Abstract
Objective
This analysis focuses on 1-year maternal and infant follow-up of a randomized trial that tested a weight management intervention conducted during pregnancy.
Methods
We randomly assigned 114 women with obesity (mean BMI 36.7 kg/m2) at a mean of 15 weeks’ gestation to a weight management intervention or usual care control condition. The intervention ended at delivery and resulted in less gestational weight gain and a lower proportion of large-for-gestational age newborns among intervention compared to control participants. The primary outcome at 12 months postpartum was maternal weight. Secondary outcomes included infant weight-for-age and weight-for-length z-scores.
Results
At 1 year, mothers in the intervention group weighed 96.3±18.6 kg, and in the control group, 99.7±19.2 kg. There was no significant difference between groups in change in weight from randomization to 1-year postpartum (b=-0.47, 95% CI [-4.03, 3.08]. There was a significant main effect of group for infant weight-for-age z-score (b=-0.40, 95% CI [-0.75,-0.05]) but not infant weight-for-length z-scores (b=-0.20, 95% CI [-0.59,0.20].
Conclusions
A gestational weight management intervention did not influence maternal weight or infant weight-for-length at 1-year postpartum. Future studies may be warranted to determine if extending prenatal interventions into the postpartum period would be beneficial for maternal and infant outcomes.
Keywords: obesity, pregnancy, weight loss maintenance
Introduction
Pregnancy is an important time in a woman’s life when her dietary and lifestyle practices influence not only her health but also that of her child’s. Maternal nutrition plays an important role with both inadequate and excessive dietary intake and gestational weight gain affecting pregnancy outcomes (1-4). Excessive gestational weight gain is not only associated with adverse pregnancy outcomes (2, 4), and postpartum weight retention with an increased BMI (4, 5), but also with a higher risk of obesity in the offspring (6). Gestational weight gain in excess of the Institute of Medicine (IOM) recommendation (7) substantially increases the risk of excessive (>10 lb) weight retention one year after delivery (8), and transitioning from overweight to obesity from early pregnancy to the first year postpartum (9). Additionally, weight retention one year after pregnancy predicts weight retention 15 years later (5). Increases in weight between pregnancies increases the risk of subsequent gestational diabetes (10-12), type 2 diabetes (12), preeclampsia (13), and cesarean delivery (14, 15). Greater gestational weight gain is also associated with higher child BMI z-scores, increased body fat and elevated systolic blood pressure for the child at three years of age (16).
Although dietary-based weight management interventions during pregnancy may reduce pregnancy weight gain and improve pregnancy outcomes (17, 18), very few trials have examined maternal and child outcomes at 1 year postpartum or beyond (19, 20). Thus, it is unknown if dietary interventions conducted solely during pregnancy influence long-term maternal and child health. The Healthy Moms study found that a comprehensive, group-based, lifestyle intervention conducted weekly during pregnancy reduced gestational weight gain among women with obesity compared to controls. We present here the one-year follow up data for the study participants and their infants. Our goal was to determine if women who received the weight management intervention during pregnancy weighed less at 1-year postpartum than controls. We also evaluated their infants to determine if there were differences between groups in weight, weight-for-length, and adiposity measures from birth to one year.
Methods
Study design
We have previously reported in detail the design and methods for the Healthy Moms trial (21), a randomized, single-site, two-arm clinical trial that tested the efficacy of an intervention program designed to minimize gestational weight gain for women with obesity. The study began recruitment in October 2009 and data collection continued until February 2013 when the last one-year visit was completed. The primary outcomes of the trial from pregnancy through the initial postpartum period have been published (18). This report presents the one-year, post-partum follow-up data for the mothers and their infants.
Study participants
We randomized 118 English-speaking, women with BMIs ≥30 kg/m2 who were receiving prenatal care from Kaiser Permanente Northwest, a closed-panel managed care organization that provides comprehensive medical care to nearly 500,000 members in Northwest Oregon and Southwest Washington. Women who had diabetes mellitus or other medical conditions requiring specialized nutritional care (for example, a history of bariatric surgery), or were greater than 20 weeks gestational age based on the pregnancy dating information available at enrollment were excluded.
Women in the weight management arm participated in a weekly, group-based diet and lifestyle intervention (described below). Women in the usual care control group received a single dietary advice session with a dietitian. All study participants were asked to return to the research clinic for outcome measures at 34 weeks gestation and postpartum with their infants at two to three weeks and one year.
The Kaiser Permanente Northwest Institutional Review Board and an independent data safety and monitoring board approved the study protocol and consent procedures. All participants provided written informed consent.
