, irrespective of protein binding. Whether PAG itself is a true uremic toxin remains unknown. Although findings in healthy volunteers have suggested that high serum levels of PAG are relatively well tolerated, at least over the short term,25 the effects of ongoing exposure to this solute in patients with CKD need additional exploration, including mechanistic studies. Furthermore, because higher serum levels of PAG result from an imbalance between intestinal exposure and renal tubular secretion, serum PAG may merely be a marker for a deleterious colonic microbial metabolism and/or tubular secretory dysfunction. Regarding tubular function, an independent association between higher mortality and diminished renal clearance of hippurate, another solute with high tubular secretion, albeit with a protein-bound fraction of >50%, has recently been shown.26 Although these observations need confirmation in other larger patient populations, they suggest that a better understanding of renal tubular function is necessary not only to identify solutes that are prone to accumulation and thus, potentially harmful but also, in an attempt to improve current risk stratification of patients with CKD on the basis of eGFR and proteinuria.
There are limitations to our study. First, the study design precludes causal inferences. Second, our study population mainly consisted of white patients. Care must be taken when extrapolating our data to other patient populations. Third, assessment of completeness of urinary collections was arbitrary. We assumed completeness of urinary collections when urinary excretion of creatinine was within 2 SDs of the mean creatinine excretion for the geographic region of this study (the INTERSALT Study).27 Fourth, we cannot formally exclude solute production within the kidney, thus potentially overestimating renal clearance. Fifth, because we do not have simultaneous measurements of serum phenylacetic acid, we cannot differentiate between potential effects of concomitant higher serum levels of phenylacetic acid and increased phase 2 metabolism to PAG. Sixth, our analyses are on the basis of single baseline biochemical measurements that are potentially subjected to variability over time. Of note, substantial variability over time would only increase the likelihood to not show a significant association between PAG and adverse outcomes.
In conclusion, high serum levels of PAG are observed in patients with more advanced CKD and a strong and independent risk factor for overall mortality and cardiovascular disease. Additional research is necessary to elucidate whether PAG contributes directly to uremic toxicity or reflects the presence of a deleterious colonic microbial metabolism and/or tubular dysfunction.
Concise Methods
Study Population
This was an ancillary analysis of the Leuven Mild-to-Moderate CKD Study, a prospective cohort to investigate the role of uremic retention solutes in patients with CKD not yet on dialysis (Clinicaltrials.gov identifier: NCT00441623).7 Prevalent patients with CKD followed at the nephrology outpatient clinic of the University Hospitals Leuven who were ≥18 years of age and able to provide consent were eligible for inclusion. Mild to moderate CKD was defined as presence of kidney damage (i.e., pathologic abnormalities or abnormalities in urine or imaging tests) or eGFR<60 ml/min per 1.73 m2 for ≥3 months, with exclusion of patients on RRT or with a history of renal transplantation. Patients were screened between November of 2005 and September of 2006. The study was performed according to the Declaration of Helsinki and approved by the ethics committee of the University Hospitals Leuven. Informed consent was obtained from all patients.
Biochemical Measurements
At inclusion, blood was taken by venous puncture for measurement of hemoglobin (grams per deciliter), albumin (grams per liter), C-reactive protein (milligrams per liter), cholesterol (milligrams per deciliter), calcium (milligrams per deciliter), phosphate (milligrams per deciliter), biointact parathyroid hormone (nanograms per liter), and creatinine (milligrams per deciliter), all measured using standard laboratory techniques. The eGFR was calculated using the CKD-EPI equation.28 We also had ancillary data available on free serum levels of p-cresyl sulfate determined as p-cresol with a dedicated gas chromatography-mass spectrometry method, allowing comparison with a protein-bound solute.7,29 Additionally, serum levels of PAG were quantified by ultraperformance liquid chromatography-tandem mass spectrometry (Acquity–Xevo TQS; Waters, Milford, MA). For sample preparation, 50 μl serum or urine, 50 μl solution of milli-Q (MQ) water:MeOH:0.01 N sodium hydroxide (75:20:5 vol/vol/vol), 20 μl internal standard mixture (PAG-d5), and 150 μl acetonitrile were thoroughly mixed in 96-well Ostro Plates (Waters). After separation by a positive pressure manifold, supernatants were collected in 2-ml collection plates. Subsequently, the organic phase was removed by a gentle stream of nitrogen for 30 minutes at 40°C. After dilution with 1000 μl