ABSTRACT
Numerous preclinical studies have demonstrated that combination immunotherapy can significantly reduce tumor growth and improve overall survival as compared to monotherapy. Furthermore, dual CTLA-4/PD-1 checkpoint blockade recently received FDA-approval for patients with metastatic melanoma, becoming the first combination immunotherapy to garner this designation in a rapidly evolving field. Despite this progress, the majority of patients do not respond to treatment, underscoring the critical need for more effective therapies. We have been investigating the mechanisms by which combination immunotherapy with an OX40 agonist plus CTLA-4 checkpoint blockade augments effector T cell responses to elicit anti-tumor immunity. Surprisingly, this approach failed to eradicate well-established tumors, in part due to the induction of anergy in cytotoxic CD8+ T cells. Further work revealed that anergic CD8+ T cells could be rescued by combining a dendritic cell-targeted vaccine with combination immunotherapy. Taken together, these data suggest that novel combinatorial immunotherapeutic strategies incorporating a vaccination strategy may be needed to generate effective anti-tumor responses in the majority of patients with metastatic disease.
KEYWORDS: checkpoint blockade, co-stimulation, immunotherapy, T cell agonist, vaccine
Cancer immunotherapy harnesses the power of the patient's own immune system to seek out and destroy tumor cells. This modality has demonstrated potent efficacy across tumors ranging from metastatic melanoma and non-small cell lung cancer (NSCLC) to renal cancer, bladder cancer, lymphoma, breast cancer, head and neck cancer, and others.1-5 One of the primary targets of immunotherapeutic drugs are a group of negative regulatory proteins, collectively known as immune checkpoints. This family of molecules, which includes cytotoxic T-lymphocyte associated protein 4 (CTLA-4), programmed cell death 1 (PD-1), lymphocyte activating 3 (LAG-3), and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), put the brakes on the immune system, thereby limiting the generation of self-reactive T cells capable of inducing potentially damaging autoimmune pathology. Unfortunately, these same suppressive pathways are often highjacked by tumors to block the generation and/or effector function of tumor-reactive T cells.6 Checkpoint blockade with antagonist (blocking) monoclonal antibodies (mAb) such as anti-CTLA-4 (aCTLA-4; e.g., ipilimumab; tremelimumab), anti-PD-1 (aPD-1; e.g., nivolumab; pembrolizumab), or anti-PD-L1 mAb (aPD-L1; e.g., atezolizumab; avelumab) releases the “brakes” on T cells to boost anti-tumor immunity and has led to enhanced long-term survival for patients.7,8 As a result, these agents have received FDA approval for a variety of indications including melanoma, NSCLC, and renal cancer.
Another type of cancer immunotherapy is costimulatory molecule activation, typically using agonist antibodies. This type of therapy is analogous to stepping on the gas (immunologically) via the provision of T cell co-stimulation through the engagement of tumor necrosis factor receptor (TNFR) family members such as OX40 (CD134), 4-1BB (CD137), CD27, or Glucocorticoid-induced tumor necrosis factor receptor (GITR; CD357).9-12 Specifically, we have been investigating the mechanisms by which OX40 co-stimulation using an agonist anti-OX40 mAb (aOX40) generates optimal cytolytic CD8+ T cell responses.13-15 We have focused primarily on OX40 given that aOX40 therapy significantly augments T cell differentiation and cytolytic function leading to enhanced anti-tumor immunity in a variety of pre-clinical tumor models including melanoma, breast, and prostate cancer.14-16 OX40 agonists have also been translated into the clinic as a first-in-human phase I clinical trial with an agonist anti-human OX40 mAb (NCT01644968) was recently completed.17 In this study, aOX40 therapy led to the regression of at least 1 metastatic lesion in 12 of 30 patients. Anti-OX40 therapy also enhanced CD4+ and CD8+ T cell proliferation and boosted tumor-specific immunity in patients with melanoma.17 Multiple clinical trials are currently exploring the safety and efficacy of OX40 agonists alone and in combination with other treatments including radiation, chemotherapy, and other immune modulating agents.
