Cardiac phosphorus spectroscopy is demonstrated to be feasible in patients at 7 T, giving higher signal-to-noise ratios and more precise quantification of the phosphocreatine to adenosine triphosphate concentration ratio than at 3 T in a group of 25 patients with dilated cardiomyopathy.
Abstract
Purpose
To test whether the increased signal-to-noise ratio of phosphorus 31 (31P) magnetic resonance (MR) spectroscopy at 7 T improves precision in cardiac metabolite quantification in patients with dilated cardiomyopathy (DCM) compared with that at 3 T.
Materials and Methods
Ethical approval was obtained, and participants provided written informe consent. In a prospective study, 31P MR spectroscopy was performed at 3 T and 7 T in 25 patients with DCM. Ten healthy matched control subjects underwent 31P MR spectroscopy at 7 T. Paired Student t tests were performed to compare results between the 3-T and 7-T studies.
Results
The phosphocreatine (PCr) signal-to-noise ratio increased 2.5 times at 7 T compared with that at 3 T. The PCr to adenosine triphosphate (ATP) concentration ratio (PCr/ATP) was similar at both field strengths (mean ± standard deviation, 1.48 ± 0.44 at 3 T vs 1.54 ± 0.39 at 7 T, P = .49), as expected. The Cramér-Rao lower bounds in PCr concentration (a measure of uncertainty in the measured ratio) were 45% lower at 7 T than at 3 T, reflecting the higher quality of 7-T 31P spectra. Patients with dilated cardioyopathy had a significantly lower PCr/ATP than did healthy control subjects at 7 T (1.54 ± 0.39 vs 1.95 ± 0.25, P = .005), which is consistent with previous findings.
Conclusion
7-T cardiac 31P MR spectroscopy is feasible in patients with DCM and gives higher signal-to-noise ratios and more precise quantification of the PCr/ATP than that at 3 T. PCr/ATP was significantly lower in patients with DCM than in control subjects at 7 T, which is consistent with previous findings at lower field strengths.
Introduction
Heart failure is a global health problem that causes widespread morbidity and mortality (1). Heart failure due to dilated cardiomyopathy (DCM) is characterized by increased ventricular volume and global impairment of systolic function (2). Phosphorus 31 (31P) magnetic resonance (MR) spectroscopy provides unique insight into cardiac energetics in vivo but is a technique with intrinsically low signal-to-noise ratio (SNR) because of low metabolite concentrations (3); a low gyromagnetic ratio, γ31P; and relatively long T1 relaxation times. These factors lead to undesirable variability in human spectra and impede single-subject comparisons (3–5). 31P MR spectroscopic studies in patients with DCM have demonstrated derangement of cardiac energetics characterized by a reduction in the phosphocreatine (PCr) to adenosine triphosphate (ATP) concentration ratio (PCr/ATP), which may be superior to the New York Heart Association functional class or left ventricular (LV) ejection fraction for prediction of mortality in patients with DCM (6,7).
Whole-body 7-T imagers capable of cardiac MR imaging recently have become available, and 31P MR spectroscopy has been shown to be feasible in healthy volunteers at 7 T (8). We hypothesize that these new systems can be used in patients with cardiac disease and that they will allow an improvement in the quality of 31P MR spectroscopy, enabling detection of small changes in metabolite concentrations or studies in small patient groups, which will further the understanding of cardiac energetics. Imaging patients with cardiac disease instead of healthy volunteers poses additional challenges such as the potential inability of the patients to tolerate the length of the examination and the physiologic monitoring in the magnet bore, and a potential reduction in the fraction of myocardium within a spectroscopic voxel due to the thinning of the ventricular walls in patients with DCM, which may challenge our ability to correct for blood contamination. This study was designed to test whether the increased 31P MR spectroscopic SNR at a field strength of 7 T improves precision in cardiac metabolite quantification in patients with DCM compared with that with imaging at 3 T.