Intervention
Immediately after randomization, intervention participants received an individual dietary counseling session, followed one week later by a second individual dietary counseling session. After that, intervention participants attended weekly group meetings until they delivered, but no intervention was provided post-partum. We encouraged participants to keep daily food and activity records and to turn them in weekly at the group meeting. The group session format has previously been described in detail but included a check-in, a nutrition topic and/or exercise topic, a behavior change topic, and a goal-setting segment for the next week with a plan for how to meet the goals (22). The weight management goals were to maintain weight within 3% of randomization weight, keep energy intake (calories) within an individualized goal, adopt the Dietary Approaches to Stop Hypertension (DASH) eating pattern without sodium restriction, and exercise daily (goals 30 minutes moderate activity per day or 10,000 steps per day on pedometer).
Outcome measures
The primary one-year postpartum outcome was maternal weight change, defined as weight measured at one-year postpartum minus weight at randomization (mean gestational age 14.9±2.6 weeks). We also evaluated infant weight and length change from birth to 1 year of age, and infant skinfold measures at 2 weeks and 1 year of age.
Staff who were blinded to the participants study group measured maternal weight (kilograms, kg) at randomization, 34 weeks gestation, and 2 weeks and 1-year postpartum and infant weight, length, and skin fold measures at 2 weeks and 1 year of age. At each time point, the participants were weighed on a high quality digital scale with their shoes removed. Height was measured once at baseline (centimeters, cm) with a calibrated, wall-mounted stadiometer. Infant birth weights (grams) were collected from the medical record. At 2 weeks and 1 year of age, infant weight, length, and skinfold measures were collected in the research clinic. Infants were weighed (grams, g) on an electronic scale (Seca model 727; Seca Corp., Hanover MD). Length was measured in centimeters on an O’ Leary Pediatric Length Board (Ellard Instrumentation, Seattle WA). Infant skinfolds were measured at the tricep and subscapular regions using Harpenden skinfold calipers (CMS Instruments, London) with readings done following a five second count after applying caliper blades and measures recorded to the nearest millimeter. Each measure was performed 3 times and the final value was the average of the three measures. Study staff were trained to collect infant measures by a study investigator (CM) who is also a Pediatrician and Neonatologist. The physician demonstrated the measure to the staff using the equipment in the research clinic and observed staff obtaining the measure to assure proper technique. We calculated infant weight-for-length, weight-for-age, and length-for-age. To determine the z-scores for these measures, we used WHO growth standards data (23). We also present the proportion of infants who had high weight for length at 1 year of age. High weight (overweight) for children ages 0-2 years is defined as a weight for length at or above the 97.7th percentile of WHO weight for recumbent length growth standards (24).
Missing maternal weights were filled when possible with data from the medical records. We have previously shown that maternal weights and heights as documented in the medical record correspond well to those collected in the research setting (25). We performed a similar analysis for infant weights (n=64) and lengths (n=64). We found that the concordance of the medical record and research infant weights was extremely high (concordance correlation coefficient (CCC)=.96), suggesting that these data are exchangeable; however, this was not so for infant lengths (CCC=.71). A previous study has also shown that clinically obtained length measures tend to underestimate measures of under- and overweight in children under 2 years of age (26). Therefore, only research measures were used for infant length outcomes. Skinfold measures are not collected as part of routine medical care; thus, fewer infants could be included in both skinfold and weight-for-length analyses (n=44 intervention, n=41 control) than in analyses requiring weight only (n=51 intervention, n=52 control).
To be included in the analysis the measured maternal weights had to have occurred within +/- 90 days of 1 year postpartum. Infant weight measures were accepted from 11 to 14 months of age. The WHO program for calculating z-scores is age specific, which allows for variability in infant age in the calculation. If participants had both a weight collected in the research clinic and a medical record weight in the specified window, then preference was given to the weight measured in the research clinic. The timing of the collection of weight measures did not differ significantly between the groups (1 day difference on average, p=.86).
Statistical analyses
We performed an intention to treat (ITT) analysis using all available data for the 114 participants according to the group to which they were randomly assigned at the baseline visit. We used generalized estimating equations (GEE) to account for the repeated measurements across time within individuals for each outcome (27). An advantage of GEE is that it allows us to include participants with missing data across time, as GEE uses all available data to estimate the working correlation matrix parameters (27-29). GEE is consistent with an ITT approach in that all randomized participants with at least one data point are included in the analysis; participants will vary in the number of data points they can contribute (1, 2, or 3) and are not dropped from the analysis if missing at follow-up. Time was modeled as a categorical within-subjects variable. Group assignment was a categorical between-subjects variable. Our focal effect was time by group interaction, which would indicate differential change over time. For the baby weight analyses, we included baby gender and gestational age at birth, as covariates. For z-score baby weight analyses, we included only gestational age at birth since gender is a component of the z-score. We examined whether there was a difference between the intervention and control groups on the proportion of infants who had high weight for length at one year of age, defined as at or above the 97.7th percentile of WHO weight for recumbent length growth standards, using multiple logistic regression. No covariates were included for maternal weight.
