Abstract
Heme oxygenase-1 (HO-1) catalyzes the degradation of heme, which may be involved in the pathogenesis of AKI. Length polymorphisms in the number of GT dinucleotide repeats in the HO-1 gene (HMOX1) promoter inversely associate with HMOX1 mRNA expression. We analyzed the association between allelic frequencies of GT repeats in the HMOX1 gene promoter and postoperative AKI in 2377 white patients who underwent cardiac surgery with cardiopulmonary bypass. We categorized patients as having the short allele (S; <27 GT repeats) or long allele (L; ≥27 GT repeats), and defined AKI as an increase in serum creatinine ≥0.3 mg/dl within 48 hours or ≥50% within 5 days, or the need for RRT. Compared with patients with the SS genotype, patients with the LL genotype had 1.58-fold (95% confidence interval, 1.06 to 2.34; P=0.02) higher odds of AKI. After adjusting for baseline and operative characteristics, the odds ratio for AKI per L allele was 1.26 (95% confidence interval, 1.05 to 1.50; P=0.01). In conclusion, longer GT repeats in the HMOX1 gene promoter associate with increased risk of AKI after cardiac surgery, consistent with heme toxicity as a pathogenic feature of cardiac surgery-associated AKI, and with HO-1 as a potential therapeutic target.
Keywords: acute renal failure, heme oxygenase, human genetics
Toxicity mediated by elevated levels of free heme and iron is a key contributor to AKI in a variety of animal models,1–3 and in humans undergoing surgery with cardiopulmonary bypass (CPB).4,5 Heme oxygenase (HO), the rate-limiting enzyme in the degradation of heme, has important antioxidant, anti-inflammatory, and antiapoptotic functions. HO-1 is the inducible form, whereas HO-2 is constitutively expressed.
HO-1 has a central role in the pathophysiology of AKI in animal models,6–8 but data on HO-1 in human AKI are sparse. Polymorphisms in the number of guanosine thymidine dinucleotide [(GT)n] repeats in the promoter of the HO-1 gene (HMOX1) are inversely associated with HMOX1 mRNA expression and HO enzyme activity.9,10 Longer (GT)n repeats are associated with increased cardiovascular events and mortality in patients with diabetes mellitus, ESRD, and peripheral artery disease.9,11,12 However, no study has evaluated the association between the number of (GT)n repeats and risk of AKI in a large cohort of patients. We therefore investigated this association in a carefully phenotyped, multicenter cohort of adult patients undergoing cardiac surgery with CPB. We hypothesized that longer (GT)n repeats would be associated with an increased risk of AKI.