Materials and Methods
Study Cohort
This study was approved by the Solihull ethics committee (REC Ref 13/WM/0155) and all participants gave written informed consent. Patients were eligible for inclusion if they had a clinical diagnosis of DCM and an LV ejection fraction of less than 50%, as measured with the Simpson biplane method from echocardiographic data. In total, 101 patients were considered for this study. Patients were excluded if they did not wish to participate (22 patients), if their heart was not beating in sinus rhythm (seven patients had atrial fibrillation), if they had valvular heart disease (five patients), or if they had a contraindication to MR imaging at 3 T or 7 T (21 for implanted cardiac devices or cardiac resynchronization therapy implants, 18 for metal from previous surgery, and three for tattoos). Another exclusion criterion was coronary artery disease, but none of the patients had it. The exclusion criteria for 3-T MR imaging were familiar to the clinical care team already (atrial fibrillation, valvular disease, implantable cardioverter defibrillators or cardiac resynchronization therapy devices), but given the relatively strict screening criteria for 7-T MR imaging, approximately 40% of potential patients who completed the laboratory volunteer screening form were found to have a safety contraindication to MR imaging at 7 T. The LV ejection fraction was verified from the first study sequence, and patients would have been excluded if they had had an LV ejection fraction greater than 50% according to cardiac MR imaging, but no patients did. In total, 25 patients with DCM (mean age ± standard deviation, 54 years ± 12, 68% men) were enrolled in the study, and 10 age- and sex-matched healthy control subjects (mean age, 52 years ± 12, 80% men) with no history of cardiac disease were enrolled for 31P MR spectroscopy at 7 T only.
Clinical Measurements
All patients answered the Minnesota Heart Failure questionnaire (9), which is a quality-of-life score with 21 questions used to assess heart failure symptoms over the preceding 4 weeks, with scores ranging from 0 (no effect) to 5 (great effect). Height and weight were recorded, and body mass index was calculated. Blood pressure was recorded (Dinamap-1846-SX; Critikon, Tampa, Fla). Venous blood was drawn for brain-type natriuretic peptide levels. Participants underwent a 6-minute walk test (10).
31P MR Spectroscopic Protocol
In this study, we compared best-in-class 3-T methods against our newest 7-T hardware and methods to quantify the real-world improvement. Each patient underwent 31P MR spectroscopic imaging with a 3-T imager (Trio; Siemens, Erlangen, Germany) and a heart-liver coil (5) and with a 7-T imager (Siemens) and a 16-channel coil (Rapid Biomedical, Würzberg, Germany) (11). The heart-liver coil comprises a 28 × 27–cm2 rectangular 31P transmit loop (also used for hydrogen 1 [1H] transmit and receive) and a loop/butterfly quadrature 31P receive pair (12 × 15−cm loop and 23 × 12−cm butterfly) connected through a hardware quadrature combiner to a single receive channel. The 16-channel coil comprises a rigid 26 × 28−cm2 rectangular 31P transmit element and a flexible set of 16 overlapping 4-cm diameter circular receive elements in a 4 × 4 grid.
Imaging was performed by two operators (V.S., a clinician with 3 years of experience in cardiac MR imaging and either C.T.R. or W.T.C., with 8 and 4 years of experience in 31P MR spectroscopy, respectively). The 3-T coil was chosen because, of the coils available in our laboratory, it historically has performed best in vivo (3,5,12,13), and this was confirmed with phantom imaging sequences. (Specifically, at 3 T, the heart-liver coil has a low drop-off in transmit performance throughout the heart [40% drop-off between 8-cm and 12-cm depth vs 64% for a 10-cm loop]; it has a good receive SNR at the depth of the heart [approximately 10 cm], measured as 6% better than that with an eight-element receive array in healthy volunteers [12]; and it benefits from a larger head-to-foot and left-to-right field of view compared with those of smaller loop coils, making coil placement less critical than with a 10-cm loop [8]). At 7 T, the 16-element array was chosen because phantom imaging showed that it gives more uniform transmit performance and greater SNR at the depth of the heart (approximately 10 cm), as shown in figure 2 of reference 11. Participants were imaged prone at 3 T (required for the coil) and supine at 7 T (for improved comfort). Both sequences were performed sequentially on the same day to minimize any physiologic variation. Spectroscopic sequences were not gated to avoid potential bias due to mistriggering, particularly at 7 T (14). (Although we note that recent studies in Oxford were not gated at 3 T, and not gating avoids the potential for artifacts from mistriggering, which also often occurs during a 28-min sequence in patients at 3 T). Control subjects underwent 31P MR spectroscopy as described at 7 T only, because we already have characterized the performance of the 3-T heart-liver coil extensively (3,5).
