During microwave tumor ablation of hepatocellular carcinoma, hepatic veins and arteries were more resistant to occlusion than portal veins, but only hepatic arterial patency within an ablation zone was related to local tumor progression.
Abstract
Purpose
To characterize vessel occlusion rates and their role in local tumor progression in patients with hepatocellular carcinoma (HCC) who underwent microwave tumor ablation.
Materials and Methods
This institutional review board approved, HIPAA-compliant retrospective review included 95 patients (75 men and 20 women) with 124 primary HCCs who were treated at a single center between January 2011 and March 2014. Complete occlusion of the portal veins, hepatic veins, and hepatic arteries within and directly abutting the ablation zone was identified with postprocedure contrast material–enhanced computed tomography. For each vessel identified in the ablation zone, its size and antenna spacing were recorded and correlated with vascular occlusion with logistic regression analysis. Local tumor progression rates were then compared between patent and occluded vessels for each vessel type with Fisher exact test.
Results
Occlusion was identified in 39.7% of portal veins (29 of 73), 15.0% of hepatic veins (six of 40), and 14.2% of hepatic arteries (10 of 70) encompassed within the ablation zone. Hepatic vein occlusion was significantly correlated with a smaller vessel size (P = .036) and vessel-antenna spacing (P = .006). Portal vein occlusion was only significantly correlated with a smaller vessel size (P = .001), particularly in vessels that were less than 3 mm in diameter. Local tumor progression rates were significantly correlated with patent hepatic arteries within the ablation zone (P = .02) but not with patent hepatic (P = .57) or portal (P = .14) veins.
Conclusion
During microwave ablation of HCC, hepatic veins and arteries were resistant to vessel occlusion compared with portal veins, and only arterial patency within an ablation zone was related to local tumor progression.
© RSNA, 2016
Introduction
Thermal tumor ablation is now considered first-line therapy for small hepatocellular carcinomas (HCCs) in patients with cirrhosis (1–3). The most common thermal ablation modality used worldwide is radiofrequency (RF) ablation, which has been the reference standard for 2 decades (4). One drawback of RF ablation is its susceptibility to the heat-sink effect of nearby blood vessels, which can protect adjacent tissue from damage and lead to incomplete tumor destruction (5). As a result, randomized RF ablation clinical trials demonstrated rates of local tumor progression (LTP) greater than 50% (6). Modern high-powered microwave ablation systems more effectively overcome this heat-sink effect by rapidly generating higher temperatures at greater depths compared with RF ablation (7). Microwave systems use this intrinsic heating advantage to create larger ablation zones and margins in the presence of nearby vasculature (7–9).
The improved heating capability of microwave ablation has raised concerns about treatment of tumors near critical hepatic vasculature. The overarching goal of thermal ablation is to effectively treat the tumor with an ablative margin to minimize the risk for LTP (10). Because perivascular locations have been associated with higher LTP rates, an aggressive approach may be needed for adequate treatment (10–12). However, thrombosis of adjacent large blood vessels can result in hepatic infarcts (13–15). Determining which vessels are at risk for thrombosis during an ablation is essential to find a balance between treatment efficacy and safety, but not enough is known about how portal veins, hepatic veins, and hepatic arteries respond to ablation-induced thermal damage to guide treatments for either clinical goal.
Previous studies showed that vessel size, vessel-antenna spacing, and hepatic vessel type are associated with vascular occlusion after thermal ablation (16–19). However, these conclusions were derived from phantom or nontumor porcine liver models. Developing a more clinically applicable ablation strategy required interrogating a database of patients who underwent microwave ablation. The purpose of our retrospective study was to characterize vessel occlusion rates and their role in LTP in patients with HCC who underwent microwave tumor ablation.
Materials and Methods
Patient Selection
Institutional review board approval was obtained to de-identify a clinical database for research purposes, and a waiver of informed consent was granted. All percutaneous microwave ablations of primary HCC performed at a single center between January 2011 and March 2014 were included in this analysis. HCC was diagnosed on the basis of the Organ Procurement and Transplantation Network/United Network for Organ Sharing class 5 criteria (20). A total of 124 tumors were treated in 95 patients (75 men and 20 women). The mean patient age was 60.6 years (range, 44–82 years), and the mean model for end-stage liver disease (MELD) score was 10.2 (range, 5.7–18.3) before ablation. The mean tumor diameter was 21.2 mm ± 8.3 (range, 7.7–36.5 mm). The underlying causes of HCC included hepatitis C infection (n = 45), combined hepatitis C infection and alcoholic liver disease (n = 18), alcoholic liver disease (n = 10), cryptogenic cirrhosis (n = 6), nonalcoholic steatohepatitis (n = 6), hepatitis B infection (n = 3), autoimmune hepatitis (n = 2), primary biliary cirrhosis (n = 2), alpha-1 antitrypsin deficiency (n = 2), and hemochromatosis (n = 1). Percutaneous microwave ablation was determined to be the best treatment option for each patient by a multidisciplinary team of radiologists, hepatologists, oncologists, and transplant surgeons at a consensus conference. In a prior study, we reported on 75 patients who were included in this study (21). The previous publication documented the safety and technical success of microwave ablation, whereas this study characterizes rates of vessel occlusion during microwave ablation and their relation to LTP.