The study was powered at .80 and a two-tailed alpha level of .05 to detect the anticipated difference (Cohen’s d=.40) between the arms in maternal weight change from randomization to 1 year postpartum with 160 participants through one year.
Across the cohort, we explored whether gestational weight gain (maternal weight change from randomization to 34 weeks) was predictive of postpartum weight retention (maternal weight change from randomization to one year postpartum) using multiple linear regression. For this analysis, which was not driven by the participants’ random allocation to intervention or control, we adjusted for confounding by BMI, parity, and age as these factors have previously be shown to be significantly associated with gestational weight gain (8). We also included arm as a covariate. Prior to analysis, we examined the distributions of the data to check that the assumptions of the analyses were met and for the presence of outliers that may have had an undue influence on the results.
Results
Figure 1 shows the flow of participants through the study. We randomized 118 women. Two participants withdrew within a week after randomization and 2 miscarried, leaving 114 in the cohort. One-year postpartum weight measures were available for 89 women (75 from the research clinic and 14 from the medical record). Nine women were pregnant at follow up, and data were missing for 16 women. Our participants were primarily White (86%) women who had at least a high school education (Table 1). Their mean BMI at study entry (randomization) was 36.7 kg/m2; over half were classified with Class 2 or 3 obesity. Women who returned to the research clinic for follow up at one year, did not differ significantly from those without a follow up visit (Table 1;Χ2(1)= 0.21, p=.65). One-year weights were available for 43 (77%) intervention and 46 (79%) control participants.
Figure 1.

CONSORT Diagram
The number of intervention participants with measured weights available at 34 weeks gestation (n=55) is 1 greater than that noted in the diagram of an earlier report (n=54) [Obesity (2014) 22, 1989–1996)]. The error is in the previously published figure only and does not change the results.
Table 1.
Healthy Moms cohort baseline characteristics
| Had One Year Visit (n=75) ±SD or N (%) | Did Not Have One Year Visit (n=39) ±SD or N (%) | p | Full cohort (n=114) ±SD or N (%) | |
|---|---|---|---|---|
|
| ||||
| Group | 0.70 | |||
|
| ||||
| Control | 37 (49%) | 21 (54%) | 58 (51%) | |
|
| ||||
| Intervention | 38 (51%) | 18 (46%) | 56 (49%) | |
|
| ||||
| Maternal (n=114) | ||||
| Maternal age (years) | 32.1±5.1 | 31.2±4.5 | 0.32 | 31.8±4.9 |
|
| ||||
| Gestational age at randomization (weeks) | 15.1±2.5 | 14.4±2.7 | 0.14 | 14.9±2.6 |
|
| ||||
| Weight (kg) | 99.0±16.5 | 101.0±12.8 | 0.51 | 99.7±15.3 |
|
| ||||
| BMI (kg/m2) | 36.8±5.4 | 36.6±3.9 | 0.85 | 36.7±4.9 |
|
| ||||
| BMI category (kg/m2) | 0.65 | |||
|
| ||||
| 30-34.9 | 34 (45%) | 16 (41%) | 50 (44%) | |
|
| ||||
| 35-39.9 | 24 (32%) | 16 (41%) | 40 (35%) | |
|
| ||||
| 40+ | 17 (23%) | 7 (18%) | 24 (21%) | |
|
| ||||
| Parity | 1.00 | |||
|
| ||||
| Nulliparous | 35 (47%) | 18 (46%) | 53 (46%) | |
|
| ||||
| Parous | 40 (53%) | 21 (54%) | 61 (54%) | |
|
| ||||
| Race | 0.46 | |||
|
| ||||
| White | 66 (88%) | 32 (82%) | 98 (86%) | |
|
| ||||
| Black | 2 (3%) | 3 (8%) | 5 (4%) | |
|
| ||||
| Other | 7 (9%) | 4 (10%) | 11 (10%) | |
|
| ||||
| Education | 0.61 | |||
|
| ||||
| High School Graduate/GED | 18 (24%) | 12 (31%) | 30 (26%) | |
|
| ||||
| Technical School Graduate | 9 (12%) | 4 (10%) | 13 (11%) | |
|
| ||||
| College Graduate or more | 46 (61%) | 20 (51%) | 66 (58%) | |
|
| ||||
| Missing | 2 (3%) | 3 (8%) | 5 (4%) | |
|
| ||||
| Infant (n=114) | ||||
| Gestational age of baby at birth, weeks | 39.0±1.8 | 39.4±1.2 | 0.29 | 39.1±1.6 |
|
| ||||
| Sex of baby | 0.32 | |||
|
| ||||
| Male | 45 (60%) | 19 (49%) | 50 (44%) | |
|
| ||||
| Female | 30 (40%) | 20 (51%) | 64 (56%) | |
Maternal weight outcomes at one year
Figure 2 graphically displays maternal weight change by study arm from randomization through 1 year postpartum. We found a significant overall time by group interaction (Χ2=19.08, p<.001). Consistent with what we reported previously (18), the intervention group gained less weight during pregnancy than the control group, and weighed less both at 34 weeks gestation (mean difference=-3.4 kg, 95% CI [-5.0,-1.8], time point A) and at 2 weeks postpartum (-2.7 vs +1.2 kg, mean difference=-3.9 kg 95% CI [-5.9,-1.9], time point B) than at randomization.