As previously described, localization was performed and subject-specific B1 maps were computed (5,8). Spectra were recorded by using a chemical-shift imaging pulse sequence (three-dimensional phase-encoded “ultrashort echo time” chemical shift imaging) with matrix, 16 × 16 × 8; voxel size, 15 × 15 × 25 mm3; acquisition weighting with 10 averages (k = 0); and repetition time, 1 second (8). At7 T, excitation was at 400 V (ie, 3.2 kW), giving a field of approximately 10 µT in the interventricular septum, and hence, a flip angle of approximately 30° there. At 3 T, flip angles were matched to those at 7 T by using the subject-specific B1 maps. Excitation was centered at −250 Hz at 3 T and at more than +266 Hz at 7 T (both relative to PCr). A 25-mm-thick saturation band suppressed the signal from the anterior chest wall. At 7 T, this was set to the maximum voltage permissible (equivalent to the maximum power permissible) within the radiofrequency heating limits for each subject.
Spectra from a voxel overlying the midventricular septum were fitted by an expert in MR spectroscopy (W.T.C.) under the guidance of another expert in MR spectroscopy (C.T.R.) by using a custom Matlab (Mathworks, Natick, Mass) implementation of the advanced method for accurate, robust, and efficient spectroscopic (AMARES) fitting (15), with prior knowledge specifying 11 Lorentzian peaks (α, β, γ-ATP, PCr, phosphodiester, and 2 × 2, 3-diphosphoglycerate), fixed amplitude ratios, and literature values for the scalar couplings for the multiplets. This set of prior knowledge has been used successfully in several previous 31P MR spectroscopic studies in Oxford, UK (5). The residual after fitting the spectrum typically showed no features above the noise level, suggesting that this is an adequate description of the spectra. We then corrected for blood contamination (16) and partial saturation (17) by using T1 values from the literature (5,8). The final PCr/ATP was taken as PCr/γ-ATP by discounting α-ATP, because it overlaps nicotinamide adenine dinucleotide phosphate (NADPH) and β-ATP because it was not fully excited at 7 T and had a phase artifact in some subjects at 3 T. Finally, the spectral SNR was determined (18), and the uncertainty in metabolite concentrations was expressed by their Cramér-Rao lower bounds (19); Cramér-Rao lower bounds give the theoretical minimum for a parameter’s 95% confidence limits.
Additional Cardiac MR Imaging Sequences
LV volume stacks were recorded for all subjects at 3 T by using a 32-channel cardiac coil to acquire steady-state free precession cine images, which were analyzed by using software (Fusing cmr42; Circle Cardiovascular Imaging, Calgary, Canada) as previously described (20). All patients had an LV ejection fraction less than 50%, which is consistent with their measurements before enrollment. To determine midventricular peak systolic circumferential strain and diastolic strain rate, myocardial tagging was performed (21,22) and analyzed by using software (CimTag2D; Auckland Medical Research, Auckland, New Zealand) (23).
Statistical Analysis
Statistical analysis was performed with software (SPSS; IBM, Chicago, Ill). Data were tested for normality by using the D’Agostino and Pearson omnibus normality tests and were presented as means ± standard deviation. Two-group comparisons for normally distributed data were analyzed by using the Welch t test, or with the paired Student t test, where appropriate, while nonnormally distributed data were analyzed with the Mann Whitney U test. Correlation was assessed with the Pearson or Spearman correlation coefficient, as appropriate. P values less than .05 were considered to indicate a significant difference.
Results
Participant Characteristics
Demographic, clinical, and imaging data are shown in Table 1. Although 72% of patients were taking β blockers and 92% were taking angiotensin converting enzyme inhibitors or angiotensin II receptor blockers, there was no significant difference in heart rate or blood pressure compared with control subjects. As expected, the mean LV ejection fraction was significantly lower in patients with DCM than in control subjects (35% ± 10 vs 65% ± 3, P < .0001), and patients with DCM had significantly increased end-diastolic volumes compared with control subjects (295 mL ± 123 vs 164 mL ± 31, P < .0001). The peak circumferential systolic strain was significantly impaired in patients compared with control subjects (−10% ± 4 vs −19% ± 2, P < .0001) as was the peak diastolic strain rate (45 sec−1 ± 22 vs 90 sec−1 ± 7, P < .0001). Patients with DCM had higher blood brain-type natriuretic peptide levels and achieved significantly shorter distances on the 6-minute walk test than did control subjects (see Table 1). Hence the patients with DCM recruited to this study had signs of significant LV dysfunction on exertion but remained clinically compensated at rest, supported by a normal resting cardiac output (5.8 L/min ± 1.4).
Table 1.
Demographic, Clinical, and Imaging Characteristics of Study Participants

Note.—Unless otherwise indicated, values are means ± standard deviation.
*Data are number of patients, with percentage in parentheses.
†For these four patients who were less than 35 years old at the time of diagnosis with no risk factors for coronary disease, a clinical decision was taken not to investigate further because the pretest probability was low.