Microwave Ablation Procedure
Ablations were performed by one of five board-certified abdominal radiologists (J.L.H. and F.T.L., with 10 and 19 years of experience, respectively) with 1–19 years of experience in percutaneous ablation at study inception. All ablations were performed under general anesthesia. A high-powered, gas-cooled microwave ablation system (Certus 140; NeuWave Medical, Madison, Wis) that used one to three antennas was used in all patients. Antenna placement was performed with ultrasonographic (US) (GE E9; GE Medical Systems, Waukesha, Wis) and/or computed tomographic (CT)/fluoroscopy (GE Optima 580; GE Medical Systems) guidance. Contrast material–enhanced CT was performed immediately after the procedure to assess for technical success or complications. If treatment was determined to be incomplete in the form of residual enhancing tumor or inadequate ablation zone coverage, additional power delivery cycles were performed until complete ablation with 5-mm margins was achieved. Follow-up imaging was performed with either contrast-enhanced CT or magnetic resonance (MR) imaging at target intervals of 1, 3, 6, and 9 months after ablation, with yearly surveillance imaging performed thereafter.
Analysis of Ablation Effects on Vessels and Recurrence Rates
Pre-, intra-, and postprocedure images for each case were evaluated by one of two radiology residents who were blinded to clinical data and follow-up results, had 3 (M.C.) and 4 (M.H.L.) years of experience in abdominal imaging, and were familiar with tumor ablation techniques and pre- and postprocedure imaging. Axial images from the most recent preprocedure contrast-enhanced CT or MR imaging examination were first reviewed to assess the presence of adjacent hepatic vessels that were larger than 1 mm in diameter and were discretely identifiable in the vicinity of the index tumor. The sagittal and coronal planes were utilized as needed to identify landmarks and confirm the location and origin of vessels of interest. Vessels were categorized by their type (ie, portal vein, hepatic vein, or hepatic artery), size, and distance to the center of the index tumor. Vessel size was measured from outer edge to outer edge at the widest diameter found within the ablation zone (Fig 1). If multiple antennas were used, the geometric center was set as the reference point to measure vessel-antenna spacing. Immediate postablation contrast-enhanced CT images were then analyzed to measure ablation zone size and determine whether or not the previously identified vessels were occluded in the ablation zone. All patients were then assessed for the presence of LTP at follow-up imaging up to 4 years (22). All data from patients who died from causes unrelated to liver disease or underwent transplantation were excluded from the analysis.
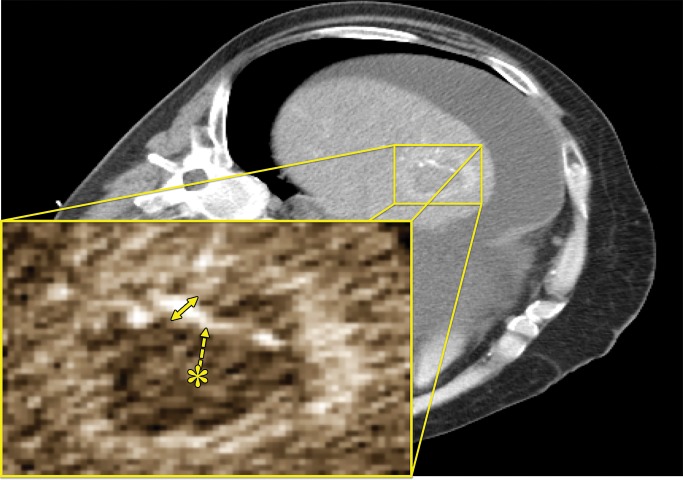
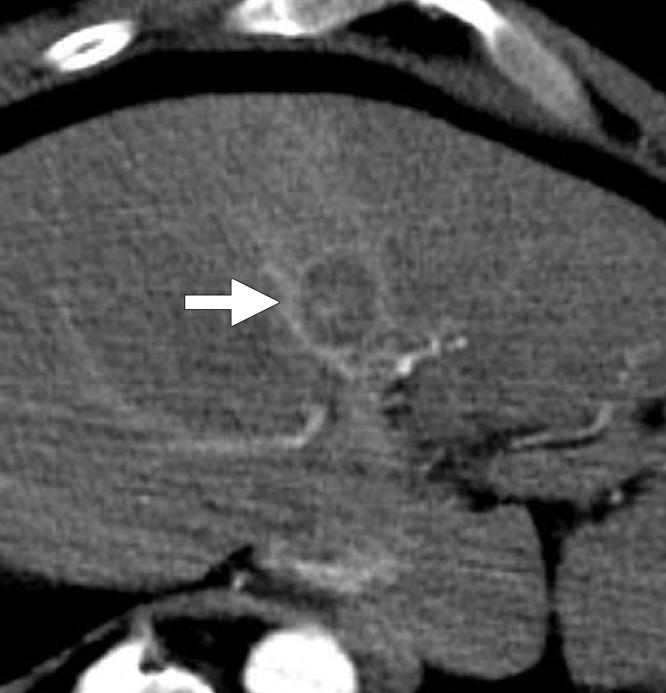
Figure 1:
CT image shows measurements of a patent hepatic artery encompassed within a microwave ablation zone (inset). Vessel-antenna spacing (dashed arrow) was measured from the center of the ablation zone (*) to the nearest vessel edge, and vessel size was measured at its widest diameter (arrow) within the ablation zone.
Statistical Analysis
Baseline descriptive statistics were collected (J.C.), with means, standard deviations, and ranges generated for the following metrics: age, sex, MELD score, tumor diameter, power delivery settings, ablation zone diameter, preablation vessel size, and vessel-antenna spacing. Mixed effects analysis of variance was used to compare mean values across each vessel group. Significant results were subject to posthoc Tukey test to identify individual differences between vessel group characteristics. Total occlusions for each vessel type were summed, and uni- and multivariate logistic regression analyses were used to analyze the likelihood of postablation vessel patency for each vessel type by vessel diameter and vessel-antenna spacing. Overall LTP rates were also correlated to patient and ablation treatment parameters with multivariate logistic regression (patient age, MELD score, number of liver lesions, ablation diameter, total ablation power, and follow-up period) and Fisher exact test for patent vessels (hepatic veins, portal veins, and hepatic arteries). Two-tailed P values of less than .05 were considered to indicate a significant difference. All statistical analyses were performed with GraphPad Prism version 5.0a (GraphPad, San Diego, Calif) and MedCalc version 7.4 (MedCalc, Mariakerke, Belgium) with assistance from the departmental statistician.