Despite the clinical efficacy of immunotherapy the majority of patients fail to respond to treatment and die from metastatic disease, highlighting the critical need for improved immunotherapeutic regimens capable of eliciting anti-tumor immunity in a greater proportion of patients. We have been investigating the mechanisms by which combinatorial approaches can be harnessed to enhance the therapeutic efficacy of cancer immunotherapy. Specifically, we have examined how CTLA-4 blockade plus treatment with an OX40 agonist synergized to elicit T cell-mediated anti-tumor immunity. While pre-clinical studies have demonstrated that aOX40 or aCTLA-4 monotherapy exhibited limited therapeutic efficacy, work from our laboratory and others revealed that combined aOX40/aCTLA-4 or aOX40/aPD-1 therapy consistently enhanced tumor regression in multiple tumor models.18-21 We showed that dual aOX40/aCTLA-4 therapy induced potent CD8+ T cell proliferation and differentiation characterized by increased expression of effector molecules including granzyme B and IFN-γ. Surprisingly, dual therapy also elicited Th2 CD4+ T cell responses characterized by IL-4, IL-5, and IL-13 production.22 Given that Th2 cytokines are typically associated with tumor progression and metastasis, in part through M1-M2 macrophage polarization,23 we asked whether IL-4 blockade would abrogate the Th2 response and ultimately enhance tumor regression. Indeed, anti-IL-4 mAb therapy in conjunction with aOX40/aCTLA-4 prevented Th2 CD4+ polarization and further improved tumor-free survival in TRAMP-C1 tumor-bearing mice.22
Based upon these studies, we hypothesized that dual aOX40/aCTLA-4 therapy would maintain its efficacy across a broad spectrum of solid tumors since the therapy facilitates immune-mediated recognition and tumor destruction by activating existing tumor-reactive lymphocytes as opposed to pharmacological disruption of a specific molecular pathway (e.g., BRAF inhibitors in melanoma). To our surprise, dual aOX40/aCTLA-4 therapy was ineffective at controlling the growth of mammary carcinomas or other more established tumors.19 One possible reason for this is that the aberrant Th2 CD4+ T cell polarization observed following dual aOX40/aCTLA-4 therapy may promote immune dysregulation of the tumor microenvironment favoring tumor progression. We further hypothesized that IL-4 blockade would overcome this limitation. Surprisingly, IL-4 blockade had no effect on tumor growth in mammary carcinoma models (data not shown). These data led us to propose an alternative hypothesis – specifically that the defect in responsiveness might be related to an increase in tumor-mediated peripheral tolerance and subsequent induction of anergy/exhaustion in tumor-reactive CD8+ T cells (Fig. 1).
Figure 1.
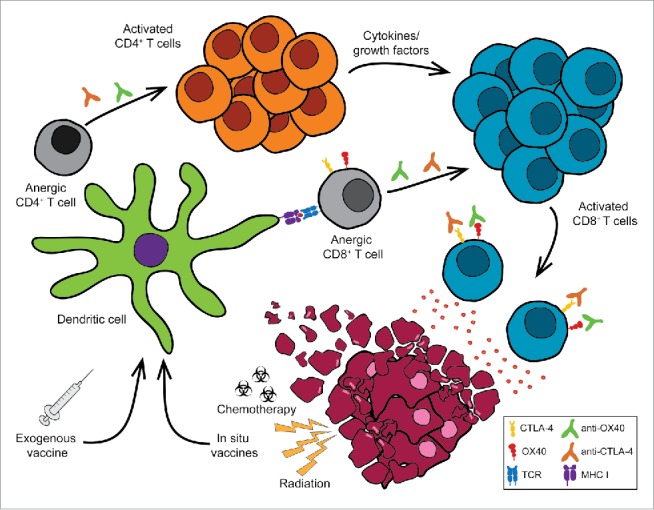
Turning the tide: restoring the function of anergic tumor-infiltrating CD8 (T) cells. Tumors can induce immune suppression through a variety of mechanisms including the induction of T cell anergy. Treatment with immunotherapy agents including T cell agonists (e.g., anti-OX40, anti-4-1BB, anti-CD27, anti-GITR mAb) and checkpoint blockade (e.g., anti-CTLA-4, anti-PD-1 mAb) enhance anti-tumor immunity leading to tumor regression. However, these combinations alone are not sufficient to restore the function of anergic cytotoxic CD8+ T cells. The addition of exogenous tumor-specific vaccines or in situ vaccination (e.g., chemotherapy, radiation therapy) plus dual anti-OX40/anti-CTLA-4 therapy can rescue anergic CD8+ T cells to promote tumor regression and significantly enhance long-term survival.
Classically, CD8+ T cell anergy is defined as the inability of a T cell to respond to T cell receptor (TCR) stimulation and may also include dysregulated cytokine secretion and/or lack of cytolytic activity. The presence of chronic antigen is one of the major contributing factors to the induction of CD8+ T cell anergy, but other factors play a role in inducing and maintaining anergy including the presence of suppressive cytokines (e.g., tumor growth factor β (TGF-β), IL-10), regulatory FoxP3+CD4+ T cells (Treg), TCR down-regulation, altered gene expression (e.g., Cbl-b, Egr-2, Egr-3), and/or epigenetic modification of the anergized CD8+ T cells.24-28 Importantly, overcoming tumor-induced anergy remains one of the key barriers that must be overcome to enhance the efficacy of tumor immunotherapy. For example, even though checkpoint blockade has been shown to stimulate tumor neoantigen-specific CD8+ T cells, if the cells have been rendered anergic, aCTLA-4 or aPD-1 may not be sufficient to restore their effector potential.