31P MR Spectroscopic Results
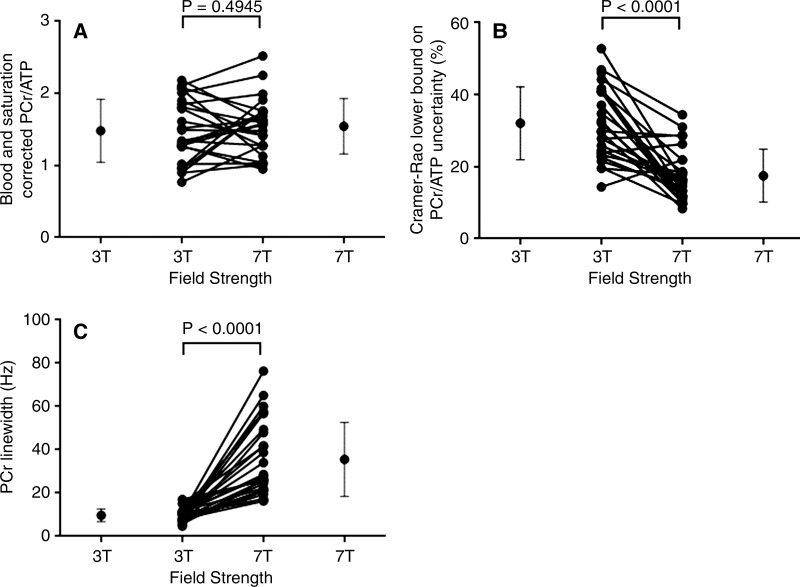
Table 2 summarizes the quantitative 31P MR spectroscopic results. As expected, there was no significant difference in the PCr/ATP at 7 T and at 3 T (1.54 ± 0.39 vs 1.48 ± 0.44, P = .49) for patients with DCM, as shown in Figure 1, A, and the 7-T PCr/ATP for the control subjects (1.95 ± 0.25) was within the accepted range (24). As demonstrated in previous lower-field-strength studies (6,7,25) the PCr/ATP was significantly lower, by 21%, in patients with DCM than in control subjects (1.54 ± 0.39 vs 1.95 ± 0.25, P = .005) at 7 T.
Table 2.
Comparison of Cardiac 31P Spectra Recorded in 25 Patients with DCM at 3 T and 7 T and 10 Healthy Control Subjects at 7 T
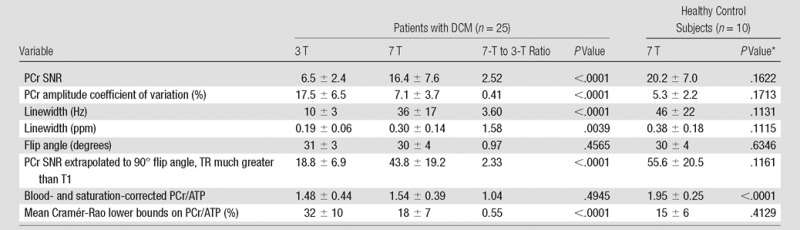
Note.—Values are means ± standard deviation, unless otherwise indicated. ppm = parts per million, TR = repetition time, T1 = longitudinal (spin-lattice) relaxation time.
*Comparison of control subjects with patients with DCM at 7 T.
Figure 1:
A, Graph shows PCR/ATP for each patient with DCM (mean age, 54 years ± 12; 68% men; further details in Table 1) at 3 T compared with that at 7 T. B, Graph shows Cramér-Rao lower bounds for each patient with DCM at 3 T compared with that at 7 T. C, Graph shows linewidth for PCr in hertz at 3 T compared with that at 7 T for each patient with DCM. Error bars at sides mark mean and standard deviation. In center, each connected pair of points shows data for one patient.