Results
The mean ablation treatment time for each patient in our cohort was 5.9 minutes ± 2.5 (range, 1–15 minutes), with a mean total power of 109.8 W ± 41.4 (range, 45–195 W distributed among one to three antennas). The mean tumor size was 21.0 mm ± 8.2, and the mean ablation zone diameter was 38.8 mm ± 10.1. All ablations were considered to be technically successful after one treatment session. The median imaging follow-up time was 14 months, and the median clinical follow-up time was 18 months, with primary technique effectiveness in 100% of tumors on the basis of images of the ablation zone. A total of 183 vessels were identified within ablation zones. A summary of the stratified results on the basis of vessel and patient characteristics is shown in Table 1.
Table 1.
Baseline Vessel and Patient Characteristics
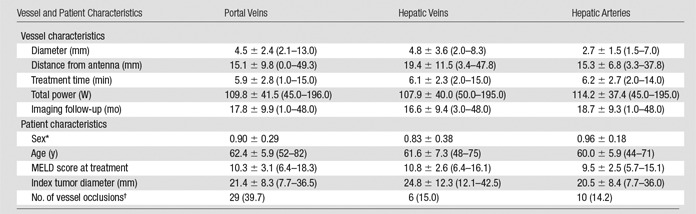
Note.—Data are the mean plus or minus standard deviation, and data in parentheses are the range. For portal veins, n = 73; for hepatic veins, n = 40; and for hepatic arteries, n = 70. Post hoc Tukey multiple comparison test was performed, with portal vein versus hepatic vein (P = .2127), portal vein versus hepatic artery (P < .001), and hepatic vein versus hepatic artery (P < .001).
*Men were coded as 1, and women were coded as 0.
†Data are the total number of vessel occlusions, and data in parenthesis are percentages.
Portal Veins
A total of 73 portal veins were encompassed within the ablation zones of 57 HCCs. Portal vein occlusion occurred in 29 of 73 vessels (39.7%) (Fig 2a–2c). Mean occluded portal vein size was 3.2 mm ± 0.8, with vessel-antenna spacing of 13.7 mm ± 9.2. The mean nonoccluded portal vein size was 5.5 mm ± 2.8, with vessel-antenna spacing of 15.8 mm ± 10.2 (Fig 2d). Logistic regression analysis showed a significant negative correlation between vessel size and occlusion rates (odds ratio [OR], 3.3; 95% confidence interval [CI]: 1.54, 5.61; P < .001). Vessel-antenna spacing showed a negative correlation, with closer spacing leading to higher rates of occlusion; however, this factor was not significant (OR, 1.02; 95% CI: 0.97, 1.08; P = .358). Univariate regression modeling showed a 50% chance of occlusion in vessels with a 3.2-mm diameter or vessel-antenna spacing less than 25.2 mm. Bivariate analysis that combined both vessel-antenna spacing and vessel size did not significantly improve determination of portal vein occlusion.
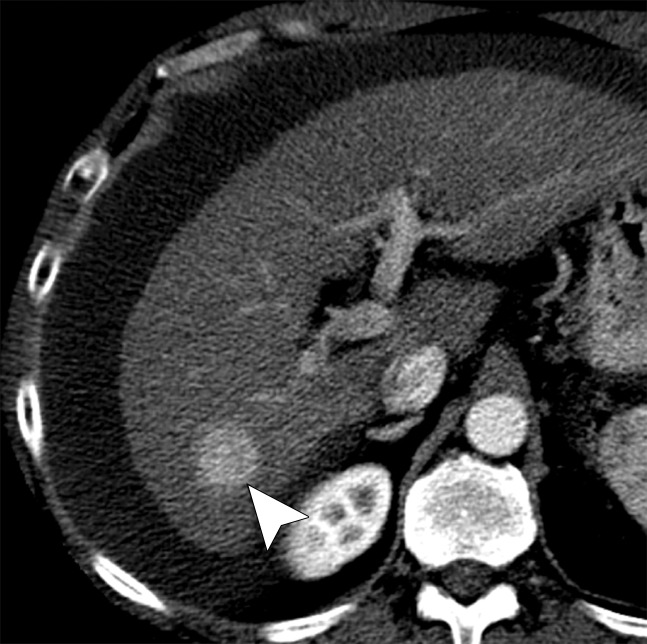
Figure 2a:
(a) Axial contrast-enhanced CT image shows an arterially enhancing 2.1-cm HCC in the right hepatic lobe (arrowhead). (b) Axial contrast-enhanced CT image obtained before ablation shows a patent posterior branch of the right portal vein (arrow) approximately 5 mm from the tumor margin. (c) Axial contrast-enhanced CT image obtained immediately after ablation shows portal vein occlusion (arrow) with an associated transient hepatic attenuation difference. (d) Graph shows patent versus occluded portal vein events as a function of vessel size and vessel-antenna spacing. Portal veins that were smaller in size were found to be significantly correlated with occlusion events (P < .001).
Figure 2c:
(a) Axial contrast-enhanced CT image shows an arterially enhancing 2.1-cm HCC in the right hepatic lobe (arrowhead). (b) Axial contrast-enhanced CT image obtained before ablation shows a patent posterior branch of the right portal vein (arrow) approximately 5 mm from the tumor margin. (c) Axial contrast-enhanced CT image obtained immediately after ablation shows portal vein occlusion (arrow) with an associated transient hepatic attenuation difference. (d) Graph shows patent versus occluded portal vein events as a function of vessel size and vessel-antenna spacing. Portal veins that were smaller in size were found to be significantly correlated with occlusion events (P < .001).
