Low-dose methylene blue increased functional MR imaging activity during sustained attention and short-term memory tasks and potentiated memory retrieval.
Abstract
Purpose
To investigate the sustained-attention and memory-enhancing neural correlates of the oral administration of methylene blue in the healthy human brain.
Materials and Methods
The institutional review board approved this prospective, HIPAA-compliant, randomized, double-blinded, placebo-controlled clinical trial, and all patients provided informed consent. Twenty-six subjects (age range, 22–62 years) were enrolled. Functional magnetic resonance (MR) imaging was performed with a psychomotor vigilance task (sustained attention) and delayed match-to-sample tasks (short-term memory) before and 1 hour after administration of low-dose methylene blue or a placebo. Cerebrovascular reactivity effects were also measured with the carbon dioxide challenge, in which a 2 × 2 repeated-measures analysis of variance was performed with a drug (methylene blue vs placebo) and time (before vs after administration of the drug) as factors to assess drug × time between group interactions. Multiple comparison correction was applied, with cluster-corrected P < .05 indicating a significant difference.
Results
Administration of methylene blue increased response in the bilateral insular cortex during a psychomotor vigilance task (Z = 2.9–3.4, P = .01–.008) and functional MR imaging response during a short-term memory task involving the prefrontal, parietal, and occipital cortex (Z = 2.9–4.2, P = .03–.0003). Methylene blue was also associated with a 7% increase in correct responses during memory retrieval (P = .01).
Conclusion
Low-dose methylene blue can increase functional MR imaging activity during sustained attention and short-term memory tasks and enhance memory retrieval.
© RSNA, 2016
Introduction
Methylene blue (U.S. Pharmacopeia grade) is a drug with a long history of safe usages that was grandfathered by the Food and Drug Administration (1). It is currently used to treat methemoglobinemia and as a surgical stain (2). The pharmacokinetic and side effects of low-dose (0.5–4.0 mg/kg) methylene blue are well known and minimal in humans (3,4). When the lipophilic methylene blue enters the mitochondria of the brain at low concentrations, it forms a renewable redox complex that can donate electrons to the electron transport chain, thereby promoting cytochrome c oxidase activity, oxygen consumption, and energy production (5–7). While methylene blue is available systemically, it produces focal effects in brain regions where mitochondrial respiration can accept more electrons because of increased energy demands (8,9). Therefore, one would expect that methylene blue produces focal functional magnetic resonance (MR) imaging effects in activated brain regions and their interacting networks.
The memory-enhancing effects of low-dose methylene blue have been demonstrated in long-term memory tasks, which were first described more than 30 years ago in healthy rats and mice and supported by more recent studies in rodents and humans (5,6,9–11). For example, in an appetitive task in healthy rats, the use of methylene blue improved spatial memory retention (6). Methylene blue also prevented chronic spatial memory impairment in a rat model of Alzheimer disease on the basis of cytochrome c oxidase inhibition, implicating causality between mitochondrial respiration and memory enhancement by methylene blue (12). Methylene blue also prevented memory impairment in rats with local inhibition of mitochondrial respiration in the posterior cingulate cortex by maintaining cingulo-thalamo-hippocampal effective connectivity (13). Besides improving mitochondrial respiration, methylene blue could have pleiotropic effects on the brain from its redox interactions with various proteins and cellular processes (14,15). However, the memory-enhancing effects of methylene blue do not depend on inhibition of tau aggregation (16,17).
In humans, a single low dose of methylene blue has been shown to enhance long-term contextual and extinction memory (18). However, the effects of methylene blue on short-term memory and sustained attention tasks have not been investigated, and the neural correlates of the effects of methylene blue in humans are unknown. The goal of this study was to use multimodal functional MR imaging to investigate the neural correlates of methylene blue in humans. We tested the hypothesis that a single low dose of oral methylene blue would increase functional MR imaging activity during sustained attention in a psychomotor vigilance task, short-term memory in a delayed match-to-sample task, and the neural networks related to the tasks. In addition, we also tested the potential effect on cerebrovascular reactivity (CVR).
Materials and Methods
The institutional review board approved this double-blinded, randomized, placebo-controlled trial. Twenty-six English-speaking participants were enrolled between September 2013 and December 2014 from the local community via advertisements. Inclusion criteria were an age of 18–65 years and English speaking. The following exclusion criteria were used: any neurologic, psychiatric, cardiovascular, hepatic, or renal disorders; a history of organ transplantation; hypersensitivity to methylene blue or thiazide diuretics/phenothiazines; glucose-6-phosphate dehydrate deficiency; a contraindication to MR imaging; colorblindness; methemoglobinemia; ingestion of any psychiatric medication (currently or within the past 5 weeks); pregnant or breastfeeding; and a history of any surgery that could interfere with normal drug absorption.