We enrolled 2464 white adult patients who underwent cardiac surgery at Brigham and Women’s Hospital and the Texas Heart Institute between 2001 and 2014 into a prospective cohort study. After excluding 87 patients based on prespecified criteria (Supplemental Figure 1), the final cohort consisted of 2377 patients. We used DNA fragment analysis to determine the number of (GT)n repeats in the HMOX1 microsatellite promoter. AKI was defined as an absolute increase in serum creatinine (SCr) ≥0.3 mg/dl above the preoperative baseline within the first 48 hours following cardiac surgery, a relative increase in SCr ≥50% above baseline within 5 days following cardiac surgery, or postoperative need for RRT during the primary hospitalization.13
Genotype Definition and Frequency
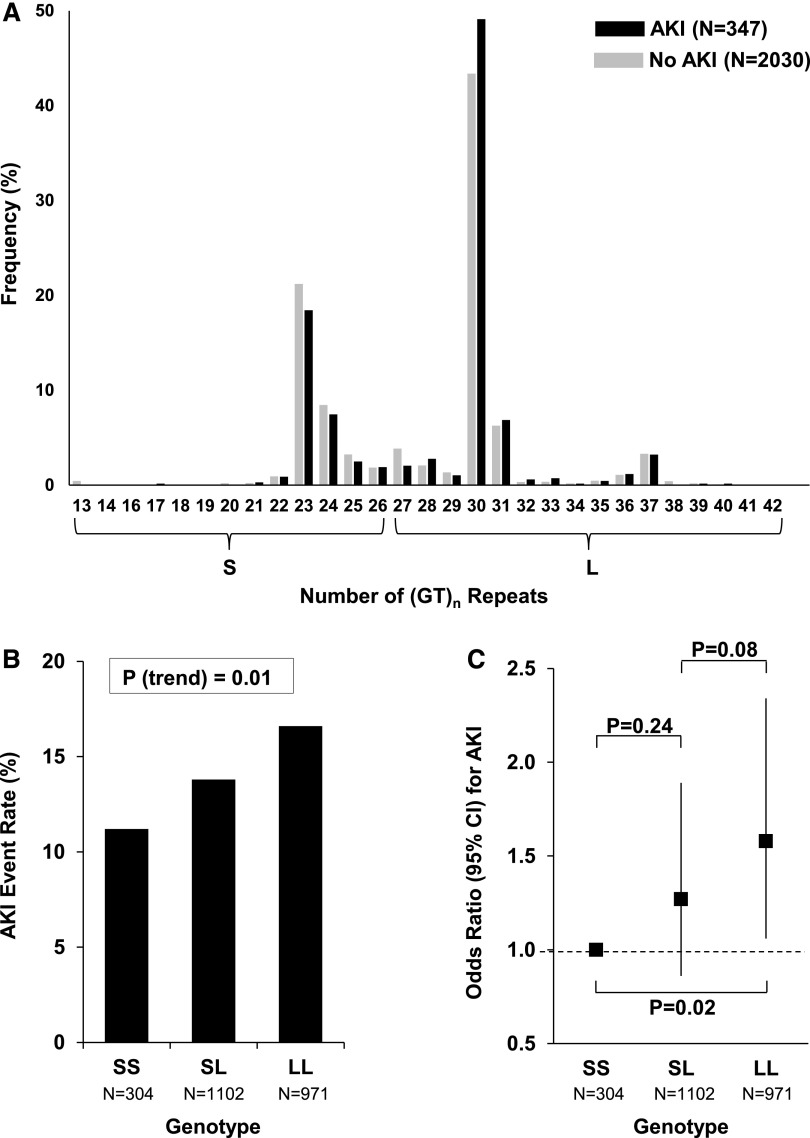
The frequency distribution of (GT)n repeats in patients with or without AKI following cardiac surgery is shown in Figure 1A. Consistent with prior studies,11,14,15 patients with <27 (GT)n repeats were classified as having the “short” (S) allele, while patients with ≥27 (GT)n repeats were classified as having the “long” (L) allele. Accordingly, patients were categorized into three mutually exclusive genotypes: SS, SL, or LL.
Figure 1.
Frequency distribution of (GT)n repeats and risk of AKI by HMOX1 genotype. (A) The number of (GT)n repeats ranged from 13 to 42 and showed a bimodal distribution, with one peak located at 23 and the other located at 30 repeats. The overall frequency distribution of (GT)n repeats was not significantly different between patients with versus without AKI (Χ2 test, P=0.09). However, longer (GT)n repeats were associated with an increased risk of AKI (Mantel–Haenszel test for trend, P=0.01). (B) Absolute risk of AKI by HMOX1 genotype. (C) Relative risk of AKI by HMOX1 genotype.
Baseline/Operative Characteristics
Baseline and operative characteristics for the overall cohort, as well as by genotype, are shown in Table 1. We did not observe any significant differences across genotypes in age, gender, preoperative renal function, comorbidities, or operative characteristics.
Table 1.