Figure 2d:
(a) Axial contrast-enhanced CT image shows an arterially enhancing 2.1-cm HCC in the right hepatic lobe (arrowhead). (b) Axial contrast-enhanced CT image obtained before ablation shows a patent posterior branch of the right portal vein (arrow) approximately 5 mm from the tumor margin. (c) Axial contrast-enhanced CT image obtained immediately after ablation shows portal vein occlusion (arrow) with an associated transient hepatic attenuation difference. (d) Graph shows patent versus occluded portal vein events as a function of vessel size and vessel-antenna spacing. Portal veins that were smaller in size were found to be significantly correlated with occlusion events (P < .001).
Figure 2b:
(a) Axial contrast-enhanced CT image shows an arterially enhancing 2.1-cm HCC in the right hepatic lobe (arrowhead). (b) Axial contrast-enhanced CT image obtained before ablation shows a patent posterior branch of the right portal vein (arrow) approximately 5 mm from the tumor margin. (c) Axial contrast-enhanced CT image obtained immediately after ablation shows portal vein occlusion (arrow) with an associated transient hepatic attenuation difference. (d) Graph shows patent versus occluded portal vein events as a function of vessel size and vessel-antenna spacing. Portal veins that were smaller in size were found to be significantly correlated with occlusion events (P < .001).
Hepatic Veins
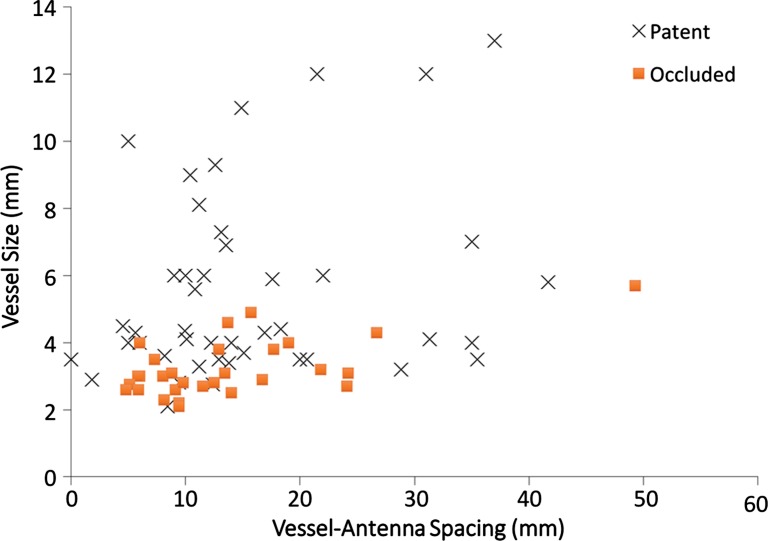
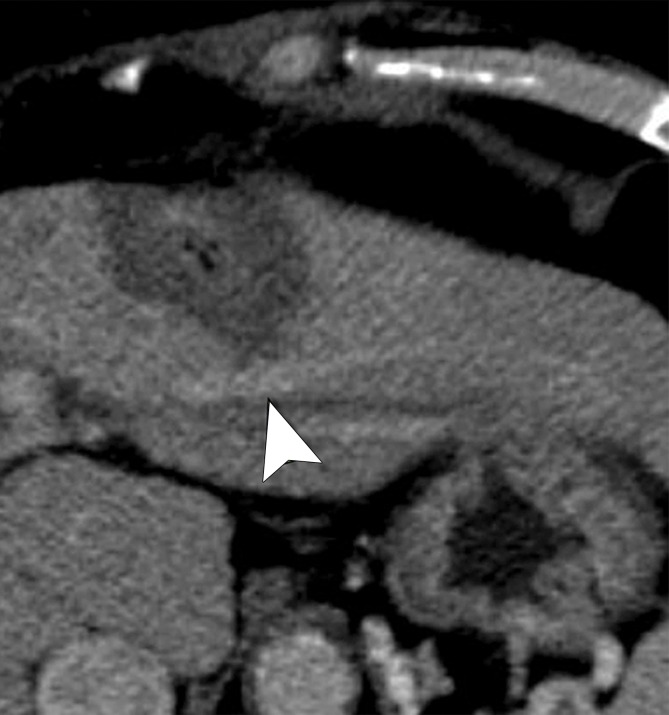
A total of 40 hepatic veins were encompassed within the ablation zones of 33 HCCs (Fig 3a). Hepatic vein occlusion occurred in six of 40 vessels (15%). The mean occluded hepatic vein size was 3.3 mm ± 0.9, with vessel-antenna spacing of 10.0 mm ± 5.6. The mean nonoccluded hepatic vein size was 4.7 mm ± 1.7, with mean vessel-antenna spacing of 21.2 mm ± 11.0 (Fig 3b). Logistic regression showed a significant negative correlation between occlusion and both vessel size (OR, 1.2; 95% CI: 1.01, 1.41; P = .036) and vessel-antenna spacing (OR, 2.1; 95% CI: 1.13, 4.55; P = .006). The univariate regression model showed a 50% chance of occlusion for a vessel size of 1.46 mm or vessel-antenna spacing of 4.25 mm. Bivariate analysis that combined vessel-antenna spacing with vessel size led to a slight, but insignificant, improvement in determining vessel occlusion.