Our studies revealed that treatment with aOX40 alone or in conjunction with CTLA-4 blockade was not sufficient to restore the function of anergic CD8+ T cells in tumor-bearing hosts.19,29 Rather, the addition of exogenous tumor-associated antigen using a dendritic cell-targeted vaccine (anti-DEC-205/HER2 mAb), along with dual aOX40/aCTLA-4 therapy was required to induce CD8+ T cell proliferation and differentiation (Fig. 1).30 We hypothesize that vaccination-mediated engagement of the TCR drives OX40 expression – a process driven by TCR ligation plus common gamma chain cytokine signaling, which enables tumor-specific CD8+ T cells to respond to the agonist aOX40 mAb.31 Furthermore, aOX40 therapy sensitized CD8+ T cells to CTLA-4 blockade, as direct CTLA-4 blockade on the responding CD8+ T cells was required to provide maximal expansion and differentiation. Most importantly, we showed that combined aOX40/aCTLA-4/vaccine immunotherapy re-invigorated tumor-reactive CD8+ T cells allowing them to traffic to the tumor site and initiate tumor regression.19 The therapeutic efficacy was associated with a Th1/Tc1 cytokine profile consisting of increased IFN-γ, TNF-α, and IL-2 cytokine production and a concomitant decrease in Th2 cytokines such as IL-4, IL-5, and IL-13. We also observed a significant increase in CCL3 (MIP-1α) and CCL4 (MIP-1β) chemokine expression,19 which have been shown to enhance CD8+ T cell differentiation and dendritic cell function.32-35 Additional studies are underway to elucidate the mechanisms by which CCL3 and/or CCL4 affect CD8+ T cell expansion and effector function following combination immunotherapy.
In summary, our data supports the use of a triple-combination immunotherapy regimen consisting of checkpoint blockade, OX40 agonist therapy, and vaccination in order to generate optimal T cell priming and tumor regression (Fig. 1). Many of the agents required to translate such an approach to the clinic are already approved or in various stages of clinical development. For example, checkpoint inhibitors have generated tremendous excitement due to their clinical efficacy and several of these agents are already approved (e.g., aCTLA-4, aPD-1 mAb) for patients with advanced disease.5,36 OX40 agonists (e.g., aOX40 mAb, recombinant OX40L) are being evaluated as monotherapies and in combination with other agents (including immunotherapies) in several ongoing clinical trials.11 The third component, cancer vaccines, encompasses a wide variety of agents and modalities including peptide or protein-based vaccines, bacterial (e.g., Listeria monocytogenes) or viral (e.g., adenovirus, poxvirus) vectors, nanoparticles, and cell-based approaches.1,37-42 Our data highlights the importance of efficiently targeting tumor-associated antigens to professional antigen presenting cells and suggests that the vaccine platform should be taken into consideration when developing translational studies aimed at inducing more effective cytotoxic T cell responses. Since not all tumors express an ideal tumor-specific antigen and given the logistical challenges of creating personalized vaccines for every patient, we are actively investigating how other modalities can be harnessed to elicit tumor-specific vaccination “in situ” Alternative approaches include the use of chemotherapies capable of inducing immunogenic cell death or radiation therapy-mediated tumor cell killing thereby releasing tumor antigens capable of priming CD8+ T cell responses.43-45 However, whether these approaches release a sufficient level of tumor-associated antigen capable of synergizing with dual aOX40/aCTLA-4 therapy remains to be determined.
Disclosure of potential conflicts of interest
W.L.R. has received commercial research grants, consulting fees, and/or royalties from Bristol-Myers Squibb, Merck, Galectin Therapeutics, and Nektar Therapeutics. S. N. L has received royalties from Galectin Therapeutics.