Baseline/operative characteristics
| HMOX1 Genotype | |||||
|---|---|---|---|---|---|
| Characteristic | All (n=2377) | SS (n=304) | SL (n=1102) | LL (n=971) | P Valuea |
| Demographics | |||||
| Age, yr | 65 [58–74] | 64 [57–73] | 65 [58–74] | 66 [58–74] | 0.49 |
| Female | 529 (22) | 69 (23) | 231 (21) | 229 (24) | 0.35 |
| Preoperative renal function | |||||
| SCr, mg/dl | 1.0 [0.9–1.2] | 1.0 [0.9–1.2] | 1.0 [0.9–1.2] | 1.0 [0.9–1.2] | 0.43 |
| eGFR, ml/min per 1.73 m2 | 72 [58–87] | 74 [59–87] | 72 [58–86] | 73 [57–87] | 0.56 |
| eGFR<60 ml/min per 1.73 m2 | 692 (29) | 83 (27) | 315 (29) | 294 (30) | 0.53 |
| eGFR=30–59 ml/min per 1.73 m2 | 652 (27) | 79 (26) | 298 (27) | 275 (28) | 0.67 |
| eGFR=15–29 ml/min per 1.73 m2 | 40 (2) | 4 (1) | 17 (2) | 19 (2) | 0.66 |
| Comorbidities | |||||
| Hypertension | 1764 (74) | 217 (71) | 834 (76) | 713 (73) | 0.21 |
| Diabetes mellitus | 739 (31) | 88 (29) | 341 (31) | 310 (32) | 0.61 |
| Chronic lung disease | 260 (11) | 35 (12) | 130 (12) | 95 (10) | 0.30 |
| Atrial fibrillation/flutter | 193 (8) | 28 (9) | 91 (8) | 74 (8) | 0.65 |
| Operative characteristics | |||||
| Type of procedure | 0.77 | ||||
| CABG alone | 1822 (77) | 241 (79) | 845 (77) | 736 (76) | |
| Valve alone | 323 (14) | 38 (13) | 151 (14) | 134 (14) | |
| Combined CABG and valve | 232 (10) | 25 (8) | 106 (10) | 101 (10) | |
| Urgent procedure | 1138 (48) | 141 (46) | 522 (47) | 475 (49) | 0.65 |
| Previous cardiac surgery | 109 (5) | 14 (5) | 50 (5) | 45 (5) | 0.99 |
| CPB time, min | 103 [75–137] | 102 [75–129] | 103 [75–137] | 104 [75–138] | 0.54 |
| Crossclamp time, min | 76 [55–101] | 76 [58–98] | 77 [55–102] | 76 [56–103] | 0.94 |
| Intraoperative pRBCs, no. of units | 0 [0–1] | 0 [0–1] | 0 [0–0] | 0 [0–1] | 0.66 |
| Intraoperative cellsaver, ml | 3 [2–197] | 3 [2–47] | 3 [2–130] | 3 [2–220] | 0.59 |
Data are presented as n (%) or median [interquartile range, 25th–75th percentile]. The S allele represents a short (<27) number of repeats, and the L allele represents a long (≥27) number of (GT)n repeats in the HMOX1 gene promoter. Shorter repeats are associated with greater HMOX1 mRNA expression and enzyme activity. eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration equation.32
P values for comparisons among the three groups were calculated using Kruskal–Wallis and Χ2 tests for continuous and categorical variables, respectively.
Absolute and Relative Risks of AKI by HMOX1 Genotype
The absolute and relative risks of AKI according to HMOX1 genotype are shown in Figure 1, B and C. The incidence of AKI among patients with the SS, SL, and LL genotypes was 11.2%, 13.8%, and 16.6%, respectively (P for trend =0.01). Patients with the LL versus the SS genotype had 1.58-fold (95% confidence interval [95% CI], 1.06 to 2.34; P=0.02) higher odds of AKI.
Univariate and Multivariate Associations between Genotype and AKI
The univariate and multivariate adjusted odds ratios for risk of AKI according to HMOX1 genotype, using additive genetic models, are shown in Table 2. Thus, odds ratios represent the incremental risk of AKI for each additional L allele. After adjusting for age, gender, preoperative eGFR, diabetes mellitus, hypertension, prior cardiac surgery, type of procedure (coronary artery bypass graft [CABG] alone, valve alone, or CABG/valve combined), CPB time, number of intraoperative packed red blood cell (pRBC) transfusions, urgent versus nonurgent procedure, and institution, the relative risk of AKI per L allele was 1.26 (95% CI, 1.05 to 1.50; P=0.01). We also investigated three alternative definitions of AKI based on increases in SCr ≥25%, ≥50%, or ≥100% within 5 days following cardiac surgery or need for RRT. In adjusted analyses, we found a significant association between HMOX1 genotype and an increase in SCr ≥25% within 5 days or need for RRT. We found similar magnitudes of association between HMOX1 genotype and larger increases in SCr (i.e., ≥50% and ≥100%), with similar odds ratios, but these findings were not statistically significant (Table 2).
Table 2.