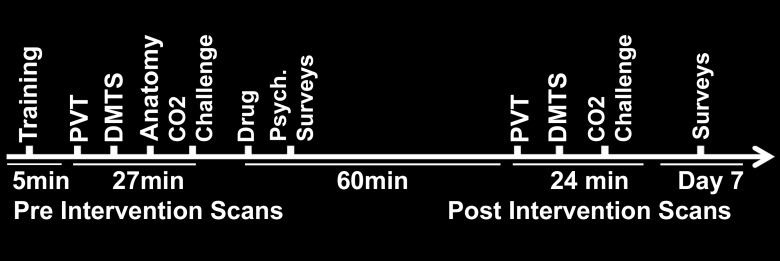
All subjects were imaged with the same paradigm order and underwent a brief training session outside the imager to become familiar with the functional MR imaging tasks (Fig 1). After the first set of MR imaging data was acquired, participants exited the imager. Then, 13 participants (the methylene blue group) were randomized to receive 280 mg (approximately 4 mg/kg) of oral U.S. Pharmacopeia-grade methylene blue (methylthioninium chloride USP; PCCA, Houston, Tex), and 13 participants (the placebo group) were randomized to receive food colorant (FD&C blue no. 2) by using a random allocation sequence generated by an independent statistician without restriction. To allow for drug absorption in accordance with previous bioavailability studies, a second set of identical MR imaging data was acquired 60 minutes later (3,4). Participants were asked to urinate before, but not after, taking the immediate-release opaque capsules and to not urinate again until after the MR imaging study to avoid compromising the blinding as a result of any urine coloration caused by ingestion of methylene blue. To minimize interference with drug intake, subjects were asked to eat a very light meal the morning of the MR imaging study and to avoid any type of caffeine intake. The 280-mg dose selection was estimated as 4 mg/kg for an average body weight of 70 kg. Methylene blue is a memory-enhancing drug in animals and humans after a single dose in the low-dose range of 0.5–4 mg/kg, but it has opposite effects at doses greater than 10 mg/kg and displays a hormetic dose response (15). The capsules were stored in sequentially labeled plastic containers by a separate research nurse, who used the randomization key created by the statistician; this nurse did not participate in any other aspect of the study. The study remained blinded until the analysis for the participants, research nurses, and those assessing outcomes was concluded.
Figure 1:
Schematic shows the timeline of functional MR imaging and surveys, including psychomotor vigilance task (PVT) and delayed match-to-sample (DMTS) task.
Subjects completed the positive and negative affect schedule (PANAS) and a crossword puzzle after acquisition of baseline images and while waiting to undergo postintervention imaging. The PANAS and delayed recall of the words in the crossword puzzle were retested after 7 days by way of electronic communication. The Consolidated Standards of Reporting Trials, or CONSORT, flowchart is shown in Figure E1 (online). Thirty-six subjects signed the consent form, but the following eight subjects were excluded: two had undiagnosed hypertension, one was morbidly obese, two had a history of transplantation or bowel surgery that disqualified them from the study, one was undergoing chemotherapy, and two withdrew because they were unavailable for the time required for imaging. Two subjects from the methylene blue group (15 were originally enrolled) were excluded from the final analysis because of technical problems (failure of response system in the first enrolled subject) and refusal to participate in the CO2 challenge.
Experimental Tasks and Image Acquisition
The psychomotor vigilance task and delayed match-to-sample paradigms were adapted to functional MR imaging from the standard test battery in the psychology experiment building language software (Figs 2b, 3d) (19). A detailed description of the tasks is given in Appendix E1 (online). We included feedback in the psychomotor vigilance and delayed match-to-sample tasks to keep subjects motivated to perform. During imaging, the psychomotor vigilance and delayed match-to-sample tasks were modeled with e-Prime 2.0 (Psychology Software Tools, Sharpsburg, Pa), and real-time behavioral data for the reaction times (psychomotor vigilance task) and retrieval phase response (delayed match-to-sample task) were logged. The MR imager automatically triggered the start of the psychomotor vigilance task and delayed match-to-sample task paradigms. Stimuli were presented by way of a liquid crystal display projector, which was visible to the subject by a mirror mounted to the head coil. The subject responded via a custom fiber-optic button system that interfaced with e-Prime. Resting CVR was the last task performed.
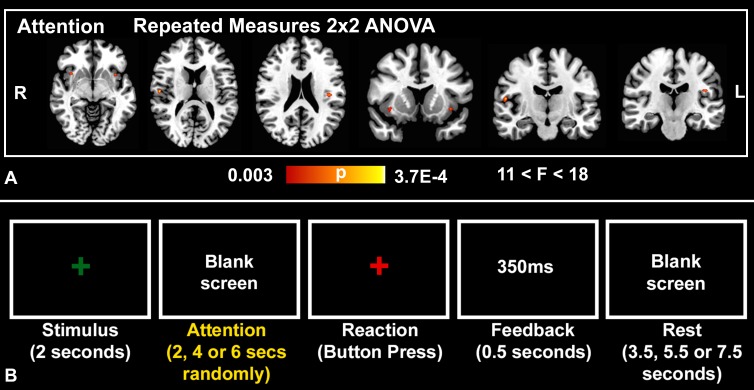
Figure 2:
Psychomotor vigilance Task. A, Repeated-measures analysis of variance (ANOVA) results of the attention phase of the psychomotor vigilance task superimposed to a standard brain template (colors indicate P values of significant voxels, cluster-based P < .05; cluster size, K ≥ 10) shows positive drug × time interactions in favor of a methylene blue effect in the bilateral anterior and posterior insular cortices (n = 26, with 24 degrees of freedom). B, Representative block from the psychomotor vigilance task shows the stimulus, attention, reaction, feedback, and rest phases as demarcated by set times.