Figure 2.
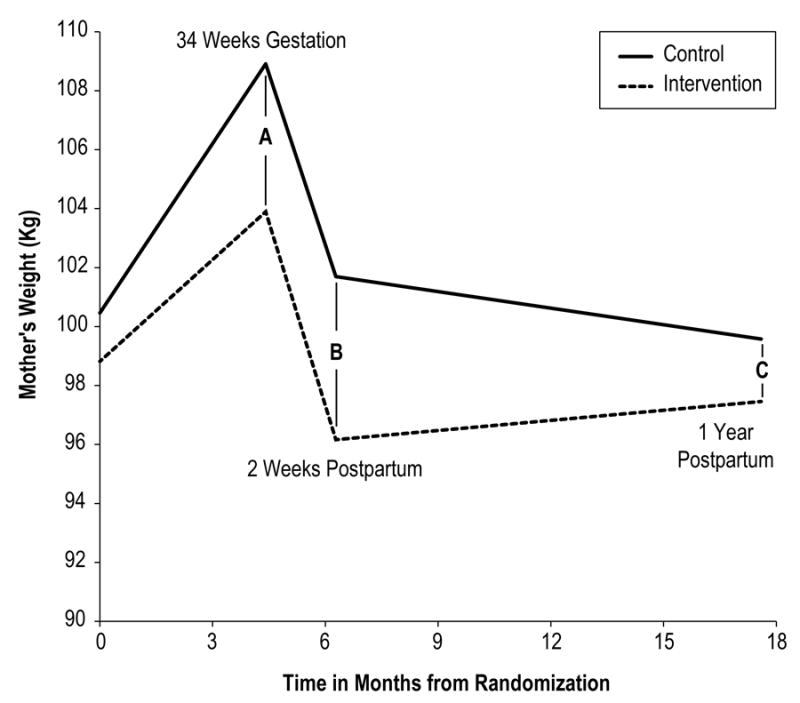
Maternal weight change from randomization to one year postpartum A, B, and C refer to the difference between the maternal weights at each time point.
From randomization to one year postpartum, there was no significant difference between the intervention and control groups in change in weight (-1.4 kg vs -0.9 kg, respectively, mean difference -0.5 kg, 95% CI [-4.0, 3.1], Table 2, time point C). There was a significant difference in maternal weight change from 2 weeks to one year postpartum (Figure 2, time points B to C), such that the intervention group had an increase in weight between 2 weeks and one year postpartum (96.2 to 97.5 kg, mean change = +1.3 kg); whereas, the control group had a decrease in weight (101.7 to 99.6 kg, mean change -2.1 kg) (adjusted effect size 3.4 kg, 95% CI [0.1, 6.8]).
Table 2.
Maternal and infant one-year outcomes
| Adjusted Mean (SE) | ||||
|---|---|---|---|---|
| Baseline | 1 Year after Birth | Change in Adjusted Mean | Difference in Adjusted Mean Change [95% CI] | |
| Maternal Weight (Kg)a | ||||
| Intervention | 98.8 (2.0) | 97.5 (2.5) | -1.3 | -0.5 [-4.0, 3.1] |
| Control | 100.5 (2.0) | 99.6 (2.5) | -0.9 | |
| Infant Weight (Kg)b | ||||
| Intervention | 3.50 (0.06) | 9.86 (0.12) | 6.36 | 0.02 [-0.37,0.41] |
| Control | 3.70 (0.07) | 10.04 (0.17) | 6.34 | |
| Weight for age z-scoreb | ||||
| Intervention | 0.41 (0.11) 65.9%tile | 0.44 (0.11) 67.0%tile | 0.03 1.1%tiles | 0.33 [-0.07,0.73] 10.7%tiles |
| Control | 0.81 (0.14) 79.1%tile | 0.51 (0.13) 69.5%tile | -0.30 -9.6%tiles | |
| Weight for length z-scorec | ||||
| Intervention | -0.16 (0.15) 43.6%tile | 0.52 (0.11) 69.9%tile | 0.68 26.3%tiles | 0.10 [-0.35,0.54] 4.3%tiles |
| Control | 0.03 (0.13) 51.2%tile | 0.62 (0.15) 73.2%tile | 0.59 22%tiles | |
| Length for age z-scorec | ||||
| Intervention | -0.09 (0.15) 46.4%tile | -0.08 (0.14) 46.8%tile | 0.01 0.4%tiles | -0.01 [-0.51, 0.50] 0%tiles |
| Control | 0.18 (0.16) 57.1%tile | 0.19 (0.20) 57.5%tile | 0.01 0.4%tiles | |
| Sum of triceps + subscapular skinfold thicknesses (mm)c | ||||
| Intervention | 16.32 (0.57) | 19.85 (0.78) | 3.53 | 0.21 [-2.58, 2.99] |
| Control | 15.86 (0.58) | 19.18 (0.62) | 3.32 | |
Baseline=Randomization
Baseline=Birth
Baseline=2 weeks after birth
Note: Corresponding percentile given for each Z score measure (%tile); difference in percentile units also provided for Z score measures (%tiles)
The proportion of study participants who were at or below their randomization weight at 1 year postpartum did not differ by intervention arm, Χ2(2)=0.98, p=.61. Overall, 56% in the Intervention group and 58% in the control group were at or below their randomization weight at 1 year postpartum, and 24% and 19%, respectively weighed >4.54 kgs (10 pounds) more than when they entered the study. When considering data for the cohort as a whole, independent of covariates and group assignment, we found gestational weight gain to be positively associated with postpartum weight retention (b=0.8 kg, 95% CI [0.4, 1.3], p<.001).