Risk of AKI following cardiac surgery based on HMOX1 genotype
| Event Rates, n (%) | Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
| End Point | All (n=2377) | SS (n=304) | SL (n=1102) | LL (n=971) | Odds Ratioa (95% CI) | P Value | Odds Ratioa (95% CI) | P Value |
| Primary end point | ||||||||
| AKI | 347 (14.6) | 34 (11.2) | 152 (13.8) | 161 (16.6) | 1.25 (1.05 to 1.49) | 0.01 | 1.26 (1.05 to 1.50) | 0.01 |
| Secondary end points | ||||||||
| Increase in SCr ≥25% in 5 d or RRT | 492 (20.7) | 51 (16.8) | 222 (20.2) | 219 (22.6) | 1.19 (1.02 to 1.38) | 0.03 | 1.18 (1.02 to 1.38) | 0.03 |
| Increase in SCr ≥50% in 5 d or RRT | 171 (7.2) | 19 (6.3) | 72 (6.5) | 80 (8.2) | 1.20 (0.95 to 1.52) | 0.13 | 1.20 (0.94 to 1.53) | 0.14 |
| Increase in SCr ≥100% in 5 d or RRT | 48 (2.0) | 5 (1.6) | 21 (1.9) | 22 (2.3) | 1.18 (0.77 to 1.83) | 0.45 | 1.20 (0.76 to 1.89) | 0.43 |
AKI was defined as an absolute increase in SCr ≥0.3 mg/dl above baseline within the first 48 hours following cardiac surgery, a relative increase in SCr ≥50% above baseline within 5 days following cardiac surgery, or postoperative need for RRT.13
Additive genetic models for each L allele. Adjusted models include age, gender, preoperative eGFR, diabetes mellitus, hypertension, prior cardiac surgery, type of procedure (CABG alone, valve alone, or CABG/valve combined), CPB time, number of intraoperative pRBC transfusions, urgent procedure, and institution.
Sensitivity Analysis
We conducted a sensitivity analysis in which the S allele was defined as <25 repeats and the L allele was defined as ≥25 repeats.16,17 In analyses adjusted for the same covariates as above, we found a significant association between HMOX1 genotype and risk of AKI, with an odds ratio of 1.25 (95% CI, 1.03 to 1.50; P=0.02) per L allele. Similar findings were observed using 29 repeats as the cutoff, with an odds ratio of 1.29 (95% CI, 1.09 to 1.54; P=0.004) per L allele.
Subgroup Analyses
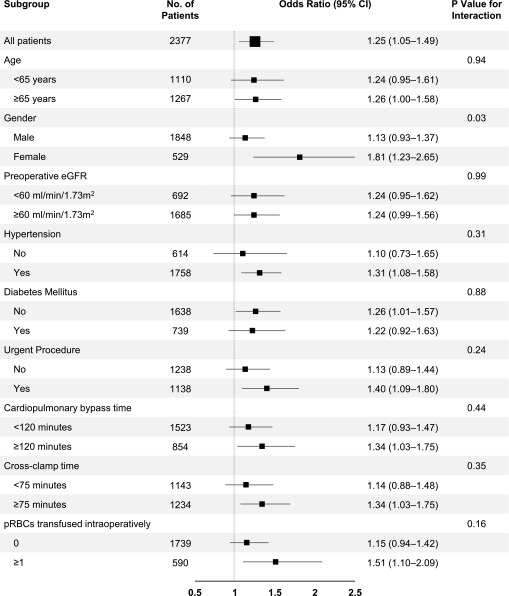
To investigate the possibility of effect modification due to differences in baseline or operative characteristics, we compared the association between HMOX1 genotype (using 27 repeats as the cutoff) and risk of AKI in subgroup analyses (Figure 2). We found a significantly stronger association between HMOX1 genotype and risk of AKI in females compared with males, and a nonsignificant trend toward a stronger association in patients who were transfused ≥1 versus 0 units of pRBCs intraoperatively.
Figure 2.
Forest plot showing risk of AKI following cardiac surgery based on HMOX1 genotype–subgroup analyses. Odds ratios were computed using additive genetic models, and therefore represent the incremental risk of AKI with each additional L allele.
Plasma Free Hemoglobin and Other Plasma Markers
Finally, to explore possible mechanisms responsible for the association between HMOX1 genotype and risk of AKI following CPB, we measured plasma levels of free hemoglobin, ferritin, transferrin saturation, and catalytic iron longitudinally in a subgroup of 192 patients at high risk of postoperative AKI. At each of the three postoperative time points, we found a trend toward higher plasma free hemoglobin levels with each additional L allele (Supplemental Figure 2). Postoperative levels of ferritin, transferrin saturation, and catalytic iron did not differ by genotype (data not shown).