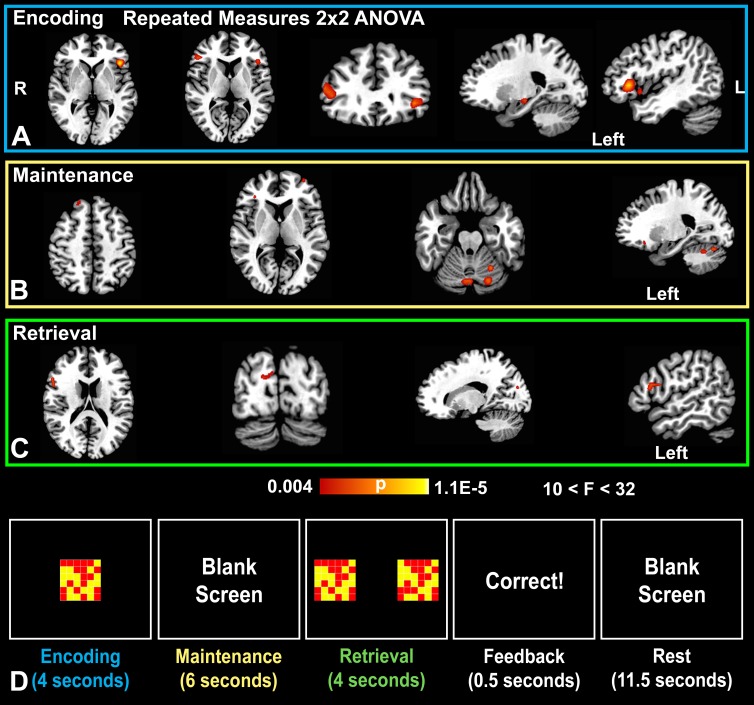
Figure 3:
Delayed match-to-sample task. Repeated-measures ANOVA results of the delayed match-to-sample task (colors indicate P values of significant voxels, cluster-based P < .05; K ≥ 10) superimposed to a standard brain template. A, Probability map overlays show positive drug × time interactions in favor of a methylene blue effect in the bilateral inferior frontal gyri during the encoding phase, B, in the right superior frontal gyrus, left middle frontal gyrus, and posterior cerebellum during the maintenance phase, and, C, in the right inferior frontal gyrus and cuneus during the retrieval phase (n = 26, with 24 degrees of freedom). D, Representative blocks from the delayed match-to-sample task show the encoding, maintenance, retrieval, feedback, and rest phases as demarcated by set times.
MR imaging was performed with a Siemens Tim Trio 3.0-T machine (Siemens Medical solutions, Erlangen, Germany) with a 12-channel head coil. The following standard three-dimensional magnetization-prepared rapid acquisition with gradient echo anatomic T1-weighted MR imaging parameters were used at baseline for registration: resolution, 1 × 1 × 1 mm; repetition time msec/echo time msec, 2200/2.8; inversion time, 766 msec; matrix, 176 × 256; 208 sections; and flip angle, 13°.
For delayed match-to-sample and psychomotor vigilance tasks, blood oxygen level–dependent (BOLD) functional MR imaging was performed with the following gradient echo-planar imaging sequence: voxel size, 1.72 × 1.72 × 4.00 mm; 2000/30; matrix, 128 × 128; field of view, 220 × 220 mm; flip angle, 90°; parallel acceleration factor, 2; and 29 sections (4-mm section thickness), with no section gap. For delayed match-to-sample and psychomotor vigilance tasks, 210 and 284 time points were acquired, respectively, and required 7 and 9.53 minutes, respectively.
For CVR studies, cerebral blood flow (CBF) was measured with a pseudo-continuous arterial-spin labeling (PCASL) sequence with the following parameters: echo-planar imaging; voxel size, 3.44 × 3.44 × 4 mm; 4500/16 msec; matrix, 64 × 64; field of view, 220 × 220 mm; flip angle, 90°; and 23 sections (4-mm section thickness), with no section gap. During the CO2 challenges, 80 tag-control pairs were acquired (6 min).
To evaluate the potential effects of methylene blue on CVR, mean CBF was measured before and after intervention by using 5% CO2 in air for 5 minutes with a nonrebreather mask at rest to evaluate the effects of methylene blue on vasodilation. The subject was moved out of the imager to place the mask only for the CO2 challenge. The paradigm was 1 minute of air and 5 minutes of 5% CO2, for a total of 6 minutes.
Image Analysis and Processing
Functional MR imaging data from psychomotor vigilance task, delayed match-to-sample task, and CVR were realigned, coregistered to the structural volume, normalized to a standard Montreal Neurological Institute template, and spatially smoothed (8-mm full-width one-half maximum) by using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/). Motion correction for head movement was applied. General linear modeling with the canonical hemodynamic response function was used to model an event in each cognitive task at the first subject level. Events were modeled with box-car functions to generate separate contrasts at the first level for the encoding, maintenance, and retrieval components of the delayed match-to-sample task; the attention component of the psychomotor vigilance task; and the hypercapnia period for the CVR by using intertrial intervals of rest or the nonhypercapnia phase as baseline.
For the CVR and mean CBF only, PCASL data for CO2 challenge were also processed with the ASL data processing toolbox (https://cfn.upenn.edu/∼zewang/ASLtbx.php) to generate absolute mean CBF maps and preprocess data for CVR analysis (15). The ASL data processing toolbox applies motion correction, realignment, and a validated CBF quantification model. The blood-brain barrier partition coefficient is 0.9, blood T1 value is 1.49 seconds, gray matter T1 value is 1.4 seconds, and labeling efficiency is 0.8. Subject-space mean CBF maps were then normalized to a standard Montreal Neurological Institute template with SPM software. CVR was calculated with the preprocessed PCASL data by using percentage signal change after 5% CO2 from air baseline. Time courses were visually inspected to ensure response to CO2 challenge with Mango version 3.4 (http://ric.uthscsa.edu/mango/). Given the expected variability to gas response among subjects, we did not separate baseline air from 5% CO2 in air when calculating the mean CBF maps.