Figure 3a:
Cirrhosis and HCC in a 61-year-old man. (a) Axial contrast-enhanced CT image obtained after ablation shows a patent left hepatic vein branch (arrowhead) approximately 2.2 cm from the center of the ablation zone. (b) Graph shows patent versus occluded hepatic veins as a function of vessel size and vessel-antenna spacing. Hepatic veins that were smaller in size and had smaller vessel-antenna spacing were found to be significantly correlated with occlusion in terms of both vessel size (P = .036) and vessel-antenna spacing (P = .006).
Figure 3b:
Cirrhosis and HCC in a 61-year-old man. (a) Axial contrast-enhanced CT image obtained after ablation shows a patent left hepatic vein branch (arrowhead) approximately 2.2 cm from the center of the ablation zone. (b) Graph shows patent versus occluded hepatic veins as a function of vessel size and vessel-antenna spacing. Hepatic veins that were smaller in size and had smaller vessel-antenna spacing were found to be significantly correlated with occlusion in terms of both vessel size (P = .036) and vessel-antenna spacing (P = .006).
Hepatic Arteries
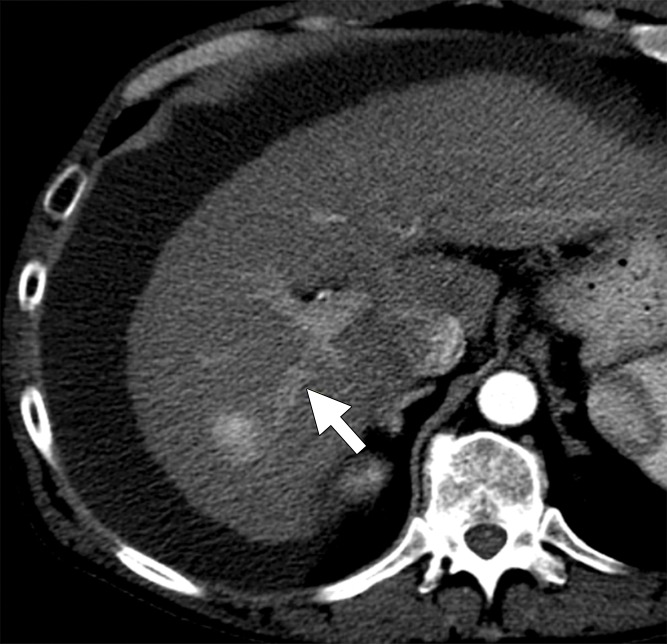
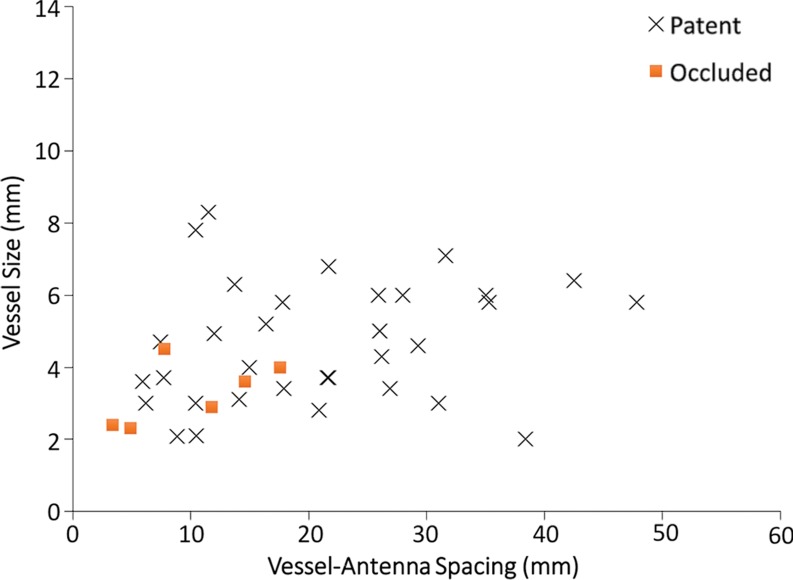
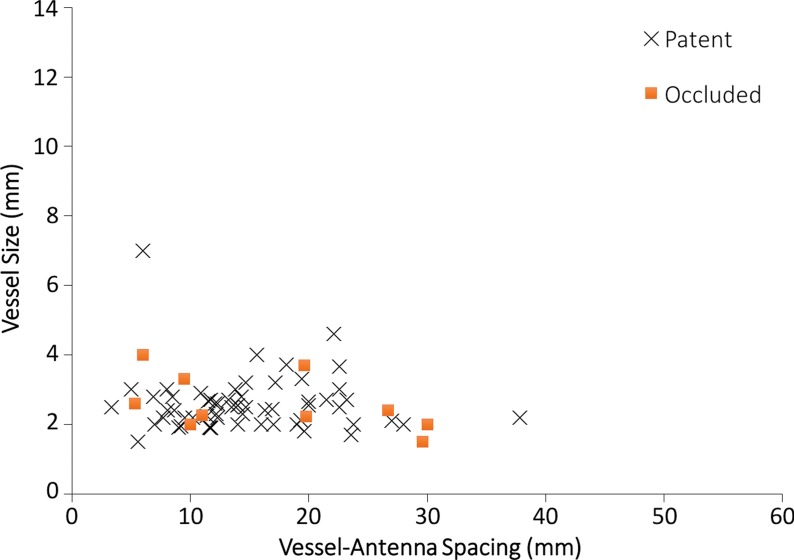
A total of 70 hepatic arteries were encompassed within the ablation zones of 57 HCCs (Fig 4a). The hepatic arteries were significantly smaller than portal (P < .001) and hepatic (P < .001) veins. Hepatic artery occlusion occurred in 10 of 70 vessels (14.2%), and the mean occluded hepatic artery size was 2.6 mm ± 0.7, with a vessel-antenna distance of 16.8 mm ± 9.6. The mean nonoccluded hepatic artery size was also 2.6 mm ± 0.8, with a vessel-antenna distance of 14.8 mm ± 6.5 (Fig 4b). Logistic regression showed no correlation between vessel occlusion and vessel size (OR, 0.98; 95% CI: 0.43, 2.22; P = .961) vessel-antenna spacing (OR, 1.04; 95% CI: 0.88, 1.05; P = .434). Similarly, combining both variables offered no improvement in association.