Thus, in this prospective cohort study, we report that patients with longer (GT)n repeats in the HMOX1 gene promoter had an increased risk of AKI following cardiac surgery. The risk of AKI was increased 1.25-fold (95% CI, 1.05 to 1.49; P=0.02) for each additional L allele, and individuals with the LL genotype had a 1.58-fold (95% CI, 1.06 to 2.34; P=0.02) increased risk of AKI compared with patients with the SS genotype. Further, these associations remained after adjusting for covariates included in multivariable models.
The potent renoprotective effects of HO-1 have been well established in a wide variety of experimental settings, including models of renal injury induced by ischemia-reperfusion18 and LPS,8 and are reviewed in detail elsewhere.6 Proposed mechanisms include: inhibition of autophagy19; anticoagulant effects20; increased synthesis of ferritin,21 which sequesters free iron; and production of carbon monoxide and biliverdin, which have antioxidant and renoprotective properties.22,23 Additionally, a recent study demonstrated the importance of myeloid-specific HMOX1 expression in the regulation of immune cell trafficking after ischemia-reperfusion injury.24
In contrast to these comprehensive studies in animal models of renal injury, data on HO-1 in human AKI are sparse. In several small studies, HO-1 protein levels were shown to be elevated in plasma samples taken from patients with AKI.25,26 Although intriguing, plasma HO-1 levels are difficult to interpret in this context due to confounding by severity of illness. We therefore used a Mendelian randomization approach, since it limits bias. This approach has been used to study the association between HMOX1 genotype and a variety of outcomes, including cardiovascular events and mortality,11 incident CKD,14 and chronic allograft nephropathy,27 but the association with AKI was evaluated in only a single small study. Askenazi et al. found no association between the number of (GT)n repeats in the HO-1 gene promoter and risk of AKI in 117 premature infants.28 However, with only 34 AKI events, the investigators likely did not have sufficient power to detect an association.
We conducted several subgroup analyses and found a significantly stronger association between HMOX1 genotype and risk of AKI in females compared with males, and a trend toward a stronger association in patients transfused ≥1 versus 0 units of pRBCs intraoperatively. The association with transfusions is particularly interesting, since pRBCs undergo hemolysis over time, resulting in the release of free hemoglobin.29 The association with gender has not been reported in prior human studies to the best of our knowledge; however, Pósa et al. found greater HO-1 expression and activity in female compared with male rat aorta and left ventricle. They concluded that gender-related differences in HO-1 activity may play a role in the sexual dimorphism of susceptibility to cardiovascular disease.30
The mechanisms responsible for our observation of greater risk of AKI in patients with longer (GT)n repeats could not be determined in this observational study. However, a highly plausible mechanism involves decreased HMOX1 expression in patients with longer (GT)n repeats,9,10 resulting in diminished capacity to catabolize toxic free heme. While the mechanisms of cardiac surgery-associated AKI are complex and multifactorial, hemolysis is likely a major contributor.4,5 CPB induces hemolysis by exposure of red blood cells to nonphysiologic surfaces, shear stress generated by pumps and suction systems, and frequent transfusion of pRBCs.29,31 HO-1 mitigates the effects of hemolysis by catabolizing heme into carbon monoxide, biliverdin, and iron. The latter becomes sequestered by ferritin, which is upregulated by HO-1. Thus, it is likely that patients with decreased HMOX1 expression caused by longer (GT)n repeats have an increased susceptibility to heme-related, cardiac surgery-associated AKI.
We acknowledge several limitations of our study, including the observational design. Although we measured plasma free hemoglobin levels longitudinally in a subcohort of patients and documented higher levels in patients with longer (GT)n repeats, we did not measure other potentially relevant plasma markers, such as HO-1 protein levels or bilirubin levels. However, the inverse association between the number of (GT)n repeats in the HMOX1 gene promoter and HMOX1 mRNA expression has been well documented in prior studies.9,10 Finally, we did not have data on long-term renal outcomes, such as incidence or progression of CKD.
In conclusion, we found that longer (GT)n repeats in the HMOX1 gene promoter, which are known to affect HMOX1 expression, were associated with an increased risk of AKI following cardiac surgery. Future studies are indicated to explore pharmacologic targeting of HMOX1 expression as a strategy for AKI prevention in humans.
