ABSTRACT
Objective: From December 2013 to January 2014, a large number of medias in China reported negative information about Hepatitis B vaccine (HepB) safety issues using eye-catching titles, such as “3 infants in Hunan inoculated with HepB occurred adverse event, and 2 died,” and that caused crisis of confidence in vaccination, which we called “HepB event.” The progress of “HepB event” could be divided into 3 stages which were initiation, peak and ending stages. In order to evaluate the influence of “HepB event” on the attitudes of participants toward Hepatitis B vaccine safety and their intention of vaccinating their children in different stages, and provide evidence for authority departments as soon as possible to take measures to prevent decrease of HepB coverage rate, a quick field investigation was carried out. Methods: Using convenience sampling methods during the initiation, peak and ending stages of the “HepB event.” Results: In the 3 stages of the “HepB event,” the awareness rate of the event among participants was rapidly rising, showing that the participants paid great attention to the event, and the information was spread very quickly. The proportion of participants who knew the event but thought that the Hepatitis B vaccine was unsafe were 31%, 37% and 26% respectively in 3 stages. In addition, the acceptance of vaccination by the participants was influenced, the proportion of participants who would like to delay or reject vaccinating their children was up to 43% in the peak stage of the event. Conclusions: The “HepB event” had impacted on the participants' confidence in the safety of Hepatitis B vaccine. For such event, relevant authority departments need effectively communicate with the media and the public, and promptly issue positive information and the investigation result, thereby reducing the negative impact of the event, and improve the vaccine confidence among the public.
KEYWORDS: Hepatitis B vaccine, media, vaccination, vaccination hesitance
Introduction
From December 2013 to January 2014, a large number of medias in China reported negative information about Hepatitis B vaccine (HepB) for the safety issues, using eye-catching titles, such as “3 infants in Hunan province inoculated with HepB occurred adverse event, and 2 died,” and that caused crisis of confidence in vaccination among the public, which we called “HepB event.” The progress of “HepB event” could be divided into 3 stages. The first stage was initiated on 11th December 2013, a local media in Hunan province reported that 2 children died following HepB vaccination.1 Two batches of HepB 2 produced by Kangtai Company (KTC) in China were suspended by China Food and Drug Administration (CFDA) on 13th December 2013. After that, there were much more similar events about newborn babies died after HepB vaccination reported from other provinces in China. The second stage started on 19th December 2013, the vaccines from KTC was widely suspended by CFDA and National Health and Family Planning Commission,3 then there were increasingly more concerns on the safety of HepB vaccine, and the media reports were comprehensively upgraded. The third stage started since January 03, 2014, the government announced that the vaccine passed the quality inspection, and the cases were unrelated to the HepB vaccination,4 then the negative reports were gradually quieted.
In order to evaluate the attitudes of the participants toward HepB safety and their intention of vaccinating their children, and provide evidence for authority departments as soon as possible to take actions to prevent decrease of HepB coverage rate, a quick field evaluation including 3 investigations was carried out during the 3 stages respectively (on December 19, 2013, December 29, 2013 and January 12, 2014).
Results
Awareness rate of the HepB event in participants
In total, 93, 151 and 132 participants were successfully interviewed in the 3 investigations respectively. In the first investigation, awareness rate of the “HepB event” among participants was only 18% (17/93). The awareness rate increased to 77% (117/151) in the second investigation when the HepB manufactured by KTC was completely suspended by the government and the information was widely reported by media. The awareness rate reached to the peak of 90% (119/132) during the third investigation, after the government announced that the infants' death was unrelated to the Hepatitis B vaccine. After media reported the suspension of the use of KTC vaccine, the awareness rate was 84% (97/116) among the participants aged 20–39 years, and 56% (19/34) among participants aged 40 y or above (χ2 = 12, P < 0.01). The awareness rate among participants with a college degree or above (86%, 95/110) was higher than that participants with middle school education background or below (53%, 21/40) (χ2 = 19, P < 0.01). The awareness rate among the participants with citizenships registered in Beijing (83%, 97/117) was higher than that among the participants with citizenships registered beyond Beijing (58%, 19/33) (χ2 = 9.4, P<0.01). However, the awareness rate among male participants (74%, 46/62) was not statistically different from that among female participants (80%, 69/86) (χ2 = 0.76, P > 0.05) (Fig. 1).