Statistical Analysis
Student two-sample t test was applied to compare the age, education level, handedness, and pretreatment PANAS scores between the intervention and placebo groups by using P < .05 as a significance threshold. A two-sided Fisher exact test was used to compare sex distribution in groups. The difference between the placebo and methylene blue groups for the psychomotor vigilance task, delayed match-to-sample task, CVR, and mean CBF was also tested at baseline with two-sample t tests (Z > 2.3 and whole-brain significance of at least P < .05).
For second-level, random effects analysis, the first-level contrasts of the delayed match-to-sample task, psychomotor vigilance task, and mean CBF were used in a 2 × 2 repeated-measures ANOVA with drug (methylene blue versus placebo) and time point (before vs after administration of the drug) as factors to assess drug × time between-group interactions (20). The following contrast for the F-test interaction was generated at the second level: methylene blue × post intervention – methylene blue × pre intervention ≠ placebo × post intervention – placebo × pre intervention. Our ANOVA analyses took into consideration the potential increase in function that may result from repeating the task by statistically subtracting the placebo change (post intervention – pre intervention) from the change in the methylene blue group. A whole-brain uncorrected P < .005 and 10-voxel threshold (K ≥ 10) was applied. This methodology was used in numerous studies that assessed the effects of nicotine with functional MR imaging, as is reviewed in table 9.2 of the chapter by David et al (21). Then, we used an a priori hypothesis based on prior meta-analyses and task descriptions evidence to examine clusters that fell, at most, 20 mm from previously described peaks or regions (22–27). Small-volume correction was conducted with 5-mm radius spheres in these peaks, and a cluster-corrected family-wise error of P < .05 was used as a significance threshold (28). For the resting hypercapnia analysis (CVR and mean CBF), we generated regions of interest for the whole brain from nodes of the default mode network (29,30). We also included regions of interest from the delayed match-to-sample task and psychomotor vigilance task analysis. For the PCASL data set, we excluded infratentorial nodes or regions close to the tentorium that were not imaged or were very susceptible to artifacts. Equivalent Z scores that were SPM software–generated from F statistics and the directionality of the F contrasts were determined by plotting the contrast estimates with SPM software. Group-level analysis of these processed mean CBF maps were then conducted with SPM software and paired within group analyses. Absolute mean CBF percent changes for 5% CO2 inhalation were obtained as an index for CVR.
For the PCASL data only (CVR and mean CBF regions of interest), we also conducted separate within-group analyses as an exploratory approach by using paired t tests in addition to ANOVA analysis. Z statistical parametric maps were cluster corrected by using Z scores of at least 2.3 and a whole-brain significance of at least P < .05. All nonfunctional MR imaging statistical analysis was performed with SPSS 22 (IBM, Armonk, NY). Functional MR imaging statistical analysis was performed with SPM8 software.
All paradigm programming and testing, image processing, and statistical analyses were primarily performed by P.R. (with 5 years of experience); partly performed by W.Z. (with 5 years of experience); and revised and approved by F.G.L, T.Q.D., and J.L.L. (each with more than 25 years of experience). D.W.B. performed all analyses of psychologic surveys.
Results
The enrolled age range was 22–62 years, but only six subjects were older than 30 years, and they were evenly distributed between groups (see Table 1). Age, sex, length of education, PANAS scores, and handedness did not differ between the methylene blue and placebo groups (P > .05) (Table 1). All subjects who received methylene blue reported voiding blue urine after leaving the imaging center, which served as a delayed marker confirming that they received the drug. No subjects who received the placebo reported urine discoloration.
Table 1.
Characteristics of Subjects in the Placebo and Methylene Blue Groups
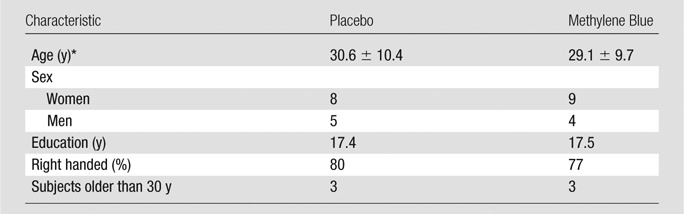
Note.—Unless otherwise indicated, data are the number of subjects.
*Mean plus or minus standard deviation.
Psychomotor Vigilance Task
Before the intervention, both the methylene blue and placebo groups had increased BOLD functional MR imaging activity in the following task-related regions: right anterior cingulate gyrus, right middle frontal gyrus, right lentiform nucleus, bilateral anterior insula, thalami, inferior parietal lobules, midbrain, occipital lingual gyri, and left pre- and postcentral gyri. There was also deactivation of the default mode network regions, including the medial prefrontal cortex, posterior cingulate cortex, precuneus, and bilateral inferior parietal cortices. Before intervention, there was no difference between the methylene blue and placebo groups.
Methylene blue was associated with increased BOLD functional MR imaging activity in the bilateral anterior and posterior insular cortices during the attention phase of the psychomotor vigilance task. Comparisons across drug and time showed that the administration of methylene blue resulted in a significant drug (placebo vs methylene blue) × time (before vs after drug administration) interaction in the bilateral anterior and posterior insular cortices (Table 2, Fig 2). Behaviorally, we found no significant drug × time interaction in reaction times in the psychomotor vigilance task between the placebo (230 msec ± 11 before and 220 msec ± 7 after drug administration) and methylene blue (230 msec ± 10 before and 230 msec ± 11 after drug administration) groups (F = 0.65, P = .43) or within-group paired differences (P = .9).
Table 2.