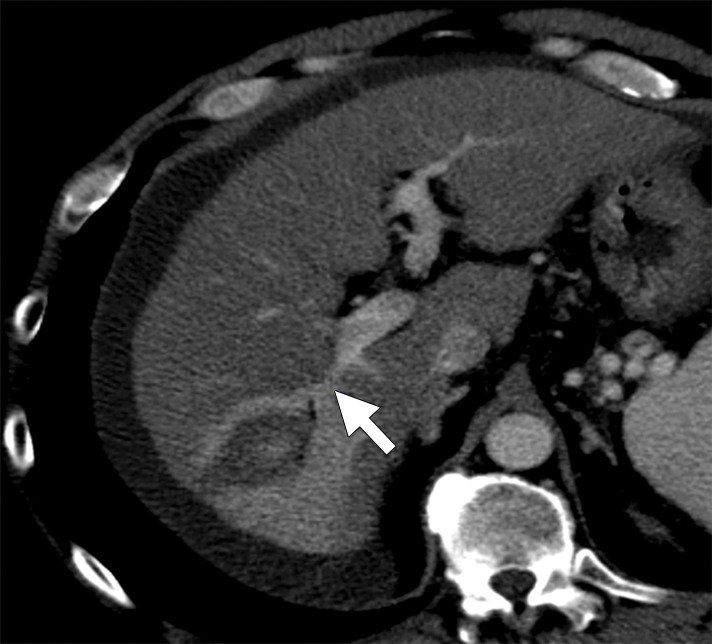
Figure 4a:
Hepatitis C infection and HCC in a 64-year-old man. (a) Axial contrast-enhanced CT image obtained immediately after ablation shows that the hepatic artery (arrow) remains patent despite its close proximity (6 mm) to the center of the ablation zone. (b) Graph shows vessel occlusion and patency as a function of hepatic artery size and vessel-antenna spacing. The hepatic arteries thrombosed at a rate similar to that of the hepatic veins (15% vs 14.2%, respectively).
Figure 4b:
Hepatitis C infection and HCC in a 64-year-old man. (a) Axial contrast-enhanced CT image obtained immediately after ablation shows that the hepatic artery (arrow) remains patent despite its close proximity (6 mm) to the center of the ablation zone. (b) Graph shows vessel occlusion and patency as a function of hepatic artery size and vessel-antenna spacing. The hepatic arteries thrombosed at a rate similar to that of the hepatic veins (15% vs 14.2%, respectively).
Local Tumor Progression
Tumor progression was identified in 12 of 124 (9.6%) treated HCCs during the follow-up period (1–48 months). A summary of the regression analysis for risk factors associated with LTP is shown in Table 2. Patent hepatic arteries within an ablation zone were significantly correlated with an increased risk for LTP compared with the presence of occluded hepatic arteries (OR, 5.86; 95% CI: 1.34, 25.64; P = .02). There was no significant difference in LTP rates between occluded and patent hepatic veins (OR, 1.27; 95% CI: 0.11, 14.60; P = .57) or between occluded and patent portal veins (OR, 3.67; 95% CI: 0.82, 16.45; P = .14).
Table 2.
Regression Models for Risk Factors Associated with LTP
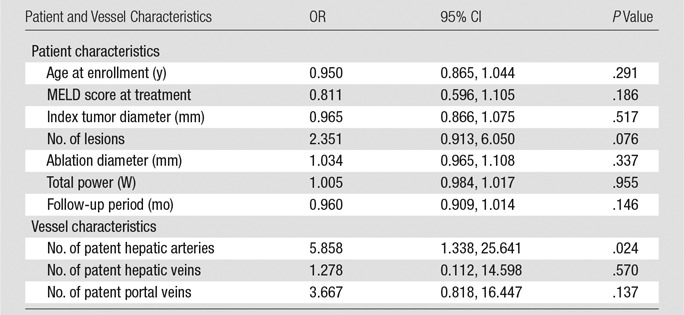
Note.—For patient characteristics, P values were calculated with multivariate logistic regression model, and for vessel characteristics, P values were calculated with Fisher exact test.
Complications
No large segmental infarcts were identified in our patient population. There were 21 deaths (21.1%) during the follow-up period, one of which occurred 1 week after the procedure as a result of aspiration pneumonia, for which the patient refused treatment. Fourteen deaths were attributed to end-stage liver disease, and five resulted from multiorgan failure after liver transplantation. There were no major procedure-related complications in this patient cohort. Minor complications included a pneumothorax, which required evacuation followed by a pleural blood patch, and an asymptomatic main portal vein thrombus, which resolved with low-dose warfarin therapy.
Discussion
Currently, there is a lack of information about how larger hepatic vessels respond to the heating of tissue within a microwave ablation zone and the relationship of hepatic vessels with LTP. The results of our study demonstrate that portal vein occlusion significantly correlates with a smaller vessel diameter (< 3.2 mm), while hepatic veins are significantly correlated with both a smaller vessel diameter (< 1.5 mm) and decreased vessel-antenna spacing (< 4.3 mm) after microwave ablation of HCC. Portal veins occluded at a rate nearly twice that of hepatic veins but did not lead to complications other than the single asymptomatic case of thrombosis in the main portal branch. Likewise, hepatic artery occlusion did not lead to lobar infarctions but was found to significantly correlate with a decreased risk for LTP.