References
- [1].Lizee G, Overwijk WW, Radvanyi L, Gao J, Sharma P, Hwu P. Harnessing the power of the immune system to target cancer. Annu Rev Med 2013; 64:71-90; PMID:23092383; http://dx.doi.org/ 10.1146/annurev-med-112311-083918 [DOI] [PubMed] [Google Scholar]
- [2].Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol 2014; 11:24-37; PMID:24247168; http://dx.doi.org/ 10.1038/nrclinonc.2013.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016; 8:328rv4; PMID:26936508; http://dx.doi.org/ 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cimino-Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncology 2015; 29:375-85; PMID:25979549 [PubMed] [Google Scholar]
- [5].Curti BD, Urba WJ. Clinical deployment of antibodies for treatment of melanoma. Mol Immunol 2015; 67:18-27; PMID:25746916; http://dx.doi.org/ 10.1016/j.molimm.2015.01.025 [DOI] [PubMed] [Google Scholar]
- [6].Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 2001; 19:225-52; PMID:11244036; http://dx.doi.org/ 10.1146/annurev.immunol.19.1.225 [DOI] [PubMed] [Google Scholar]
- [7].Melero I, Berman DM, Aznar MA, Korman AJ, Perez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer 2015; 15:457-72; PMID:26205340; http://dx.doi.org/ 10.1038/nrc3973 [DOI] [PubMed] [Google Scholar]
- [8].Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 2015; 33:1974-82; PMID:25605845; http://dx.doi.org/ 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol 2009; 9:271-85; PMID:19319144; http://dx.doi.org/ 10.1038/nri2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res 2013; 19:1044-53; PMID:23460535; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Linch SN, McNamara MJ, Redmond WL. OX40 Agonists and Combination Immunotherapy: Putting the Pedal to the Metal. Front Oncol 2015; 5:34; PMID:25763356; http://dx.doi.org/ 10.3389/fonc.2015.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sanmamed MF, Pastor F, Rodriguez A, Perez-Gracia JL, Rodriguez-Ruiz ME, Jure-Kunkel M, Melero I. Agonists of Co-stimulation in Cancer Immunotherapy Directed Against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol 2015; 42:640-55; PMID:26320067; http://dx.doi.org/ 10.1053/j.seminoncol.2015.05.014 [DOI] [PubMed] [Google Scholar]
- [13].Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol 2005; 23:23-68; PMID:15771565; http://dx.doi.org/ 10.1146/annurev.immunol.23.021704.115839 [DOI] [PubMed] [Google Scholar]
- [14].Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol 2003; 3:609-20; PMID:12974476; http://dx.doi.org/ 10.1038/nri1148 [DOI] [PubMed] [Google Scholar]
- [15].Redmond WL, Weinberg AD. Targeting OX40 and OX40L for the treatment of autoimmunity and cancer. Crit Rev Immunol 2007; 27:415-36; PMID:18197805; http://dx.doi.org/ 10.1615/CritRevImmunol.v27.i5.20 [DOI] [PubMed] [Google Scholar]
- [16].Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol 2004; 4:420-31; PMID:15173831; http://dx.doi.org/ 10.1038/nri1371 [DOI] [PubMed] [Google Scholar]
- [17].Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, Walker J, Gonzalez I, Meeuwsen T, Fox BA, et al.. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 2013; 73:7189-98; PMID:24177180; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood 2009; 113:3546-52; PMID:18941113; http://dx.doi.org/ 10.1182/blood-2008-07-170274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Linch SN, Kasiewicz MJ, McNamara MJ, Hilgart-Martiszus IF, Farhad M, Redmond WL. Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T-cell anergy and promotes survival in tumor-bearing mice. Proc Natl Acad Sci U S A 2016; 113:E319-27; PMID:26729864; http://dx.doi.org/ 10.1073/pnas.1510518113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guo Z, Wang X, Cheng D, Xia Z, Luan M, Zhang S. PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One 2014; 9:e89350; PMID:24586709; http://dx.doi.org/ 10.1371/journal.pone.0089350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Linch SN, Redmond WL. Combined OX40 ligation plus CTLA-4 blockade: More than the sum of its parts. Oncoimmunology 2014; 3:e28245; PMID:25050194; http://dx.doi.org/ 10.4161/onci.28245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res 2014; 2:142-53; PMID:24778278; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0031-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009; 16:91-102; PMID:19647220; http://dx.doi.org/ 10.1016/j.ccr.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol 2014; 35:51-60; PMID:24210163; http://dx.doi.org/ 10.1016/j.it.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science 2012; 335:723-7; PMID:22267581; http://dx.doi.org/ 10.1126/science.1214277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity 2005; 22:275-84; PMID:15780985; http://dx.doi.org/ 10.1016/j.immuni.2005.01.010 [DOI] [PubMed] [Google Scholar]