Brain Regions with Increased functional MR imaging Drug × Time Interaction during the Psychomotor Vigilance Task in the Methylene Blue Group Relative to Baseline and Placebo Group
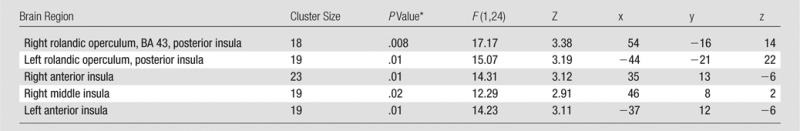
Note.—All regions fall under “attention.” n = 26, with 24 degrees of freedom. Uncorrected P < .005. Montreal Neurological Institute, K ≥ 10 (1.719 × 1.719 × 4 mm3 per voxel).
*Small-volume cluster-corrected, family wise error (P < .05).
Delayed Match-to-Sample Task
Before intervention in both the methylene blue and placebo groups, the delayed match-to-sample task showed increased BOLD functional MR imaging activation in task-related regions. In the encoding phase, this activation included the bilateral occipital lobes, basal ganglia, thalami, parietal lobules, anterior cingulate gyrus, and cerebellum. The maintenance phase resulted in similarly increased activation in the bilateral middle frontal gyri, inferior parietal lobules, right inferior frontal gyrus, left occipital lobe, and left insula. During retrieval, both groups showed increased activity in the bilateral anterior insula, thalami, occipital lobes, middle frontal gyri, parietal lobules, parahippocampal gyri, and right anterior cingulate gyrus. The encoding, maintenance, and retrieval phases also showed expected decreased activity in the medial prefrontal cortex, posterior cingulate cortex, precuneus, and bilateral inferior parietal cortices, findings indicative of task-induced down regulation of the default mode network. There were no significant differences in functional MR imaging activations between the methylene blue and placebo groups before intervention, supporting task stability and reliability. The subsequent analysis focused on posttreatment functional MR imaging differences.
Comparison across drug and time of the functional MR imaging data showed that different networks were influenced by methylene blue during the encoding, maintenance, and retrieval phases of the delayed match-to-sample task. During encoding, there was a significant drug (placebo vs methylene blue) × time (before vs after drug administration) interaction in the bilateral inferior frontal gyri, left parahippocampal gyrus/hippocampus, right superior parietal lobule, and left inferior frontal gyrus (Fig 3a, Table 3). During the maintenance phase, there was also a significant drug × time interaction in the bilateral posterior cerebella (declive and crus 1), left midbrain, left middle and inferior frontal gyri, and right superior frontal gyrus (Fig 3b, Table 3). The retrieval phase had the least number of significant drug × time interactions, but we detected small clusters in the right inferior frontal gyrus and right occipital visual cortex (cuneus) (Fig 3c, Table 3).
Table 3.
Brain Regions with Increased Functional MR imaging Drug × Time during the Delayed Match-to-Sample Task in the Methylene Blue Group Relative to Baseline and the Placebo Group
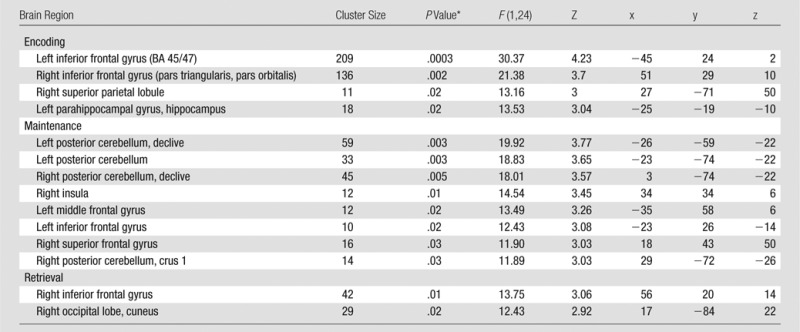
Note.—n = 26, with 24 degree of freedom. Uncorrected P < .005. Montreal Neurological Institute, K ≥ 10 (1.719 × 1.719 × 4 mm3 per voxel).
*Small-volume cluster-corrected, family wise error (P < .05).
Retrieval Behavior Response
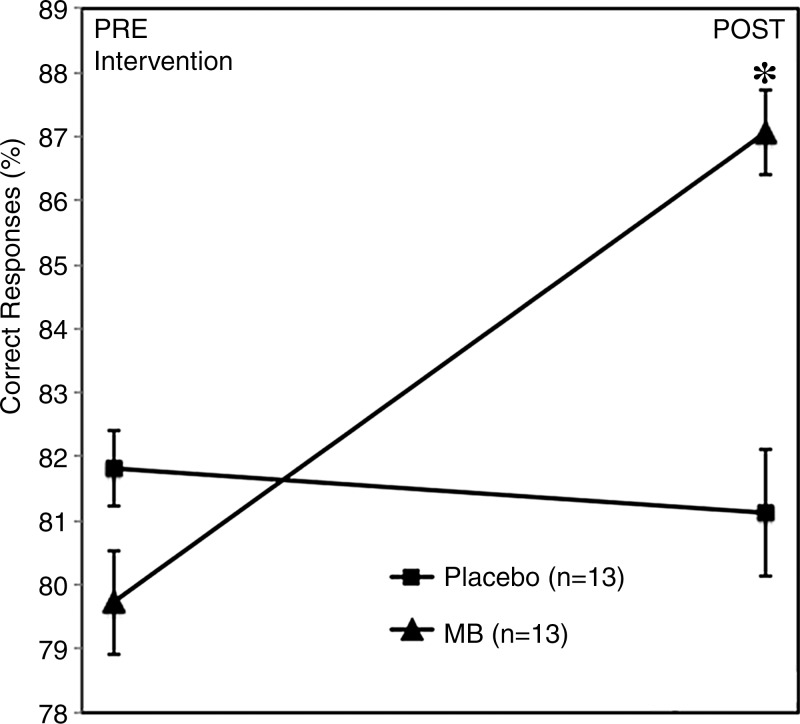
There was an approximately 7% increase in the correct number of behavioral responses after administration of methylene blue (P < .01 with two-tailed paired t test) and no change after administration of the placebo (Fig 4). Repeated-measures 2 × 2 ANOVA of the percentage of correct response × drug interaction trended but was not significant (F = 2.96, P = .09). There was no significant difference in the time subjects used to log a response (P > .05).