The mechanism behind the increased rate of occlusion in portal veins compared with hepatic arteries and hepatic veins is likely related to differences in flow pattern, flow velocity, and the total amount of blood flow. Portal veins have slower blood flow because of drainage into high-resistance hepatic sinuses. This relatively sluggish flow is exacerbated in patients with cirrhosis and portal hypertension, who have even higher sinus pressures and slower antegrade portal vein flow (23). Slow flow is less effective at dissipating heat, resulting in vessel occlusion (18). Conversely, the drainage pathway of hepatic veins is into the lower resistance inferior vena cava. Caval blood flow is also subject to transmitted backpressure from the heart throughout the cardiac cycle, resulting in a substantially faster and more pulsatile flow pattern compared with that in the portal veins. In addition, hepatic veins carry more blood flow per vessel because all of the blood flow exiting the liver traverses the hepatic veins, whereas hepatic inflow is divided between the hepatic arteries and portal veins. Subsequently, hepatic veins dissipate heat more quickly, leading to fewer occlusions (19). Hepatic arteries, which were as resistant to occlusion as hepatic veins, are also characterized by high-flow velocity and pulsatility (24). The presence of patent hepatic arteries was significantly correlated with increased rates of LTP, a finding likely due to the heat-sink effect, which preserves microvascular tumor invasion (25).
Our data are consistent with those from prior studies that characterized rates of vessel occlusion within thermal ablation zones, with notable exceptions. Previous animal studies showed that vessels smaller than 3 mm in diameter were more likely to be occluded during microwave or RF ablation (16,17). Our study showed the 3-mm vessel size cutoff to be accurate in humans, but only for portal veins. Hepatic veins were found to be more resistant to occlusion, with vessels larger than 1.5 mm in diameter being relatively protected from occlusion. The increased rate of portal vein occlusion compared with hepatic vein occlusion within the ablation zone (39.7% vs 15.0%, respectively), with equivalent size distributions, is also similar to the results of previous in-vivo studies (19).
After RF ablation, LTP has been linked to the presence of nearby vessels (11). A previous study demonstrated that increased arterial enhancement of HCC at preablation contrast-enhanced CT is a prognostic factor for LTP (26). The authors hypothesized that the increased hepatic arterial supply to a tumor corresponds to a less differentiated, more aggressive HCC that is more likely to recur, even after ablation (27). Another possible explanation is that increased arterial enhancement results from the presence of larger hepatic arteries, which have a greater heat-sink effect and are more difficult to thrombose and, thus, preserve nests of tumor. Regardless of the mechanism, physicians should consider a more aggressive treatment approach to tumors with robust hepatic arterial supply. Microwave ablation, which has a higher tissue heating rate than RF ablation, may effectively occlude more of these vessels. Other possible ablation strategies include increasing ablation time and the number of antennas to deliver a higher thermal dose to the tumor tissue and combining ablation with intraarterial therapies, such as transarterial chemoembolization.
The primary limitation of our study is that it was performed with a unique microwave ablation system that is able to use multiple synchronized antennas. Thus, our results are difficult to generalize to clinical trials that use single-antenna systems and deliver less energy to target tissues (28,29). There are methods to quantify thermal dose response in ex-vivo tissue to account for different energy delivery approaches, but data on the relationship with blood vessels are limited (30). The retrospective nature of our study also precludes the collection of additional data that may be relevant to vessel occlusion, namely blood flow velocity. A prospective vessel occlusion study may benefit from obtaining Doppler US measurements or performing quantitative MR imaging to assess blood flow velocity between vessel groups before an ablation procedure. Lastly, our study did not look at the long-term data involving vessel patency. Aside from the previously mentioned main portal vein thrombosis, it is unknown whether the acutely occluded vessels remained occluded or if they eventually recannulized.
In conclusion, our study shows that, during microwave tumor ablation of HCC, hepatic veins and arteries are more resistant to occlusion than are portal veins, but only hepatic arterial patency within an ablation zone is related to LTP. Portal veins occluded at twice the rate of hepatic veins within an ablation zone. Despite being substantially smaller, hepatic arteries occluded at the same rQuesate as did hepatic veins, likely because of their faster velocity and more pulsatile blood flow. Additional studies that incorporate blood flow data and thermal dose models will be necessary to fully characterize the effect of blood vessels within an ablation zone and their relationship to LTP.
Advances in Knowledge
■ Portal veins occlude more often than hepatic veins (39.7% vs 15.0%, respectively) during microwave ablation of hepatocellular carcinoma.
■ Smaller vessel size (odds ratio, 1.2; P = .04) and vessel-antenna spacing (odds ratio, 2.1; P = .01) are significantly associated with hepatic vein occlusion, but only smaller vessel size was significantly associated with portal vein occlusion (odds ratio, 3.3; P = .001).
■ The presence of patent hepatic arteries within an ablation zone significantly correlates with a higher rate of local tumor progression compared with occluded hepatic arteries (odds ratio, 5.9; P = .02).
Implications for Patient Care
■ Portal veins occlude more frequently than do hepatic veins and should be treated with caution during microwave ablation, particularly in patients with cirrhosis or compromised liver function.
■ The presence of patent hepatic arteries within an ablation zone correlates with local tumor progression.
Received November 18, 2015; revision requested January 29, 2016; revision received February 29; accepted March 17; final version accepted April 1.
C.L.B. supported by the National Cancer Institute (R01 CA142737), and J.C. supported by the National Cancer Institute (F30 CA165548) and the National Center for Advancing Translational Sciences (UL1TR000427).