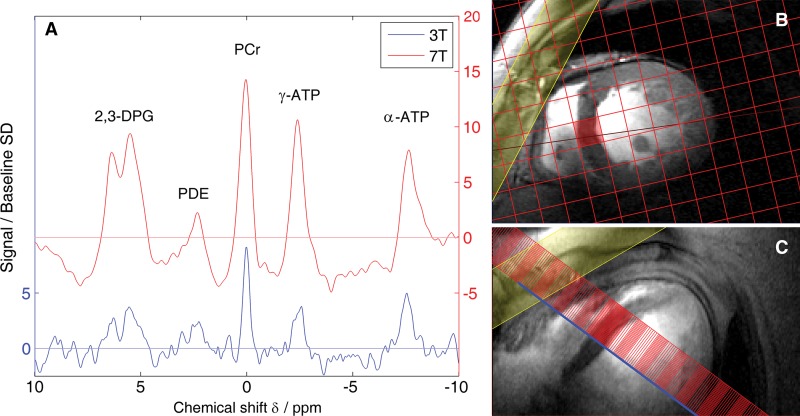
Typical spectra for a patient with DCM (Fig 2) show the increased SNR at 7 T. The SNR for PCr was 2.5 times higher at 7-T field strength than at 3 T. Cramér-Rao lower bounds were 45% lower at 7 T than at 3 T, showing that the higher quality spectra obtained at 7 T enable more precise metabolite quantification (Fig 1b). Note, however, that the mean PCr linewidth was higher at 7 T (36 Hz) than at 3 T (10 Hz). The 2.5 times higher SNR in spite of this increase in linewidth (Fig 1c) suggests that using optimized per-subject B0 shim settings may further improve the quality of cardiac 31P MR spectroscopy at 7 T.
Figure 2:
A, Graph shows comparison of spectra in a typical patient (57-year-old woman) at 3 T and 7 T. These spectra have had a matched filter applied and have been normalized to mean baseline noise, so the PCr peak height is, by definition, the PCr SNR. Increase in SNR at 7 T is readily apparent. B, Corresponding mid-short axis localizer image acquired at 7 T. C, Corresponding four-chamber localizer image acquired at 7 T. The spectrocopy matrix is overlaid in red, and the voxel plotted in A is highlighted. The yellow-shaded region denotes the regional saturation slab used to suppress signal from overlying skeletal muscle.
Correlations of LV Functional Parameters with the PCr/ATP
The 7-T PCr/ATP correlated with LV end-diastolic volume (r = −0.59, P = .0002), LV end-systolic volume (r = −0.60, P = .0001), LV ejection fraction (r = 0.51, P = .002), peak circumferential systolic strain (r = −0.44, P = .012), and peak diastolic strain rate (r = 0.38, P = .034). This suggests that, as remodeling parameters and mechanical function of the LV deteriorate, so does the myocardial energy deficit.
Discussion
All participants imaged at 3 T also successfully completed the 7-T sequence, demonstrating that cardiac 7-T MR imaging and spectroscopy is well tolerated by patients. Cardiac 31P MR spectroscopy showed a 2.5 times increase in SNR at 7 T compared with our best methods at 3 T. The PCr/ATP was similar at both field strengths, excluding any new bias at 7 T. The Cramér-Rao lower bounds (measuring uncertainty) of PCr/ATP showed a 2.2 times improvement at 7 T.
The higher SNR at 7 T can be used (a) to obtain higher SNR spectra, (b) to increase spatial resolution (eg, for investigating regional differences), or (c) to decrease the acquisition time (eg, to allow dynamic studies under more acute stress conditions than could be tolerated for a full 28-minute protocol). The increased precision (ie, decreased Cramér-Rao lower bounds) of PCr/ATP also may aid separation of subject groups, either providing greater confidence in the difference between two groups or allowing the identification of smaller between-group differences.
As found in previous studies at lower field strengths, at 7 T the PCr/ATP of patients with DCM was significantly lower than that of control subjects (6,7,25). Although we did not acquire control data at 3 T for this study, previous work by our group has shown average PCr/ATP in healthy control subjects to be 2.07 ± 0.38 (5), which would be significantly higher than the 3-T PCr/ATP of 1.48 ± 0.44 in our patients with DCM and which would be comparable to that of our 7-T control group (P = .37). Our 7-T results are also consistent with findings from lower-field 31P MR spectroscopic studies (6), showing that the PCr/ATP correlates with the LV ejection fraction. We further observed that the correlations with the PCr/ATP extended also to other markers of LV remodeling such as LV end-diastolic volume and LV end-systolic volume and more subtle earlier markers of LV dysfunction such as impaired peak systolic strain and impaired diastolic strain rates. These findings suggest that the increased precision of measuring PCr/ATP at 7 T might improve the ability of phosphorus spectroscopy to deliver biochemical insights through comparison with other important cardiac parameters in future studies.
The mean ± standard deviation of PCr/ATP in control subjects here was 1.95 ± 0.25 (7 T, control subjects, 16-element coil), which we can compare with 2.08 ± 0.33 (7 T, control subjects, 10 cm coil [8]) with 1.71 ± 0.48 (3 T, control subjects, 10 cmcoil [8]) and with 2.07 ± 0.38 (3 T, control subjects, heart-liver coil, average of three voxels [5]). The standard deviation of the PCr/ATP decreases with increased field strength and with more sophisticated radiofrequency coils. This is consistent with increased measurement precision where the measurement precision is less than the biologic variability.