Figure 4:
Analysis of delayed match-to-sample task performance change (after intervention to before intervention) during the retrieval phase shows a significant increase in correct responses only in the methylene blue (MB) group (P = .01). Error bars = standard error of mean.
CVR and Mean CBF
Group analysis revealed no significant difference in CVR and absolute mean CBF between the methylene blue and placebo groups for percentage signal change before intervention. Brain-wise comparisons also showed no significant clusters for CVR and mean CBF by using the same thresholds from the delayed match-to-sample and psychomotor vigilance task analysis applied to the normalized maps. There was no significant group × time or within-group difference in CVR or mean CBF during the CO2 challenge between the methylene blue and placebo groups, including whole-brain analyses, clusters of the delayed match-to-sample and psychomotor vigilance tasks, and nodes of the default mode network. For example, with a precuneus region of interest, there was no drug × time interaction for the mean plus or minus standard deviation of the percentage signal change after 5% CO2 exposure from baseline room air for the placebo (1.4% ± 0.6 before and 1.5% ± 0.4 after drug administration) and methylene blue (1.3% ± 0.3 before and 1.4% ± 0.5 after drug administration) groups (F = 0.016, P = .9) or within groups for the methylene blue (P = .4) versus placebo (P = .7) groups. There was also no significant drug × time interaction in whole-brain mean CBF plus or minus standard deviation between the placebo (63.0 mL/100 g/min ± 14 before and 60.6 mL/100 g/min ± 14 after drug administration) and methylene blue (61.4 mL/100 g/min ± 14 before and 56.7 mL/100 g/min ± 10 after drug administration) groups (F = 0.85, P = .37) or by using within-group paired differences for the methylene blue group (P = .07) versus the placebo group (P = .12). Figure E2 (online) shows group average mean CBF maps.
Surveys
From a follow-up PANAS questionnaire administered 1 week after drug administration, there was no significant interaction between the treatment group and pre- and post-affect scores (F = 0.459, P = .505) with repeated-measures ANOVA. There was also no significant difference between the placebo (15.6 ± 0.9) and methylene blue (16.6 ± 0.8) groups (F = 0.698, P = .412) in the number of recalled words from a crossword puzzle completed immediately after administration of methylene blue or the placebo on the day of MR imaging.
Discussion
In this randomized study, low-dose methylene blue increased functional MR imaging activity during sustained attention and short-term memory tasks and potentiated memory retrieval. Specifically, the major findings showed that low-dose methylene blue increased (a) insular functional MR imaging activity during sustained attention of the psychomotor vigilance task and (b) functional MR imaging activity in the encoding, maintenance, and retrieval neural networks of the delayed match-to-sample task. These findings suggest that methylene blue can modulate certain brain networks related to sustained attention and short-term memory after a single low oral dose. At low concentrations in the brain, methylene blue acts as an electron cycler that produces regional selectivity effects in brain regions where mitochondrial respiration can accept more electrons because of increased energy demands (8,9). Accordingly, we found that methylene blue mainly produced regional functional MR imaging effects in task-related brain regions and their interacting networks.
To our knowledge, there is no previous study that suggests that low-dose methylene blue may modify sustained attention or reaction time consistent with our behavioral results. However, we found that methylene blue may still have some effect in regions of the underlying neural networks. Administration of methylene blue was associated with increased functional MR imaging activity in the bilateral anterior and posterior insular cortex during the attention phase of the psychomotor vigilance task. The insular cortex is located at the junction of the frontal, parietal, and temporal lobes and serves as a central regulatory hub that integrates motor control and sensory, autonomic, and salient stimuli, which are important for sustained attention (22,25). Multiple functional neuroimaging studies have linked attention deficits in patients with schizophrenia and bipolar disorder to abnormalities in the insular cortex (31). Our findings of an association of methylene blue intake with increased activity in the insular cortex are consistent with the hypothesis that methylene blue may modulate an important hub of active psychomotor vigilance task networks. In a previous rodent study, methylene blue potentiated stimulus-evoked functional MR imaging response in the somatosensory cortex during forepaw stimulation (8). Similarly, in our study, methylene blue potentiated stimulus-evoked functional MR imaging response in the insular cortex, which contributes to sustained attention during the psychomotor vigilance task.
During the encoding phase, there was strong activation in the bilateral inferior frontal gyri, left parahippocampal gyrus/hippocampus, right superior parietal lobule, and left inferior frontal gyrus after administration of methylene blue. Because these regions were activated during memory task encoding, this finding suggests that methylene blue potentiated frontoparietal networks that are important for encoding, such as the bilateral inferior frontal gyri and right superior parietal lobule, and limbic networks that are important for short-term memory, including the parahippocampal gyrus and hippocampus. The large clusters in bilateral inferior frontal gyri have been shown to play fundamental roles in the encoding of new memories (32,33). It is possible that improved metabolic activity within these regions as mediated by methylene blue may improve visuospatial memory retention performance, visual discrimination learning, and object recognition memory, as was previously reported in studies on rodents that were treated with methylene blue (7,11).