Infant outcomes
Among the 114 infants born to our study participants, 44% were male (Table 1). The average gestational age at birth was 39.1 wks (+/- 1.6 wks), and 5 (4.4%) were born preterm (<37 weeks gestation). As previously reported (18), the mean birthweights and weight for gestational age z-scores of intervention and control group newborns did not differ significantly; however, the intervention group had fewer large-for-gestational-age infants (birth weight greater than 90th percentile for gestational age, 9% vs. 26%; odds ratio [OR]=0.28, 95% CI [0.09, 0.84]; p=.02).
The unadjusted mean infant weight, length, weight-for-age, length-for-age, weight-for-length, and skinfold measures by study arm for birth, 2 weeks and 1 year are shown in Table 3.
Table 3.
Infant measures at birth, 2 weeks, and 1 year of age
| Intervention | Usual care | |||||
|---|---|---|---|---|---|---|
| Mean (SD)a | n | Mean (SD)a | n | Main Effect of Group [95% CI]b | Difference in Adjusted Meane Change [95% CI] | |
| Weight (kg) | ||||||
| Birth | 3.48 (0.48) | 56 | 3.68 (0.67) | 58 | ||
| 2 weeks of age | 3.89 (0.57) | 55 | 4.15 (0.58) | 57 | -0.02 [-0.17,0.12] | |
| 1 year of age | 9.83 (0.93) | 51 | 10.01 (1.24) | 52 | 0.02 [-0.37,0.41] | |
| -0.20 [-0.38,-0.02]c | ||||||
| Weight for age z-score | ||||||
| Birth | 0.37 (0.98) 64.4%tile | 56 | 0.74 (1.40) 77.0%tile | 58 | ||
| 2 weeks of age | -0.10 (1.02) 46.0%tile | 55 | 0.41 (0.84) 65.9%tile | 57 | -0.03 [-0.23,-0.17] | |
| 1 year of age | 0.37 (0.74) 64.4%tile | 51 | 0.51 (0.94) 69.5%tile | 52 | 0.33 [-0.07, 0.73] | |
| -0.40 [-0.75,-0.05]d | ||||||
| Length (cm) | ||||||
| 2 weeks of age | 52.63 (2.35) | 46 | 53.44 (2.40) | 46 | ||
| 1 year of age | 74.94 (2.71) | 44 | 75.86 (3.51) | 41 | -0.43 [-1.66,0.80] | |
| -0.50 [-1.38,0.39]c | ||||||
| Length for age z-score | ||||||
| 2 weeks of age | -0.17 (1.17) 43.3%tile | 46 | 0.24 (1.10) 59.5%tile | 46 | ||
| 1 year of age | -0.15 (0.97) 44.0%tile | 44 | 0.20 (1.31) 57.9%tile | 41 | -0.01 [-0.51,0.50] | |
| -0.27 [-0.68,0.15]d | ||||||
| Weight for length z-score | ||||||
| 2 weeks of age | -0.16 (1.07) 43.6%tile | 46 | 0.02 (0.91) 50.8%tile | 46 | ||
| 1 year of age | 0.53 (0.72) 70.2%tile | 44 | 0.60 (0.92) 72.6%tile | 41 | 0.10 [-0.35,0.54] | |
| -0.20 [-0.59,0.20]d | ||||||
| Sum of triceps + subscapular skinfold thicknesses (mm) | ||||||
| 2 weeks of age | 16.31 (3.90) | 45 | 15.82 (3.89) | 46 | ||
| 1 year of age | 19.79 (5.18) | 44 | 19.13 (4.00) | 41 | 0.21 [-2.57,2.99] | |
| 0.47 [-1.13, 2.06]c | ||||||
Means and standard deviations are crude (observed) values.