Concise Methods
Study Design
We enrolled patients undergoing cardiac surgery into a prospective cohort study at Brigham and Women’s Hospital (Boston, MA) and the Texas Heart Institute (Houston, TX) between 2001 and 2014. All patients provided written informed consent, including consent specifically for genetic studies, and all protocols were approved by the respective institutional review boards.
Study Patients
Subjects with a preoperative hematocrit <25% or those who received transfusion of leukocyte-rich blood products within 30 days before surgery were not enrolled. We restricted our analyses to subjects of white ancestry to avoid the potential influence of population stratification on the observed associations. Additionally, we excluded the following groups of patients: cardiac surgery without CPB; emergency surgeries; missing preoperative SCr data; missing postoperative SCr data (patients were required to have at least one SCr value within 5 days following cardiac surgery to be included); preoperative eGFR <15 ml/min per 1.73 m2; history of renal transplantation; and ESRD (Supplemental Figure 1).
Data and Sample Collection
Data were collected for each enrolled subject during their hospitalization using detailed case report forms that included: (1) preoperative demographic characteristics and comorbidities; (2) surgical characteristics; and (3) postoperative data, including daily SCr values for the first 5 days after surgery. Data were subjected to automated range and logic checking, as well as a manual audit of records to ensure data quality.
Plasma was collected preoperatively and on postoperative days 1 through 5, and stored at −80°C within 2 hours of collection. DNA was extracted from white blood cells using standard protocols.
HMOX1 Genotyping
We used DNA fragment analysis to determine the number of (GT)n repeats in the HMOX1 microsatellite promoter. The HMOX1 locus containing the GT repeat was amplified using PCR with fluorescently labeled primers. All amplicons were separated by size using capillary electrophoresis on an ABI3730 sequencer. Data analysis was performed with GeneMapper 5 software (Thermo Fisher Scientific, Vernon Hills, IL), including automatic sizing and allele calling. Two individuals were Sanger sequenced and the number of repeats was counted. This information was used to convert amplicon lengths to repeat numbers. Consistent with prior studies, we used 27 repeats as the cutoff for genotype classification.11,14,15 Patients with <27 (GT)n repeats were classified as having the S allele, while patients with ≥27 (GT)n repeats were classified as having the L allele.
End Points
The prespecified primary end point was postoperative AKI, defined as an absolute increase in SCr ≥0.3 mg/dl above baseline within the first 48 hours following cardiac surgery, a relative increase in SCr ≥50% above baseline within 5 days following cardiac surgery, or postoperative need for RRT. These criteria are identical to those established by the Kidney Disease Improving Global Outcomes Work Group,13 with the exception of 5 days instead of 7 days. Secondary end points included the following alternative definitions of AKI: increases in SCr ≥25%, ≥50%, or ≥100% above baseline within 5 days following cardiac surgery or need for RRT.
Subgroup Analyses
We evaluated the association between HMOX1 genotype and risk of AKI in the following subgroups: age (<65 versus ≥65 years); gender; preoperative eGFR (<60 versus ≥60 ml/min per 1.73 m2); hypertension; diabetes mellitus; urgent procedure; CPB time (<120 versus ≥120 minutes); crossclamp time (<75 versus ≥75 minutes); and pRBCs transfused intraoperatively (0 versus ≥1 unit).
Sensitivity Analyses
We conducted two sensitivity analyses in which the S allele was defined as <25 repeats and the L allele was defined as ≥25 repeats,16,17 and in which the S allele was defined as <29 repeats and the L allele was defined as ≥29 repeats.
Plasma Measurements
We measured plasma levels of free hemoglobin, ferritin, transferrin saturation, and catalytic iron in a subcohort of 192 patients at high risk of postoperative AKI. Patients were considered to be at high risk of AKI if they had a baseline eGFR ≤30 ml/min per 1.73 m2 or any two of the following: baseline eGFR = 31–60 ml/min per 1.73 m2, diabetes mellitus, left ventricular ejection fraction ≤40%, previous cardiac surgery, combined CABG/valve procedure, urgent procedure, and preoperative intraaortic balloon pump.4 The above measurements were performed on plasma samples collected at four time points: preoperatively, at the end of CPB, and on postoperative days 1 and 3. The details of these measurements are reported elsewhere.4 In brief, plasma free hemoglobin levels were measured using an ELISA kit (catalog no. MBS 564144; MyBioSource, San Diego, CA). Ferritin levels were measured using a two site immunoenzymometric assay kit (Tosoh Corporation, Japan). Transferrin saturation (%) was calculated as the ratio of total iron/ total iron binding capacity, multiplied by 100. Catalytic iron was measured using the modified bleomycin assay. The interassay coefficient of variation for all assays, estimated using blinded split samples from study patients, was <10%.