During the maintenance phase, there was strong activation in the bilateral posterior cerebelli, left middle and inferior frontal gyri, and right superior frontal gyrus after methylene blue administration. These clusters were previously described as being part of the visuospatial working memory network that facilitates retention of the stimulus (23,34,35). The clusters in the right superior frontal gyrus and left middle and inferior frontal gyri also correspond to areas of the dorsolateral prefrontal cortex, a key component of the working memory network (36,37).
The retrieval phase showed fewer activated regions after methylene blue administration relative to other phases of the delayed match-to-sample task. These methylene blue effects involved the right inferior frontal gyrus and the right occipital visual cortex, areas that play important roles in the visual examination of stimulus and memory retrieval (23,24). Together, these detected neuroimaging correlates of methylene blue administration may have contributed to improved visual discrimination and object recognition within the visuospatial working memory network that led to a 7% increase in behavioral performance. In short, our results suggest that, in humans, low doses of methylene blue enhance working memory processing in the brain.
Group analysis revealed no significant difference in CVR in the activated areas associated with the delayed match-to-sample and psychomotor vigilance tasks, a finding consistent with an animal functional MR imaging study that used 5% CO2 inhalation and showed that low-dose methylene blue does not affect CVR in rats (8). These findings indicate that low-dose methylene blue does not exert an observable effect on vascular reactivity in the brain. On the other hand, low-dose methylene blue increases brain oxygen consumption, as was measured with in vitro and in vivo animal models (5,8,38). Therefore, the task-based functional MR imaging signals modified by methylene blue administration may more likely be attributed to changes in tissue oxygen consumption, as would be the case if methylene blue enhanced mitochondrial respiration rather than changes in basal CBF or vascular tone (14).
There are several limitations of this study. As a proof-of-concept study, the sample size was relatively small. However, this is not unusual in multimodal functional MR imaging studies with a large number of data points. Our primary goals were to use a single low dose of methylene blue, which has been shown to improve memory in animals and humans in different memory paradigms, and investigate the neuroimaging correlates of the effects of methylene blue on neural networks (5,18). Future studies will include a larger sample and chronic methylene blue dosing. We did not measure methylene blue in the blood, but a previous study showed that methylene blue reached its maximum concentration in whole blood 1 hour after oral methylene blue administration in healthy subjects (3). It is possible that methylene blue could have age-dependent and resting-state effects; future studies will investigate these effects.
In conclusion, multimodal functional MR imaging data from this randomized, double-blinded, placebo-controlled clinical study support the hypothesis that a single low dose of methylene blue modulates functional MR imaging activity during sustained attention and working memory in the human brain. The results support the notion that methylene blue enhances memory performance and functional MR imaging activity in brain regions associated with a visuospatial short-term memory task. These findings are consistent with behavioral measurements in the same subjects. This work provides a neuroimaging foundation to pursue clinical trials of methylene blue in patients undergoing healthy aging and those with cognitive impairment, dementia, or other conditions who may benefit from drug-induced memory enhancement.
Advances in Knowledge
■ Compared with control subjects, a low dose of oral methylene blue increased functional MR imaging response in the bilateral insular cortex during a sustained attention task (Z = 2.9–3.4, P = .01–.008).
■ Compared with control subjects, oral administration of low-dose methylene blue increased functional MR imaging response during the encoding, maintenance, and retrieval components of a short-term memory task in multiple clusters in the prefrontal, parietal, and occipital cortex (Z = 2.9–4.2, P = .03–.0003).
■ Compared with control subjects, a single low dose of oral methylene blue led to a 7% increase in short-term memory retrieval (P = .01).
■ Administration of low-dose methylene blue did not alter cerebrovascular reactivity (P > .05).
Implication for Patient Care
■ The neuroimaging biomarkers of methylene blue in healthy humans give insight into the drug mechanism and serve as a foundation for future clinical trials in healthy elderly populations and populations with disease.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments
We thank Che Kelly, RN, and the staff members of the Research Imaging Institute for their support in completing this study.
Received December 30, 2015; revision requested February 17, 2016; revision received March 4; accepted March 22; final version accepted April 5.
Supported by National Center for Advancing Translational Sciences (UL1TR001120) and Julio C. Palmaz, MD, Endowment for Excellence in Radiology Research.
Disclosures of Conflicts of Interest: P.R. disclosed no relevant relationships. W.Z. disclosed no relevant relationships. D.W.B. disclosed no relevant relationships. W.A. disclosed no relevant relationships. J.E.G. disclosed no relevant relationships. J.L. disclosed no relevant relationships. J.L.L. disclosed no relevant relationships. F.G.L. disclosed no relevant relationships. T.Q.D. disclosed no relevant relationships.
