Abstract
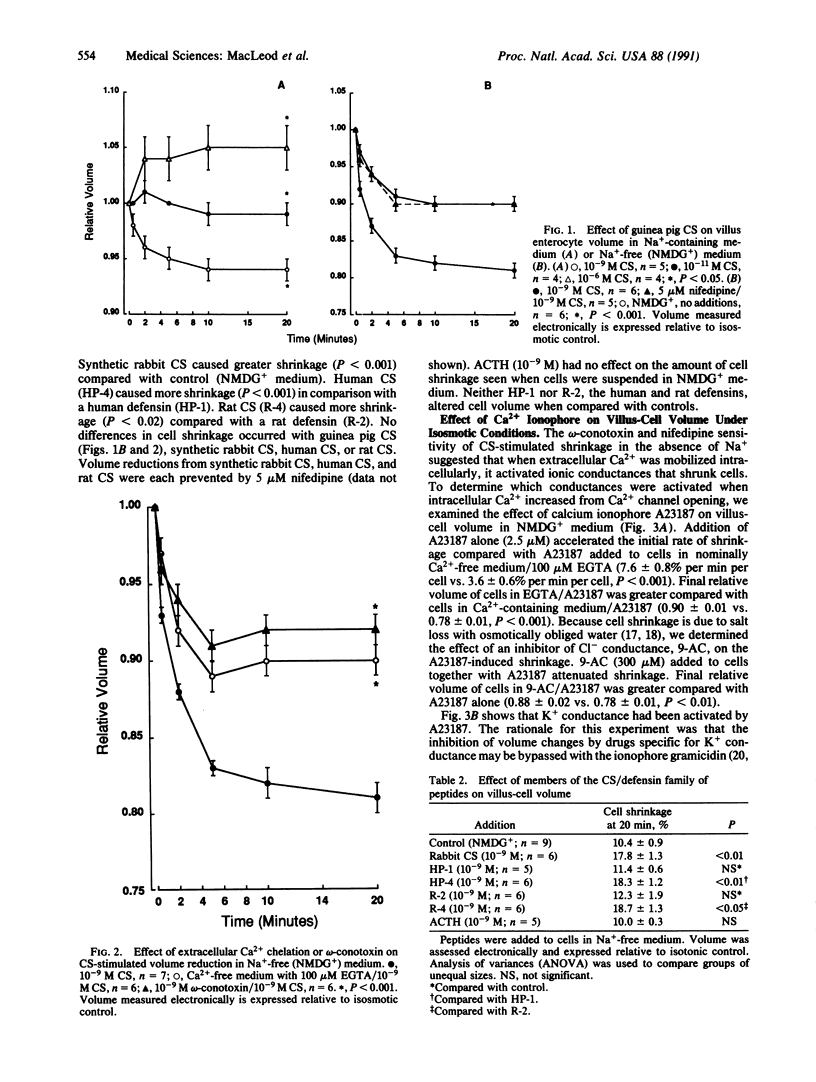
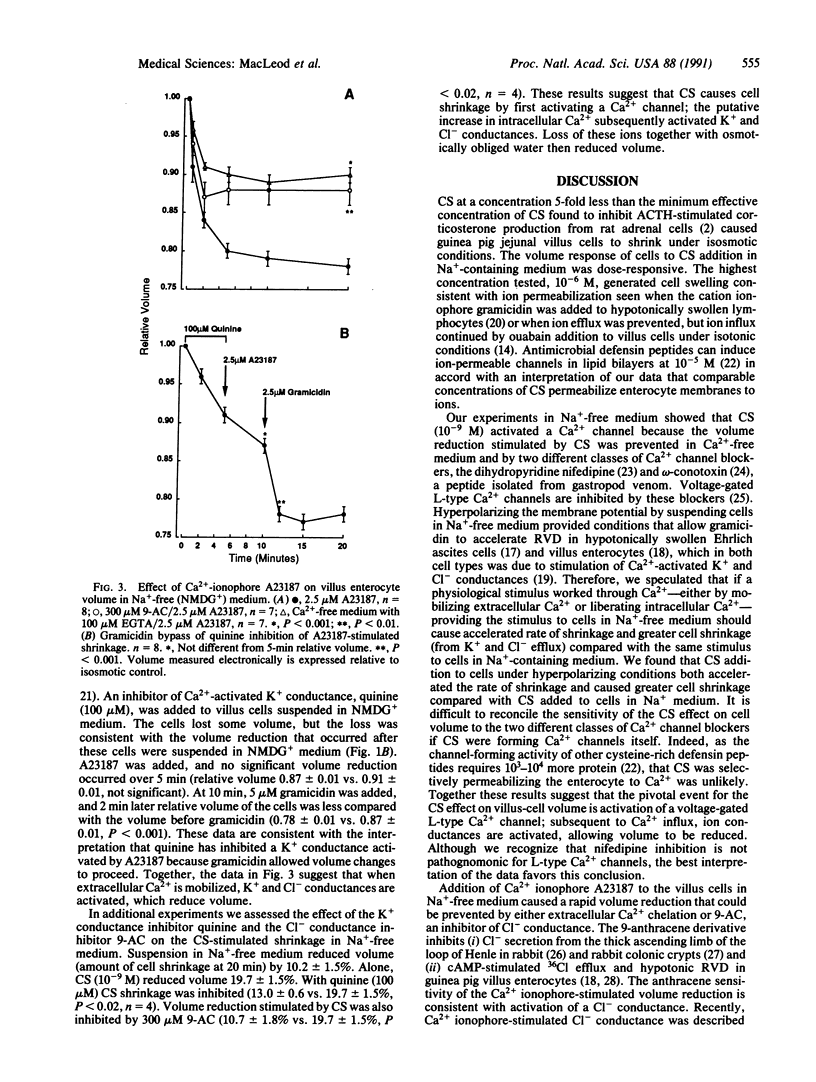
We studied cell-volume changes caused by adding corticostatin (CS) or defensin-like peptides to villus enterocytes isolated in suspension from guinea pig jejunum. Guinea pig CS (10(-9) M) added to villus cells in Na(+)-containing medium reduced volume, but immediate cell swelling was caused by 10(-6) M guinea pig CS. In Na(+)-free N-methyl-D-glucamine-containing medium 10(-9) M guinea pig CS accelerated the initial rate of shrinkage compared with cells in N-methyl-D-glucamine-containing medium alone as well as causing greater cell shrinkage. Guinea pig CS-stimulated cell shrinkage was prevented by a Ca2(+)-channel blocker--5 microM nifedipine, by chelation of extracellular Ca2+ with 100 microM EGTA, or by omega-conotoxin (10(-9) M). The Ca2+ ionophore A23187 (2.5 microM) reduced volume when added to villus cells in N-methyl-D-glucamine-containing medium; this action was prevented by EGTA, or quinine--an inhibitor of K+ conductance, or 9-anthracenecarboxylic acid--a Cl- channel blocker, suggesting that the volume reduction occurred because K+ and Cl- conductances were activated. Guinea pig CS-stimulated volume reduction was also prevented by 100 microM quinine or 9-anthracenecarboxylic acid. We conclude that jejunal villus enterocytes possess a Ca2(+)-activated Cl- conductance and a K+ conductance that need not be stretch-activated. Corticostatic peptides cause volume reduction in villus cells by activating L-type Ca2+ channels; other defensin-like peptides were without effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L. J., Olivera B. M. Calcium channel antagonists. Omega-conotoxin defines a new high affinity site. J Biol Chem. 1986 May 15;261(14):6230–6233. [PubMed] [Google Scholar]
- Donowitz M., Welsh M. J. Ca2+ and cyclic AMP in regulation of intestinal Na, K, and Cl transport. Annu Rev Physiol. 1986;48:135–150. doi: 10.1146/annurev.ph.48.030186.001031. [DOI] [PubMed] [Google Scholar]