Main effect of Group refers to the difference between arms collapsed across time (i.e., between-subjects effect) and after adjustment for covariates. These are based on the coefficients for the group term in the GEE model.
Controlling for gestational age at birth and gender.
Controlling for gestational age at birth. Note: Corresponding percentile given for each Z score measure (%tile)
Difference in Adjusted Mean Change is the difference in the change between arms after adjustment for covariates. These are based on the coefficients for the time by group interaction terms in the GEE model.
For infant weight, there were no significant differences between the intervention and control groups in the change from birth to 1 year in infant weight and weight-for-age z-score, from 2 weeks to 1 year of age in length, length-for-age z-score, or weight-for-length z-score (Table 2). Further, there were no differences between groups in the change from 2 weeks to one year of age in infant adiposity as measured by the sum of the triceps and subscapular skinfolds.
However, there were significant main effects (Table 3). Averaged across time, infants born to women in the intervention group weighed less than the control group (mean difference =-0.20 kg, 95% CI [-0.38, -0.02], p=.031) and had a lower weight-for-age z-score, (mean difference =-0.40 SD units, 95% CI [-0.75, -0.05], p=.024). There were no significant main effects for infant length, length-for-age z-score, weight-for-length z-score, or adiposity. There was also no significant difference between the intervention (n=1; 2.3%) and control (n=4; 9.8%) groups on the proportion of children at the 97.7th percentile or higher on weight for length, OR=0.22, 95% CI [0.02, 2.01], p=.18.
Discussion
The Healthy Moms trial is one of only two other interventions (19, 20) to examine maternal weight at one-year postpartum, and possibly the only trial to examine both infant weight and adiposity as measured by skinfolds at the same time. We found that while a group-based, weight management intervention focused on dietary and lifestyle changes during pregnancy resulted in less gestational weight gain among the intervention group, the maternal weight differences between the intervention and control groups were not sustained at one-year postpartum. The infants born to women in the intervention group, however, did appear to experience a benefit as fewer were born large for gestational age and there was a modest sustained difference in weight over the course of the first year of life.
The one-year maternal weight findings of our study are similar to those of the two other pregnancy management trials with 1-year postpartum follow up, which also found no significant weight differences between groups at 1-year postpartum (19, 20). However, unlike our study, neither of those trials had a significant impact on reducing pregnancy weight gain among women with obesity. Why the significant difference in weight seen in the initial postpartum period in our study did not persist through the first year postpartum is unclear; however, the lack of continued intervention in the postpartum period is a likely possibility. The slow rise in weight noted among the intervention group after the 2 week postpartum visit reflects the slow rise observed in most adult weight loss studies (30), suggesting relapse to pre-intervention behavior following the end of intervention contacts.
At one year postpartum, over half of women in both the intervention and control groups had returned to or were below their baseline weight (measured at 15 weeks gestation on average). These proportions are higher than those observed at 1 year in the Fit for Delivery study (35.4% of intervention and 28.1% of control participants, p=.18) whose intervention also focused only on the prenatal period (20). However, it is hard to compare the numbers directly as that study assessed return to self-reported, pre-pregnancy weight. Both of these trials confirmed the finding from prior retrospective cohort analyses (8, 9) that gestational weight gain is a strong predictor of postpartum weight retention.
The data from our trial and others suggest there is a need for weight management interventions in the postpartum period, not only to help women with postpartum weight retention lose weight, but also to provide ongoing for support those who were able to successfully manage their weight during pregnancy. The best modality for postpartum weight management is yet to be determined. Time constraints have been reported as a significant barrier for women in the postpartum period (31); therefore, testing interventions that do not require face-to face contact, such as phone and internet, would be valuable.
Weight and length trajectories in the first year of life did not differ between the intervention and control group infants. Since weight and length changes are not linear between 2 weeks and 12 months postpartum, additional weight data collected between those two time points may be needed to see subtle differences. Another possibility is that maternal weight management interventions conducted during pregnancy do not influence childhood growth trajectories and postpartum intervention with mothers and their infants is needed if affecting the childhood weight trajectory is desired.
It is unclear what factors may have contributed to the main effect observed for infant weight and weight-for-age. Since women in the intervention group gained less weight during pregnancy, one possible hypothesis is an in utero effect of maternal dietary pattern or caloric intake on offspring weight. Women in the intervention group were given a calorie intake goal and advised to follow the DASH dietary pattern, which favors foods with a lower glycemic index and less intake of saturated fats. A recent meta-analysis of randomized clinical trials among women with gestational diabetes suggests that dietary patterns with a low glycemic index lead to reduced birth weights and less insulin use (32). Another possibility is variation in infant feeding practices in the first year of life. Additional trials testing dietary interventions among pregnant women and their offspring may be able to further delineate the impact of maternal diet during pregnancy and infant diet in the first year of life on long-term risk for childhood obesity.