Statistical Analyses
Statistical analyses were performed with SAS Version 9.4 (SAS Institute Inc., Cary, NC). Data are reported as median and interquartile range (25th–75th percentiles). Baseline/operative characteristics were compared in patients across HMOX1 genotypes using Kruskal–Wallis and Χ2 tests for continuous and categorical variables, respectively. The Mantel–Haenszel test for trend was used to assess the association between longer (GT)n repeats and risk of AKI. The Cochran–Armitage test for trend was used to assess the unadjusted association between HMOX1 genotype and incidence of AKI.
Logistic regression was used to assess the association between HMOX1 genotype and AKI, using additive genetic models. Multivariate models were adjusted for age, gender, preoperative eGFR, diabetes mellitus, hypertension, prior cardiac surgery, type of procedure (CABG alone, valve alone, or CABG/valve combined), CPB time, number of intraoperative pRBC transfusions, urgent versus nonurgent procedure, and institution. Comparison of plasma free hemoglobin levels at individual time points across HMOX1 genotypes was assessed using Spearman rank correlation coefficient. All comparisons are two-tailed, with P<0.05 considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Mohan Rajapurkar, from the Department of Nephrology, Muljibhai Patel Urological Hospital, Gujuarat, India, for performing the plasma iron assays for this study.
D.E.L. is supported by grant K23DK106448 from the National Institute of Diabetes and Digestive Kidney Diseases (NIDDK) and by the University of Alabama at Birmingham-University of California, San Diego O’Brien Center for Acute Kidney Injury Research (the National Institutes of Health, grant P30-DK079337). S.C.B. is supported by grant R01HL098601 from the National Heart Lung and Blood Institute (NHLBI). J.D.M. is supported by grant R01HL118266 from the NHLBI. S.S.W. is supported by grants R01DK093574, R01DK103784, U01DK104308, and U01DK085660 from the NIDDK.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Heme Oxygenase-1 Gene Polymorphisms—Toward Precision Medicine for AKI,” on pages 3229–3231.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016010038/-/DCSupplemental.
References
- 1.Baliga R, Ueda N, Shah SV: Increase in bleomycin-detectable iron in ischaemia/reperfusion injury to rat kidneys. Biochem J 291: 901–905, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker PD, Shah SV: Evidence suggesting a role for hydroxyl radical in gentamicin-induced acute renal failure in rats. J Clin Invest 81: 334–341, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paller MS: Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol 255: F539–F544, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Rawn JD, Frendl G, Waikar SS: Increased plasma catalytic iron in patients may mediate acute kidney injury and death following cardiac surgery. Kidney Int 87: 1046–1054, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, Altintas S, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ: Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int 77: 913–920, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Nath KA: Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens 23: 17–24, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maines MD, Mayer RD, Ewing JF, McCoubrey WK Jr: Induction of kidney heme oxygenase-1 (HSP32) mRNA and protein by ischemia/reperfusion: possible role of heme as both promotor of tissue damage and regulator of HSP32. J Pharmacol Exp Ther 264: 457–462, 1993 [PubMed] [Google Scholar]
- 8.Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Rajagopalan G, Knutson KL, Badley AD, Griffin MD, Alam J, Nath KA: Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1-/- mice. Am J Pathol 170: 1820–1830, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY: Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet 111: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Hirai H, Kubo H, Yamaya M, Nakayama K, Numasaki M, Kobayashi S, Suzuki S, Shibahara S, Sasaki H: Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood 102: 1619–1621, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Chen YH, Hung SC, Tarng DC: Length polymorphism in heme oxygenase-1 and cardiovascular events and mortality in hemodialysis patients. Clin J Am Soc Nephrol 8: 1756–1763, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick P, Schillinger M, Minar E, Mlekusch W, Amighi J, Sabeti S, Schlager O, Raith