- Eisenhauer P. B., Harwig S. S., Szklarek D., Ganz T., Selsted M. E., Lehrer R. I. Purification and antimicrobial properties of three defensins from rat neutrophils. Infect Immun. 1989 Jul;57(7):2021–2027. doi: 10.1128/iai.57.7.2021-2027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall D. G., Butler D. G., Tepperman F., Hamilton J. Sodium ion transport in isolated intestinal epithelial cells. The effect of actively transported sugars on sodium ion efflux. Biochim Biophys Acta. 1974 Mar 29;339(3):291–302. doi: 10.1016/0005-2736(74)90156-4. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Clarke C. A., Dupre A., Rothstein A. Volume-induced increase of anion permeability in human lymphocytes. J Gen Physiol. 1982 Dec;80(6):801–823. doi: 10.1085/jgp.80.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy E., Lopez del Pino V., Schwenk M. Isolated intestinal mucosa cells of high viability from guinea pig. Eur J Cell Biol. 1983 Mar;30(1):132–136. [PubMed] [Google Scholar]
- Hoffmann E. K., Lambert I. H., Simonsen L. O. Separate, Ca2+-activated K+ and Cl- transport pathways in Ehrlich ascites tumor cells. J Membr Biol. 1986;91(3):227–244. doi: 10.1007/BF01868816. [DOI] [PubMed] [Google Scholar]
- Homaidan F. R., Donowitz M., Weiland G. A., Sharp G. W. Two calcium channels in basolateral membranes of rabbit ileal epithelial cells. Am J Physiol. 1989 Jul;257(1 Pt 1):G86–G93. doi: 10.1152/ajpgi.1989.257.1.G86. [DOI] [PubMed] [Google Scholar]
- Kagan B. L., Selsted M. E., Ganz T., Lehrer R. I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K. A stretch-activated K+ channel in the basolateral membrane of Xenopus kidney proximal tubule cells. Pflugers Arch. 1990 Feb;415(5):624–629. doi: 10.1007/BF02583516. [DOI] [PubMed] [Google Scholar]
- MacLeod R. J., Hamilton J. R. Regulatory volume increase in mammalian jejunal villus cells is due to bumetanide-sensitive NaKCl2 cotransport. Am J Physiol. 1990 May;258(5 Pt 1):G665–G674. doi: 10.1152/ajpgi.1990.258.5.G665. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Cordell B. Accumulation of abundant messenger ribonucleic acids during postnatal development of mouse small intestine. Gastroenterology. 1988 Jan;94(1):114–121. doi: 10.1016/0016-5085(88)90618-x. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Greco R. M., James M., Frederick D., Naftilan J., Fallon J. T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989 May;108(5):1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin H. A stretch-activated K+ channel sensitive to cell volume. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1731–1735. doi: 10.1073/pnas.86.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Mack E., Rothstein A. Ionic events during the volume response of human peripheral blood lymphocytes to hypotonic media. I. Distinctions between volume-activated Cl- and K+ conductance pathways. J Gen Physiol. 1984 Apr;83(4):497–512. doi: 10.1085/jgp.83.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. H., Dolphin A. C. G-protein regulation of neuronal voltage-activated calcium currents. Gen Pharmacol. 1989;20(6):715–720. doi: 10.1016/0306-3623(89)90317-0. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Harwig S. S., Lehrer R. I. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J Biol Chem. 1985 Apr 25;260(8):4579–4584. [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S., Ganz T., Schilling J. W., Lehrer R. I. Primary structures of three human neutrophil defensins. J Clin Invest. 1985 Oct;76(4):1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S. Purification, primary structure, and antimicrobial activities of a guinea pig neutrophil defensin. Infect Immun. 1987 Sep;55(9):2281–2286. doi: 10.1128/iai.55.9.2281-2286.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Bateman A., Zhu Q. Z., Shimasaki S., Esch F., Solomon S. Structure of a novel human granulocyte peptide with anti-ACTH activity. Biochem Biophys Res Commun. 1988 Aug 30;155(1):524–529. doi: 10.1016/s0006-291x(88)81118-5. [DOI] [PubMed] [Google Scholar]
- Wangemann P., Wittner M., Di Stefano A., Englert H. C., Lang H. J., Schlatter E., Greger R. Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407 (Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]
- Zhu Q. Z., Hu J., Mulay S., Esch F., Shimasaki S., Solomon S. Isolation and structure of corticostatin peptides from rabbit fetal and adult lung. Proc Natl Acad Sci U S A. 1988 Jan;85(2):592–596. doi: 10.1073/pnas.85.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Bateman A., Singh A., Solomon S. Isolation and biological activity of corticostatic peptides (anti-ACTH). Endocr Res. 1989;15(1-2):129–149. doi: 10.1080/07435808909039093. [DOI] [PubMed] [Google Scholar]