Disclosures of Conflicts of Interest: J.C. disclosed no relevant relationships. M.C. disclosed no relevant relationships. M.H.L. disclosed no relevant relationships. A.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultant for NeuWave Medical. Other relationships: disclosed no relevant relationships. J.L.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: Owns stock in NeuWave Medical and Cellectar Biosciences. Other relationships: disclosed no relevant relationships. F.T.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: shareholder, patent holder, member of board of directors for NeuWave Medical and patent holder and inventor royalties from Covidien. C.L.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultant for NeuWave Medical and Symple Surgical, patents and royalties from WARF, and stock in NeuWave Medical. Other relationships: disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- HCC
- hepatocellular carcinoma
- LTP
- local tumor progression
- MELD
- model for end-stage liver disease
- OR
- odds ratio
- RF
- radiofrequency
References
- 1.Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53(3):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56(4):908–943. [DOI] [PubMed] [Google Scholar]
- 3.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4(2):439–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed M, Brace CL, Lee FT, Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology 2011;258(2):351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhardwaj N, Strickland AD, Ahmad F, Atanesyan L, West K, Lloyd DM. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology 2009;41(2):168–172. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252(6):903–912. [DOI] [PubMed] [Google Scholar]
- 7.Andreano A, Huang Y, Meloni MF, Lee FT, Jr, Brace C. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys 2010;37(6):2967–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubner MG, Brace CL, Hinshaw JL, Lee FT, Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21(8 Suppl):S192–S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodd GD, 3rd, Dodd NA, Lanctot AC, Glueck DA. Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology 2013;267(1):129–136. [DOI] [PubMed] [Google Scholar]
- 10.Kosari K, Gomes M, Hunter D, Hess DJ, Greeno E, Sielaff TD. Local, intrahepatic, and systemic recurrence patterns after radiofrequency ablation of hepatic malignancies. J Gastrointest Surg 2002;6(2):255–263. [DOI] [PubMed] [Google Scholar]
- 11.Lu DSK, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 2003;14(10):1267–1274. [DOI] [PubMed] [Google Scholar]
- 12.Kang TW, Lim HK, Lee MW, Kim YS, Choi D, Rhim H. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology 2014;270(3):888–899. [DOI] [PubMed] [Google Scholar]
- 13.Meloni MF, Andreano A, Bovo G, et al. Acute portal venous injury after microwave ablation in an in vivo porcine model: a rare possible complication. J Vasc Interv Radiol 2011;22(7):947–951. [DOI] [PubMed] [Google Scholar]
- 14.Meloni MF, Andreano A, Lava M, Lazzaroni S, Okolicsanyi S, Sironi S. Segmental portal vein thrombosis after microwave ablation of liver tumors: Report of two cases. Eur J Radiol Extra 2010;76(3):e95–e98. [Google Scholar]
- 15.Livraghi T, Meloni F, Solbiati L, Zanus G; Collaborative Italian Group using AMICA system . Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol 2012;35(4):868–874. [DOI] [PubMed] [Google Scholar]
- 16.Lu DSK, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol 2002;178(1):47–51. [DOI] [PubMed] [Google Scholar]
- 17.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DSK. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol 2008;19(7):1087–1092. [DOI] [PubMed] [Google Scholar]
- 18.Chiang J, Hynes K, Brace CL. Flow-dependent vascular heat transfer during microwave thermal ablation. Conf Proc IEEE Eng Med Biol Soc 2012;2012:5582–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiang J, Willey BJ, Del Rio AM, Hinshaw JL, Lee FT, Brace CL. Predictors of thrombosis in hepatic vasculature during microwave tumor ablation of an in vivo porcine model. J Vasc Interv Radiol 2014;25(12):1965–1971.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology 2013;266(2):376–382. [DOI] [PubMed] [Google Scholar]
- 21.Ziemlewicz TJ, Hinshaw JL, Lubner MG, et al. Percutaneous microwave ablation of hepatocellular carcinoma with a gas-cooled system: initial clinical results with 107 tumors. J Vasc Interv Radiol 2015;26(1):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology 2014;273(1):241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perisic M, Ilic-Mostic T, Stojkovic M, Culafic D, Sarenac R. Doppler hemodynamic study in portal hypertension and hepatic encephalopathy. Hepatogastroenterology 2005;52(61):156–160. [PubMed] [Google Scholar]
- 24.McNaughton DA, Abu-Yousef MM. Doppler US of the liver made simple. RadioGraphics 2011;31(1):161–188. [DOI] [PubMed] [Google Scholar]
- 25.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14(3):199–208. [DOI] [PubMed] [Google Scholar]
- 26.Park Y, Kim Y-S, Rhim H, Lim HK, Choi D, Lee WJ. Arterial enhancement of hepatocellular carcinoma before radiofrequency ablation as a predictor of postablation local tumor progression. AJR Am J Roentgenol 2009;193(3):757–763. [DOI] [PubMed] [Google Scholar]
- 27.Nakashima Y, Nakashima O, Hsia CC, Kojiro M, Tabor E. Vascularization of small hepatocellular carcinomas: correlation with differentiation. Liver 1999;19(1):12–18. [DOI] [PubMed] [Google Scholar]
- 28.Brace CL, Laeseke PF, Sampson LA, Frey TM, van der Weide DW, Lee FT, Jr. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology 2007;244(1):151–156. [DOI] [PubMed] [Google Scholar]
- 29.Harari CM, Magagna M, Bedoya M, et al. Microwave ablation: comparison of simultaneous and sequential activation of multiple antennas in liver model systems. Radiology 2016;278(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mertyna P, Goldberg W, Yang W, Goldberg SN. Thermal ablation a comparison of thermal dose required for radiofrequency-, microwave-, and laser-induced coagulation in an ex vivo bovine liver model. Acad Radiol 2009;16(12):1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]