M, Endler G, Mannhalter C, Wagner O, Exner M: Haem oxygenase-1 genotype and cardiovascular adverse events in patients with peripheral artery disease. Eur J Clin Invest 35: 731–737, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 14.Chen YH, Kuo KL, Hung SC, Hsu CC, Chen YH, Tarng DC: Length polymorphism in heme oxygenase-1 and risk of CKD among patients with coronary artery disease. J Am Soc Nephrol 25: 2669–2677, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu MM, Chiou HY, Chen CL, Hsu LI, Lien LM, Wang CH, Hsieh YC, Wang YH, Hsueh YM, Lee TC, Cheng WF, Chen CJ: Association of heme oxygenase-1 GT-repeat polymorphism with blood pressure phenotypes and its relevance to future cardiovascular mortality risk: an observation based on arsenic-exposed individuals. Atherosclerosis 219: 704–708, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Wagener FA, Toonen EJ, Wigman L, Fransen J, Creemers MC, Radstake TR, Coenen MJ, Barrera P, van Riel PL, Russel FG: HMOX1 promoter polymorphism modulates the relationship between disease activity and joint damage in rheumatoid arthritis. Arthritis Rheum 58: 3388–3393, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Córdova EJ, Martínez-Hernández A, Ramírez-Bello J, Velázquez-Cruz R, Centeno F, Baca V, Orozco L: HMOX1 promoter (GT)n polymorphim is associated with childhood-onset systemic lupus erythematosus but not with juvenile rheumatoid arthritis in a Mexican population. Clin Exp Rheumatol 30: 297–301, 2012 [PubMed] [Google Scholar]
- 18.Shimizu H, Takahashi T, Suzuki T, Yamasaki A, Fujiwara T, Odaka Y, Hirakawa M, Fujita H, Akagi R: Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit Care Med 28: 809–817, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, Agarwal A: Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol 21: 1702–1712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, Nath KA: Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int 74: 47–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, Balla J, Verlander J, Darshan D, Kuhn LC, Agarwal A: Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423–4434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goebel U, Siepe M, Schwer CI, Schibilsky D, Foerster K, Neumann J, Wiech T, Priebe HJ, Schlensak C, Loop T: Inhaled carbon monoxide prevents acute kidney injury in pigs after cardiopulmonary bypass by inducing a heat shock response. Anesth Analg 111: 29–37, 2010 [DOI] [PubMed] [Google Scholar]
- 23.LeBlanc RM, Navar LG, Botros FT: Bilirubin exerts renoprotective effects in angiotensin II-hypertension. Am J Med Sci 340: 144–146, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Hull TD, Kamal AI, Boddu R, Bolisetty S, Guo L, Tisher CC, Rangarajan S, Chen B, Curtis LM, George JF, Agarwal A: Heme Oxygenase-1 Regulates Myeloid Cell Trafficking in AKI. J Am Soc Nephrol 26: 2139–2151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billings FT 4th, Yu C, Byrne JG, Petracek MR, Pretorius M: Heme Oxygenase-1 and Acute Kidney Injury following Cardiac Surgery. Cardiorenal Med 4: 12–21, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zager RA, Johnson AC, Becker K: Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol 23: 1048–1057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baan C, Peeters A, Lemos F, Uitterlinden A, Doxiadis I, Claas F, Ijzermans J, Roodnat J, Weimar W: Fundamental role for HO-1 in the self-protection of renal allografts. Am J Transplant 4: 811–818, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Askenazi DJ, Halloran B, Patil N, Keeling S, Saeidi B, Koralkar R, Ambalavanan N: Genetic polymorphisms of heme-oxygenase 1 (HO-1) may impact on acute kidney injury, bronchopulmonary dysplasia, and mortality in premature infants. Pediatr Res 77: 793–798, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT: Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 124: 465–476, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pósa A, Kupai K, Ménesi R, Szalai Z, Szabó R, Pintér Z, Pálfi G, Gyöngyösi M, Berkó A, Pávó I, Varga C: Sexual dimorphism of cardiovascular ischemia susceptibility is mediated by heme oxygenase. Oxid Med Cell Longev 2013: 521563, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takami Y, Makinouchi K, Nakazawa T, Glueck J, Benkowski R, Nosé Y: Effect of surface roughness on hemolysis in a pivot bearing supported Gyro centrifugal pump (C1E3). Artif Organs 20: 1155–1161, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.