The strengths of our study include its randomized design, use of measured weights (rather than self-reported weights), and the duration of follow up of both the mothers and their infants. One limitation is our study’s sample size, which may have contributed to our inability to detect significant differences in maternal and infant outcomes at one year. Further, we did not collect biologic and anthropometric measures to assess the impact of the intervention on maternal metabolism or body composition.
In summary, our study findings suggest that ongoing support for weight management may be required after delivery to sustain favorable weight changes attained during pregnancy. This inference is not surprising given the adult weight loss literature also shows that successful weight loss maintenance requires continued support (30). Further, it is unknown whether ongoing postpartum diet and lifestyle intervention for the mother will also affect the diet and weight of her children. Ongoing trials may be able to provide more insight into this issue (33).
What is known
Approximately half of women with obesity gain excessive amounts of weight during pregnancy and long-term postpartum weight retention is common.
Cohort studies suggest that excessive maternal weight gain during pregnancy may lead to offspring obesity.
Few randomized trials of pregnancy weight management interventions have included long-term weight outcomes for either mothers or their infants.
What this study adds
A successful weight management intervention delivered during pregnancy did not produce a lasting difference in maternal weight between intervention and control groups at 1-year postpartum.
Gestational weight management intervention may have an effect on infant weight over the first year of life.
Pregnancy weight management interventions might benefit from adding a postpartum weight loss maintenance program to prevent postpartum weight regain.
Acknowledgments
Financial source of the study: This work was supported by a grant from the National Institute of Child Health and Human Development (RO1HD058061). Clinicaltrials.gov # NCT00950235
Footnotes
Authors’ Contributions:
Kimberly K. Vesco: Involvement in the conception and design of the study, overall lead of activities, review and interpretation of data, primary author of manuscript.
Michael C. Leo: Assisted with data management, led completion of statistical analyses, assisted with interpretation of data, and with preparation, critical revision and approval of the final manuscript.
Njeri Karanja: Involvement in the conception and design of the study, led the dietary component of the intervention and infant feeding survey development, review and interpretation of data, critical revision and approval of the final manuscript.
Matthew W. Gillman: Involvement in the conception and design of the study and selection of outcome measures, review and interpretation of data, and critical revision and approval of the final manuscript.
Cindy T. McEvoy: Involvement in the conception and design of the study, assisted with newborn outcome measurement training, review and interpretation of data, and approval of the final manuscript.
Janet C. King: Involvement in the conception and design of the study, assisted with the dietary component of the intervention, reviewed and interpreted data, and critical revision and approval of the final manuscript.
Cara L. Eckhardt: Involvement in the conception and design of the study, review and interpretation of data, and critical revision and approval of the final manuscript.
K. Sabina Smith: Involvement in the conception and design of the study, review and interpretation of data, and assisted with preparation, critical revision, and approval of the final manuscript.
Nancy Perrin: Involvement in the conception and design of the study, assisted with statistical analyses, approval of the final submitted manuscript.
Victor J. Stevens: Involvement in the conception and design of the study, led the intervention team, review and interpretation of data, and assisted with preparation, critical revision, and approval of the final manuscript.
Disclosure statement: The authors declare no conflict of interest.
Prior presentation of data: Dr. Vesco presented preliminary data from this study as a poster presentation at The Obesity Society Annual Meeting, Atlanta, Georgia, November 11-16, 2013.