MQ water, 5 μl final solution was injected on the ultraperformance liquid chromatography-tandem mass spectrometry system. Chromatographic separation was performed on an Acquity CSHFluoroPhenyl Column (50×2.5 mm; 1.7-μm particle size; Waters). The mobile phase, delivered at a flow rate of 0.5 ml/min at 40°C, consisted of a gradient of 0.1% formic acid in MQ water (A) and MeOH (B). The gradient was as follows: starting with 3% B, there was a subsequent increase to 16% B within 1 minute followed by an increase to 80% B within 3 minutes and thereafter, an increase to 95% B within 30 seconds for a duration of 1 minute, after which the initial 3% B was reintroduced with equilibration for a duration of 3.5 minutes before the next injection. Ionization of PAG and the corresponding isotopologue (internal standard) was achieved in negative mode. The following multiple reaction monitoring transitions were used for quantification: PAG 263→145 and PAG-d5 268→145. Limit of detection and limit of quantification (LOQ) were 0.06 and 0.18 μM for PAG. For analysis, solute levels below the LOQ were treated as the average value of the limit of detection and the LOQ. The total, within–run, between–run, and between–day method imprecisions according to the National Committee for Clinical Laboratory Standards EP5-T guideline were 3.92%, 1.61%, 2.69%, and 2.02%, respectively, and the mean recovery was 97%. We also sampled 24-hour urinary collections when available at the time of inclusion to calculate renal clearance and 24-hour urinary excretion of PAG. Collections were considered complete when 24-hour urinary creatinine excretion was within 2 SDs (range =0.7–1.8 g) of the mean creatinine excretion for the geographic region of this study derived from the INTERSALT Study.27 Assuming steady-state conditions and negligible nonrenal clearance, 24-hour urinary excretion of PAG was considered an indirect estimate of 24-hour intestinal uptake of PAG. Furthermore, protein intake was calculated30 according to the formula by Maroni et al.31 using 24-hour urinary urea nitrogen excretion and body weight.
End Point Evaluation
After inclusion, patients were prospectively followed at the nephrology outpatient clinic at 3- to 6-month intervals until December 31, 2010. End point evaluation has been described previously7 and consisted of overall mortality and first cardiovascular event. Cause of death was classified as cardiovascular, infectious, malignancy, or other. First cardiovascular event was a composite of death from cardiac causes, nonlethal myocardial infarction, myocardial ischemia, coronary intervention, ventricular arrhythmia, ischemic stroke, and new–onset peripheral vascular disease, whichever occurred first.
Statistical Analyses
Data are expressed as means (SDs) for normally distributed variables or medians (IQRs) for non–normally distributed variables. Differences between baseline variables according to tertiles of serum PAG were tested using parametric ANOVA, Kruskal–Wallis test, or chi-squared test as appropriate. Correlations between serum PAG and other variables were calculated by Spearman rank correlation coefficients. To identify independent determinants of serum PAG, multivariate linear regression analysis was performed. Relevant demographic (i.e., age, sex, diabetes mellitus, smoking status, and body mass index) and biochemical (i.e., hemoglobin, C-reactive protein, albumin, and eGFR) parameters were first subjected to a backward elimination procedure at P<0.20 and subsequently, a final backward elimination step at P<0.05. Standardized β-coefficients were obtained after prior conversion of each variable to a Z score. In a subgroup of patients with 24-hour urinary collection, correlation and linear regression analyses were also performed with serum PAG, 24-hour urinary excretion of PAG, renal clearance of PAG, and eGFR. The Kaplan–Meier method was used to estimate cumulative incidence of the end point with the log rank test to compare differences between tertiles of serum PAG. Time to first event analysis was performed using Cox proportional hazards analysis with adjustment for age, sex, presence of diabetes mellitus, prior cardiovascular disease, and eGFR. For overall mortality, data were censored at the start of RRT (i.e., hemodialysis, peritoneal dialysis, or preemptive renal transplantation), loss to follow-up, or the end of the study observation period. With respect to cardiovascular disease, additional censoring was performed for noncardiovascular death. For all statistical analyses, P values <0.05 were considered significant. All statistical analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
Technical assistance by M. Dekens and T. Coopmans is highly appreciated.
R.P. is the recipient of a fellowship from Research Foundation, Flanders (FWO) grant 11E9813N. Part of the research has been funded by FWO grant G077514N.
Part of this work was presented at the American Society of Nephrology Kidney Week held November 3–8, 2015 (San Diego, CA).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015121302/-/DCSupplemental.