- [27].Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH, et al.. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol 2005; 6:472-80; PMID:15834410; http://dx.doi.org/ 10.1038/ni1193 [DOI] [PubMed] [Google Scholar]
- [28].Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, et al.. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 2004; 21:167-77; PMID:15308098; http://dx.doi.org/ 10.1016/j.immuni.2004.07.013 [DOI] [PubMed] [Google Scholar]
- [29].Redmond WL, Gough MJ, Weinberg AD. Ligation of the OX40 co-stimulatory receptor reverses self-Ag and tumor-induced CD8 T-cell anergy in vivo. Eur J Immunol 2009; 39:2184-94; PMID:19672905; http://dx.doi.org/ 10.1002/eji.200939348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang B, Zaidi N, He LZ, Zhang L, Kuroiwa JM, Keler T, Steinman RM. Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res 2012; 14:R39; PMID:22397502; http://dx.doi.org/ 10.1186/bcr3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Redmond WL, Triplett T, Floyd K, Weinberg AD. Dual anti-OX40/IL-2 therapy augments tumor immunotherapy via IL-2R-mediated regulation of OX40 expression. PLoS One 2012; 7:e34467; PMID:22496812; http://dx.doi.org/ 10.1371/journal.pone.0034467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Johannsen A, Genolet R, Legler DF, Luther SA, Luescher IF. Definition of key variables for the induction of optimal NY-ESO-1-specific T cells in HLA transgene mice. J Immunol 2010; 185:3445-55; PMID:20733200; http://dx.doi.org/ 10.4049/jimmunol.1001397 [DOI] [PubMed] [Google Scholar]
- [33].Gonzalez-Martin A, Gomez L, Lustgarten J, Mira E, Manes S. Maximal T cell-mediated antitumor responses rely upon CCR5 expression in both CD4(+) and CD8(+) T cells. Cancer Res 2011; 71:5455-66; PMID:21715565; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1687 [DOI] [PubMed] [Google Scholar]
- [34].Kim TK, St John LS, Wieder ED, Khalili J, Ma Q, Komanduri KV. Human late memory CD8+ T cells have a distinct cytokine signature characterized by CC chemokine production without IL-2 production. J Immunol 2009; 183:6167-74; PMID:19841187; http://dx.doi.org/ 10.4049/jimmunol.0902068 [DOI] [PubMed] [Google Scholar]
- [35].Grange M, Verdeil G, Arnoux F, Griffon A, Spicuglia S, Maurizio J, Buferne M, Schmitt-Verhulst AM, Auphan-Anezin N. Active STAT5 regulates T-bet and eomesodermin expression in CD8 T cells and imprints a T-bet-dependent Tc1 program with repressed IL-6/TGF-beta1 signaling. J Immunol 2013; 191:3712-24; PMID:24006458; http://dx.doi.org/ 10.4049/jimmunol.1300319 [DOI] [PubMed] [Google Scholar]
- [36].Wolchok JD. PD-1 Blockers. Cell 2015; 162:937; PMID:26317459; http://dx.doi.org/ 10.1016/j.cell.2015.07.045 [DOI] [PubMed] [Google Scholar]
- [37].Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J, et al.. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med 2014; 6:232ra51; PMID:24739759; http://dx.doi.org/ 10.1126/scitranslmed.3008068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ascierto ML, Melero I, Ascierto PA. Melanoma: From Incurable Beast to a Curable Bet. The Success of Immunotherapy. Front Oncol 2015; 5:152; PMID:26217587; http://dx.doi.org/ 10.3389/fonc.2015.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013; 39:38-48; PMID:23890062; http://dx.doi.org/ 10.1016/j.immuni.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Burotto M, Singh N, Heery CR, Gulley JL, Madan RA. Exploiting synergy: immune-based combinations in the treatment of prostate cancer. Front Oncol 2014; 4:351; PMID:25566495; http://dx.doi.org/ 10.3389/fonc.2014.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 2016; 16(4):219-33; PMID:26965076; http://dx.doi.org/ 10.1038/nrc.2016.16 [DOI] [PubMed] [Google Scholar]
- [42].Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW Jr. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A 2004; 101:13832-7; PMID:15365184; http://dx.doi.org/ 10.1073/pnas.0406035101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Crittenden M, Kohrt H, Levy R, Jones J, Camphausen K, Dicker A, Demaria S, Formenti S. Current clinical trials testing combinations of immunotherapy and radiation. Seminars Radiation Oncol 2015; 25:54-64; http://dx.doi.org/ 10.1016/j.semradonc.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 2015; 1:1325-32; PMID:26270858; http://dx.doi.org/ 10.1001/jamaoncol.2015.2756 [DOI] [PubMed] [Google Scholar]
- [45].Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015; 28:690-714; PMID:26678337; http://dx.doi.org/ 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]