Reference List
- 1.Bhutta ZA, Das JK, Rizvi A, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–77. doi: 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- 2.Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obstet Gynecol. 2010;202(6):574–8. doi: 10.1016/j.ajog.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Mamun AA, Callaway LK, O’Callaghan MJ, et al. Associations of maternal pre-pregnancy obesity and excess pregnancy weight gains with adverse pregnancy outcomes and length of hospital stay. BMC Pregnancy Childbirth. 2011;11:62. doi: 10.1186/1471-2393-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine and National Research Council. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 5.Sarwer DB, Allison KC, Gibbons LM, Markowitz JT, Nelson DB. Pregnancy and obesity: a review and agenda for future research. J Womens Health (Larchmt) 2006;15(6):720–33. doi: 10.1089/jwh.2006.15.720. [DOI] [PubMed] [Google Scholar]
- 6.Mamun AA, Mannan M, Doi SA. Gestational weight gain in relation to offspring obesity over the life course: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2014;15(4):338–47. doi: 10.1111/obr.12132. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Nutrition During Pregnancy. Washington D.C: National Academy Press; 1990. [Google Scholar]
- 8.Vesco KK, Dietz PM, Rizzo J, et al. Excessive gestational weight gain and postpartum weight retention among obese women. Obstet Gynecol. 2009;114(5):1069–75. doi: 10.1097/AOG.0b013e3181baeacf. [DOI] [PubMed] [Google Scholar]
- 9.Nohr EA, Vaeth M, Baker JL, Sorensen TI, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87(6):1750–9. doi: 10.1093/ajcn/87.6.1750. Erratum appears in Am J Clin Nutr. 2008 Dec;88(6):1705. [DOI] [PubMed] [Google Scholar]
- 10.Glazer NL, Hendrickson AF, Schellenbaum GD, Mueller BA. Weight change and the risk of gestational diabetes in obese women. Epidemiology. 2004;15(6):733–7. doi: 10.1097/01.ede.0000142151.16880.03. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol. 2011;117(6):1323–30. doi: 10.1097/AOG.0b013e31821aa358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteman VE, Aliyu MH, August EM, et al. Changes in prepregnancy body mass index between pregnancies and risk of gestational and type 2 diabetes. Arch Gynecol Obstet. 2011;284(1):235–40. doi: 10.1007/s00404-011-1917-7. [DOI] [PubMed] [Google Scholar]
- 13.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368(9542):1164–70. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- 14.Hoff GL, Cai J, Okah FA, Dew PC. Pre-pregnancy overweight status between successive pregnancies and pregnancy outcomes. J Womens Health (Larchmt) 2009;18(9):1413–7. doi: 10.1089/jwh.2008.1290. [DOI] [PubMed] [Google Scholar]
- 15.Paramsothy P, Lin YS, Kernic MA, Foster-Schubert KE. Interpregnancy weight gain and cesarean delivery risk in women with a history of gestational diabetes. Obstet Gynecol. 2009;113(4):817–23. doi: 10.1097/AOG.0b013e31819b33ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322–8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vesco KK, Karanja N, King JC, et al. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: a randomized trial. Obesity. 2014 Silver Spring;22(9):1989–96. doi: 10.1002/oby.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Althuizen E, van der Wijden CL, van MW, Seidell JC, van Poppel MN. The effect of a counselling intervention on weight changes during and after pregnancy: a randomised trial. BJOG. 2013;120(1):92–9. doi: 10.1111/1471-0528.12014. [DOI] [PubMed] [Google Scholar]
- 20.Phelan S, Phipps MG, Abrams B, et al. Does behavioral intervention in pregnancy reduce postpartum weight retention? Twelve-month outcomes of the Fit for Delivery randomized trial. Am J Clin Nutr. 2014;99(2):302–11. doi: 10.3945/ajcn.113.070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vesco KK, Karanja N, King JC, et al. Healthy Moms, a randomized trial to promote and evaluate weight maintenance among obese pregnant women: Study design and rationale. Contemp Clin Trials. 2012;33(4):777–85. doi: 10.1016/j.cct.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.PREMIER Intervention. 2007 http://www.kpchr.org/public/premier/intervention/Intervention.asp.
- 23.World Health Organization. The WHO Child Growth Standards. [12-3-2015];2015 http://www.who.int/childgrowth/standards/en/
- 24.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0-59 months in the United States. MMWR Recomm Rep. 2010;59(RR-9):1–15. [PubMed] [Google Scholar]
- 25.Leo MC, Lindberg NM, Vesco KK, Stevens VJ. Validity of medical chart weights and heights for obese pregnant women. EGEMS (Wash DC ) 2014;2(1):1051. doi: 10.13063/2327-9214.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rifas-Shiman SL, Rich-Edwards JW, Scanlon KS, Kleinman KP, Gillman MW. Misdiagnosis of overweight and underweight children younger than 2 years of age due to length measurement bias. MedGenMed. 2005;7(4):56. [PMC free article] [PubMed] [Google Scholar]
- 27.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 28.Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7(1-2):305–15. doi: 10.1002/sim.4780070131. [DOI] [PubMed] [Google Scholar]
- 29.Twisk J, de VW Attrition in longitudinal studies. How to deal with missing data. J Clin Epidemiol. 2002;55(4):329–37. doi: 10.1016/s0895-4356(01)00476-0. [DOI] [PubMed] [Google Scholar]
- 30.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125(9):1157–70. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicklas JM, Zera CA, Seely EW, Abdul-Rahim ZS, Rudloff ND, Levkoff SE. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy Childbirth. 2011;11:23. doi: 10.1186/1471-2393-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37(12):3345–55. doi: 10.2337/dc14-1530. [DOI] [PubMed] [Google Scholar]
- 33.Lifestyle Interventions For Expectant Moms (LIFE-Moms) [12-3-2015];Lifestyle Interventions For Expectant Moms (LIFE-Moms) 2015 https://portal.bsc.gwu.edu/web/lifemoms.