References
- 1.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW: Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer TW, Hostetter TH: Uremic solutes from colon microbes. Kidney Int 81: 949–954, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Poesen R, Windey K, Neven E, Kuypers D, De Preter V, Augustijns P, D’Haese P, Evenepoel P, Verbeke K, Meijers B: The influence of CKD on colonic microbial metabolism [published online ahead of print September 23, 2015]. J Am Soc Nephrol doi:ASN.2015030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y: Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 69: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA European Uraemic Toxin Work Group (EUTox) : Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA European Uremic Toxin Work Group (EUTox) : Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P: p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 5: 1182–1189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P: Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73: 1174–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P: The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 54: 891–901, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, Brunet P: The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 5: 1302–1308, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H: Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 31: 1771–1779, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Pletinck A, Glorieux G, Schepers E, Cohen G, Gondouin B, Van Landschoot M, Eloot S, Rops A, Van de Voorde J, De Vriese A, van der Vlag J, Brunet P, Van Biesen W, Vanholder R: Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J Am Soc Nephrol 24: 1981–1994, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seakins JW: The determination of urinary phenylacetylglutamine as phenylacetic acid. Studies on its origin in normal subjects and children with cystic fibrosis. Clin Chim Acta 35: 121–131, 1971 [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto Y, Watanabe H, Noguchi T, Kotani S, Nakajima M, Kadowaki D, Otagiri M, Maruyama T: Organic anion transporters play an important role in the uptake of p-cresyl sulfate, a uremic toxin, in the kidney. Nephrol Dial Transplant 26: 2498–2502, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Deguchi T, Kusuhara H, Takadate A, Endou H, Otagiri M, Sugiyama Y: Characterization of uremic toxin transport by organic anion transporters in the kidney. Kidney Int 65: 162–174, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW: Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 84: 585–590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirich TL, Funk BA, Plummer NS, Hostetter TH, Meyer TW: Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol 25: 615–622, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirich TL, Meyer TW, Gondouin B, Brunet P, Niwa T: Protein-bound molecules: A large family with a bad character. Semin Nephrol 34: 106–117, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman L, Jörnvall H, Bergström J: Phenylacetylglutamine and hippuric acid in uremic and healthy subjects. Nephron 55: 265–271, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Meijers BK, Bammens B, Verbeke K, Evenepoel P: A review of albumin binding in CKD. Am J Kidney Dis 51: 839–850, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, Tzen CY, Wang YC, Lin CY, Wu MS: p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant 26: 938–947, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesaffer G, De Smet R, Lameire N, Dhondt A, Duym P, Vanholder R: Intradialytic removal of protein-bound uraemic toxins: Role of solute characteristics and of dialyser membrane. Nephrol Dial Transplant 15: 50–57, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Poesen R, Viaene L, Verbeke K, Claes K, Bammens B, Sprangers B, Naesens M, Vanrenterghem Y, Kuypers D, Evenepoel P, Meijers B: Renal clearance and intestinal generation of p-cresyl sulfate and indoxyl sulfate in CKD. Clin J Am Soc Nephrol 8: 1508–1514, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shafi T, Meyer TW, Hostetter TH, Melamed ML, Parekh RS, Hwang S, Banerjee T, Coresh J, Powe NR: Free levels of selected organic solutes and cardiovascular morbidity and mortality in hemodialysis patients: Results from the Retained Organic Solutes and Clinical Outcomes (ROSCO) Investigators. PLoS One 10: e0126048, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacArthur RB, Altincatal A, Tuchman M: Pharmacokinetics of sodium phenylacetate and sodium benzoate following intravenous administration as both a bolus and continuous infusion to healthy adult volunteers. Mol Genet Metab 81[Suppl 1]: S67–S73, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Suchy-Dicey AM, Laha T, Hoofnagle A, Newitt R, Sirich TL, Meyer TW, Thummel KE, Yanez ND, Himmelfarb J, Weiss NS, Kestenbaum BR: Tubular secretion in CKD [published online ahead of print November 27, 2015]. J Am Soc Nephrol doi:ASN.2014121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Intersalt Cooperative Research Group : Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297: 319–328, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Loor H, Bammens B, Evenepoel P, De Preter V, Verbeke K: Gas chromatographic-mass spectrometric analysis for measurement of p-cresol and its conjugated metabolites in uremic and normal serum. Clin Chem 51: 1535–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Masud T, Manatunga A, Cotsonis G, Mitch WE: The precision of estimating protein intake of patients with chronic renal failure. Kidney Int 62: 1750–1756, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Maroni BJ, Steinman TI, Mitch WE: A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.