However, in patients with DCM, we observed only a slight reduction in the standard deviation of the PCr/ATP (1.48 ± 0.44 at 3 T vs 1.54 ± 0.39 at 7 T, F test P = .18). This suggests that the true biologic variability of PCr/ATP in patients with DCM might be greater than that in healthy volunteers and of a magnitude sufficient to contribute substantially to the observed scatter at 7 T. Authors of other studies have reported an increase in the standard deviation of the PCr/ATP in patients with DCM (1.41 ± 0.12) compared with control subjects (1.80 ± 0.06 [26]) and in those with severe DCM (1.44 ± 0.52) compared with control subjects (1.95 ± 0.45 [7]) and of the mean PCr concentration in patients with heart failure (8.3 ± 2.6) compared with control subjects (10.1 ± 1.3 [27]). Determining whether this is a real effect will require future studies, but if it is confirmed, then 7-T 31P MR spectroscopy could offer an enhanced ability to resolve disease-related variations in PCr/ATP among patients and could allow better resolution to changes in response to therapy.
For this comparison, we chose to compare our most widely used 3-T coil (a heart-liver loop-butterfly coil with hardware quadrature combination, Siemens product for 1.5 T, retuned for 3 T) with our best available 7-T coil (a 16-element receive array used with a software combination and the whitened singular value decomposition algorithm [28]). The improvement seen here reflects real-world improvement at 7 T compared with 3 T. This is due both to the increase in field strength and to the optimized coil at 7 T. However, we note that when we previously attempted to introduce receive array coils at 3 T, we saw an increase in field of view for the coil, but with no statistically significant change in SNR in the interventricular septum (actually a 6% loss in SNR) compared with that with the use of the heart-liver coil (12). In practice, the differences in SNR between coils optimized for cardiac applications at 3 T are typically 10%, which is much smaller than the gains due to increasing field strength from 3 T to 7 T (2.5 times increase in SNR).
In this comparison, we chose to match flip angles at 30° in the interventricular septum at both 3 T and 7 T. This is slightly lower than the Ernst flip angle at both field strengths. By using T1 values from the literature (8) for 31P-containing metabolites, one can compute that this choice of flip angle accounts for approximately 8% of the PCr SNR gain at 7 T.
Limitations
Individual-subject 7-T to 3-T PCr SNR ratios ranged from 0.68 to 6.56; this likely reflects differences in the coil-to-septum distance and in the loading of the two coils at 3 T and 7 T. Other groups have made similar observations (3,29).
The increased linewidth at 7 T relative to 3 T is likely due to the increased effect of different tissue magnetic susceptibilities at the higher field strengths (eg, at the heart-lung interface); optimized per-subject B0 shimming should be able to mitigate this effect in the future. Per-subject B0 shimming requires 1H imaging throughout the chest to measure B0 maps, followed by a shim current calculation and then cardiac 31P MR spectroscopy in the same sequence. This is possible and can give an approximately 20% decrease in the PCr linewidth, but only with sophisticated hardware (30).
In comparison to our previous work using a 10-cm loop radiofrequency coil, the 28 × 30 cm2 transmit loop in the 16-element array coil (11) provided a more uniform excitation across the heart, but with a corresponding reduction in the peak B1+. This meant that we could not reliably excite β-ATP in this study, whereas it was straightforward to do so using the 10-cm loop coil. We plan to upgrade our radiofrequency hardware in future to allow a uniform and high peak B1+.
We imaged patients in the prone position at 3 T as in previous studies, but we chose to image them in the supine position at 7 T. This was for two reasons: it improved patient comfort and it facilitated swapping between the 1H loop coil for localization and the 31P array for spectroscopy. In our experience, at 3 T, imaging prone versus supine makes no difference to the quality of 31P spectra in the interventricular septum. In a CT and MR imaging study of 16 patients (31), researchers observed no change in the position of the medial aspect of the heart, which would include our target voxel in the interventricular septum, although the anterior and lateral aspects of the myocardium moved anteriorly. In any event, if the heart did move in this way, it would have caused us to underestimate the potential gain in SNR at 7 T.
At present, very few patient implants have been tested at 7 T, which excluded 40% of potential participants in this study. In order for 7-T MR imaging to be used in larger trials, or routinely in the clinic, widespread testing of common implants will be needed.