Abbreviations:
- ANOVA
- analysis of variance
- BOLD
- blood oxygen level dependent
- CBF
- cerebral blood flow
- CVR
- cerebrovascular reactivity
- PANAS
- positive and negative affect schedule
- PCASL
- pseudo-continuous arterial-spin labeling
References
- 1.Clifton J, 2nd, Leikin JB. Methylene blue. Am J Ther 2003;10(4):289–291. [DOI] [PubMed] [Google Scholar]
- 2.Scheindlin S. Something old... something blue. Mol Interv 2008;8(6):268–273. [DOI] [PubMed] [Google Scholar]
- 3.Peter C, Hongwan D, Küpfer A, Lauterburg BH. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol 2000;56(3):247–250. [DOI] [PubMed] [Google Scholar]
- 4.Walter-Sack I, Rengelshausen J, Oberwittler H, et al. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur J Clin Pharmacol 2009;65(2):179–189. [DOI] [PubMed] [Google Scholar]
- 5.Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol 2012;96(1):32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol Biochem Behav 2004;77(1):175–181. [DOI] [PubMed] [Google Scholar]
- 7.Riha PD, Bruchey AK, Echevarria DJ, Gonzalez-Lima F. Memory facilitation by methylene blue: dose-dependent effect on behavior and brain oxygen consumption. Eur J Pharmacol 2005;511(2-3):151–158. [DOI] [PubMed] [Google Scholar]
- 8.Huang S, Du F, Shih YY, Shen Q, Gonzalez-Lima F, Duong TQ. Methylene blue potentiates stimulus-evoked fMRI responses and cerebral oxygen consumption during normoxia and hypoxia. Neuroimage 2013;72:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Lima F, Bruchey AK. Extinction memory improvement by the metabolic enhancer methylene blue. Learn Mem 2004;11(5):633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez JL, Jensen RA, Vasquez BJ, McGuinness T, McGaugh JL. Methylene-blue alters retention of inhibitory avoidance responses. Physiol Psychol 1978;6(3):387–390. [Google Scholar]
- 11.Wrubel KM, Riha PD, Maldonado MA, McCollum D, Gonzalez-Lima F. The brain metabolic enhancer methylene blue improves discrimination learning in rats. Pharmacol Biochem Behav 2007;86(4):712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callaway NL, Riha PD, Wrubel KM, McCollum D, Gonzalez-Lima F. Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats. Neurosci Lett 2002;332(2):83–86. [DOI] [PubMed] [Google Scholar]
- 13.Riha PD, Rojas JC, Gonzalez-Lima F. Beneficial network effects of methylene blue in an amnestic model. Neuroimage 2011;54(4):2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Lima F, Barksdale BR, Rojas JC. Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochem Pharmacol 2014;88(4):584–593. [DOI] [PubMed] [Google Scholar]
- 15.Bruchey AK, Gonzalez-Lima F. Behavioral, physiological and biochemical hormetic responses to the autoxidizable dye methylene blue. Am J Pharmacol Toxicol 2008;3(1):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochgräfe K, Sydow A, Matenia D, et al. Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau. Acta Neuropathol Commun 2015;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wischik CM, Staff RT, Wischik DJ, et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J Alzheimers Dis 2015;44(2):705–720. [DOI] [PubMed] [Google Scholar]
- 18.Telch MJ, Bruchey AK, Rosenfield D, et al. Effects of post-session administration of methylene blue on fear extinction and contextual memory in adults with claustrophobia. Am J Psychiatry 2014;171(10):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller ST, Piper BJ. The psychology experiment building language (PEBL) and PEBL test battery. J Neurosci Methods 2014;222:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gläscher J, Gitelman D. Contrast weights in flexible factorial design with multiple groups of subjects. http://www.sbirc.ed.ac.uk/cyril/download/Contrast_Weighting_Glascher_Gitelman_2008.pdf. Published March 18, 2008. Accessed December ##, 2015.
- 21.David SP, Sweet LH, Cohen RA, MacKillop J, Mulligan RC, Niaura R. Application of functional neuroimaging to examination of nicotine dependence. In: Cohen RA, Sweet LH, eds. Brain imaging in behavioral medicine and clinical neuroscience. New York, NY: Springer, 2011; 117–145. [Google Scholar]
- 22.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 2010;214(5-6):519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picchioni M, Matthiasson P, Broome M, et al. Medial temporal lobe activity at recognition increases with the duration of mnemonic delay during an object working memory task. Hum Brain Mapp 2007;28(11):1235–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedden T, Gabrieli JD. The ebb and flow of attention in the human brain. Nat Neurosci 2006;9(7):863–865. [DOI] [PubMed] [Google Scholar]
- 25.Mutschler I, Wieckhorst B, Kowalevski S, et al. Functional organization of the human anterior insular cortex. Neurosci Lett 2009;457(2):66–70. [DOI] [PubMed] [Google Scholar]
- 26.Habeck C, Rakitin BC, Moeller J, et al. An event-related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed-match-to-sample task. Brain Res Cogn Brain Res 2005;23(2-3):207–220. [DOI] [PubMed] [Google Scholar]
- 27.Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage 2010;49(4):3426–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1996;4(1):58–73. [DOI] [PubMed] [Google Scholar]
- 29.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005;102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 2015;111:611–621. [DOI] [PubMed] [Google Scholar]
- 31.Sepede G, Spano MC, Lorusso M, et al. Sustained attention in psychosis: Neuroimaging findings. World J Radiol 2014;6(6):261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhl BA, Bainbridge WA, Chun MM. Neural reactivation reveals mechanisms for updating memory. J Neurosci 2012;32(10):3453–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feurra M, Fuggetta G, Rossi S, Walsh V. The role of the left inferior frontal gyrus in episodic encoding of faces: An interference study by repetitive transcranial magnetic stimulation. Cogn Neurosci 2010;1(2):118–125. [DOI] [PubMed] [Google Scholar]
- 34.Grady CL, Protzner AB, Kovacevic N, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex 2010;20(6):1432–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma WJ, Husain M, Bays PM. Changing concepts of working memory. Nat Neurosci 2014;17(3):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mars RB, Grol MJ. Dorsolateral prefrontal cortex, working memory, and prospective coding for action. J Neurosci 2007;27(8):1801–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balconi M. Dorsolateral prefrontal cortex, working memory and episodic memory processes: insight through transcranial magnetic stimulation techniques. Neurosci Bull 2013;29(3):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin AL, Poteet E, Du F, et al. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS One 2012;7(10):e46585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.