Conclusions
Cardiac phosphorus spectroscopy is demonstrated to be feasible in patients at 7 T, giving higher SNRs and more precise quantification of the PCr/ATP than at 3 T in a group of 25 patients with DCM. These 7-T cardiac 31P MR spectroscopic methods provide a powerful tool that will enable us to better understand myocardial energetics, to identify differences in diseased tissue with greater confidence, or to perform studies in smaller populations than has been possible until now. For example, this technique will enable us to assess the effects of energy-sparing drugs in patients with DCM in a forthcoming clinical study.
Advances in Knowledge
■ Cardiac 7-T MR spectroscopy is feasible and well tolerated in patients.
■ The signal-to-noise ratio (SNR) of phosphorus spectroscopy in patients with dilated cardiomyopathy was 2.5 times higher at 7-T field strength (phosphocreatine SNR = 16.4 ± 7.6 at 7 T), compared with spectroscopy at 3-T field strength (phosphocreatine SNR = 6.5 ± 2.4 at 3 T).
■ The Cramér-Rao lower bounds in the uncertainty of metabolite quantification with phosphorus spectroscopy in the human heart were 45% lower at 7-T field strength compared with those at 3-T field strength (the percentage of phosphocreatine Cramér-Rao lower bounds decreased from 17.5% ± 6.5 at 3 T to 7.1% ± 3.7 at 7 T in patients with dilated cardiomyopathy).
Implication for Patient Care
■ The use of 7-T MR imagers for cardiac phosphorus spectroscopy allows more precise quantification of cardiac metabolites, which is an important step toward monitoring the metabolic state of an individual patient’s heart over time.
Received November 27, 2015; revision requested January 11, 2016; revision received March 14, 2016; accepted March 28; final version accepted April 6.
C.T.R. supported by a Sir Henry Dale Fellowship from the Wellcome Trust and Royal Society (098436/Z/12/Z). V.M.S. supported by the British Heart Foundation (FS/12/14/29354). S.G.M. and S.N. supported by the Oxford National Institute for Health Research Biomedical Research Centre Programme. S.N. supported by the British Heart Foundation Centre of Research Excellence, Oxford. M.D.R. supported by a grant from the UK Medical Research Council (RE/13/1/30181).
Disclosures of Conflicts of Interest: V.M.S. disclosed no relevant relationships. W.T.C. disclosed no relevant relationships. E.L. disclosed no relevant relationships. A.L. disclosed no relevant relationships. S.G.M. disclosed no relevant relationships. M.D.R. disclosed no relevant relationships. S.N. disclosed no relevant relationships. C.T.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: consultancy for Acuitas Medical and Perspectum Diagnostics. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ATP
- adenosine triphosphate
- DCM
- dilated cardiomyopathy
- LV
- left ventricle
- PCr
- phosphocreatine
- PCr/ATP
- phosphocreatine to adenosine triphosphate concentration ratio
- SNR
- signal-to-noise ratio
References
- 1.Roger VL. Epidemiology of heart failure. Circ Res 2013;113(6):646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francone M. Role of cardiac magnetic resonance in the evaluation of dilated cardiomyopathy: diagnostic contribution and prognostic significance. ISRN Radiol 2014;2014:365404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyler DJ, Hudsmith LE, Clarke K, Neubauer S, Robson MD. A comparison of cardiac (31)P MRS at 1.5 and 3 T. NMR Biomed 2008;21(8):793–798. [DOI] [PubMed] [Google Scholar]
- 4.Hudsmith LE, Tyler DJ, Emmanuel Y, et al. (31)P cardiac magnetic resonance spectroscopy during leg exercise at 3 Tesla. Int J Cardiovasc Imaging 2009;25(8):819–826. [DOI] [PubMed] [Google Scholar]
- 5.Tyler DJ, Emmanuel Y, Cochlin LE, et al. Reproducibility of 31P cardiac magnetic resonance spectroscopy at 3 T. NMR Biomed 2009;22(4):405–413. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer S, Horn M, Pabst T, et al. Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur Heart J 1995;16(Suppl O):115–118. [DOI] [PubMed] [Google Scholar]
- 7.Neubauer S, Krahe T, Schindler R, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 1992;86(6):1810–1818. [DOI] [PubMed] [Google Scholar]
- 8.Rodgers CT, Clarke WT, Snyder C, Vaughan JT, Neubauer S, Robson MD. Human cardiac 31P magnetic resonance spectroscopy at 7 Tesla. Magn Reson Med 2014;72(2):304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J 1992;124(4):1017–1025. [DOI] [PubMed] [Google Scholar]
- 10.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 11.Rodgers CT, Clarke WT, Berthel D, Neubauer S, Robson MD. A 16-element receive array for human cardiac 31P magnetic resonance spectroscopy at 7T [abstr]. In: Proceedings of the Twenty-Second Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2014; 2896. [Google Scholar]
- 12.Rodgers CT, Cochlin LE, Tyler DJ, Neubauer S, Robson MD. Performance of a phased array for 31P cardiac MR spectroscopy [abstr]. In: Proceedings of the Eighteenth Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2010; 1327. [Google Scholar]
- 13.Rodgers CT, Li M, MacNaught G, Semple S. Human cardiac 31P magnetic resonance spectroscopy at 3T with a receive array: is single-loop or dual-loop RF transmission superior? J Cardiovasc Magn Reson 2015;17(Suppl 1):P248. [Google Scholar]
- 14.Frauenrath T, Hezel F, Renz W, et al. Acoustic cardiac triggering: a practical solution for synchronization and gating of cardiovascular magnetic resonance at 7 Tesla. J Cardiovasc Magn Reson 2010;12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 1997;129(1):35–43. [DOI] [PubMed] [Google Scholar]
- 16.Horn M, Kadgien M, Schnackerz K, Neubauer S. 31P-nuclear magnetic resonance spectroscopy of blood: a species comparison. J Cardiovasc Magn Reson 2000;2(2):143–149. [DOI] [PubMed] [Google Scholar]
- 17.Ernst RR, Anderson WA. Application of Fourier transform spectroscopy to magnetic resonance. Rev Sci Instrum 1966;37(1):93–102. [Google Scholar]
- 18.Ernst RR, Bodenhausen G, Wokaun A. Principles of nuclear magnetic resonance in one and two dimensions. Oxford, England: Clarendon, 1987. [Google Scholar]
- 19.Cavassila S, Deval S, Huegen C, van Ormondt D, Graveron-Demilly D. Cramér-Rao bounds: an evaluation tool for quantitation. NMR Biomed 2001;14(4):278–283. [DOI] [PubMed] [Google Scholar]
- 20.Rider OJ, Lewandowski A, Nethononda R, et al. Gender-specific differences in left ventricular remodelling in obesity: insights from cardiovascular magnetic resonance imaging. Eur Heart J 2013;34(4):292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawton JS, Cupps BP, Knutsen AK, et al. Magnetic resonance imaging detects significant sex differences in human myocardial strain. Biomed Eng Online 2011;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuber M, Spiegel MA, Fischer SE, et al. Single breath-hold slice-following CSPAMM myocardial tagging. MAGMA 1999;9(1-2):85–91. [DOI] [PubMed] [Google Scholar]
- 23.Mahmod M, Bull S, Suttie JJ, et al. Myocardial steatosis and left ventricular contractile dysfunction in patients with severe aortic stenosis. Circ Cardiovasc Imaging 2013;6(5):808–816. [DOI] [PubMed] [Google Scholar]
- 24.Bottomley PA. NMR spectroscopy of the human heart. In: Encyclopedia of magnetic resonance. Chichester, England: Wiley, 2009. [Google Scholar]
- 25.Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997;96(7):2190–2196. [DOI] [PubMed] [Google Scholar]
- 26.Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J 1991;122(3 Pt 1):795–801. [DOI] [PubMed] [Google Scholar]
- 27.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A 2005;102(3):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodgers CT, Robson MD. Receive array magnetic resonance spectroscopy: Whitened singular value decomposition (WSVD) gives optimal Bayesian solution. Magn Reson Med 2010;63(4):881–891. [DOI] [PubMed] [Google Scholar]
- 29.Hardy CJ, Bottomley PA, Rohling KW, Roemer PB. An NMR phased array for human cardiac 31P spectroscopy. Magn Reson Med 1992;28(1):54–64. [DOI] [PubMed] [Google Scholar]
- 30.DelaBarre L, Neubauer S, Robson MD, Vaughan JT, Rodgers CT. B0 shimming further improves human cardiac 31P-MRS at 7 Tesla [abstr]. In: Proceedings of the Twenty-Third Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2015; 3152. [Google Scholar]
- 31.Chino JP, Marks LB. Prone positioning causes the heart to be displaced anteriorly within the thorax: implications for breast cancer treatment. Int J Radiat Oncol Biol Phys 2008;70(3):916–920. [DOI] [PubMed] [Google Scholar]