Figure 1.
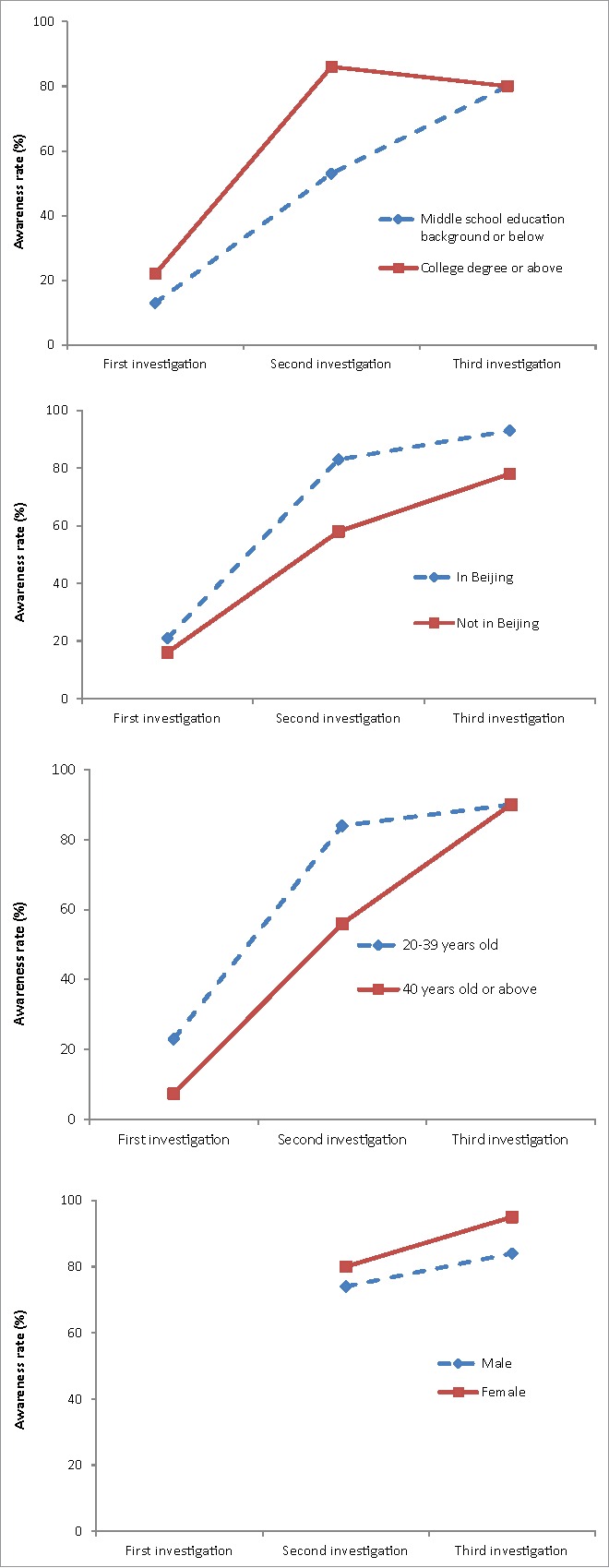
Awareness rate of the “HepB event” among different background participants from December 2013 to January 2014.
Participants' attitude and intention of vaccination
Interview with the participants knowing the event showed that the proportion of participants who were sure or suspected that the children' death was related to HepB was 65% (11/17), 77% (89/116) and 72% (86/119) during the 3 investigations respectively, there was no statistical difference between different background participants (Table 1, Table 2).
Table 1.
Different background participants' attitude and intention of vaccination during the “HepB event” from December 2013 to January 2014.
| Variable | Proportion of participants who knew the event were sure or suspected that the children' death was related to HepB(%) | Proportion of participants who believed that the HepB was unsafe (%) | Proportion of participants who knew the event and prefered to delay or reject HepB vaccination (%) |
|---|---|---|---|
| Gender | |||
| Male | 72.8(67/92) | 20.5(24/117)* | 30.4(28/92) |
| Female | 76.4(107/140) | 31.7(51/161) | 32.1(45/140) |
| Age groups(years) | |||
| 20–39 | 75.5(154/204) | 26.5(72/272)* | 32.6(62/190) |
| 40–75 | 66.0(31/47) | 15.3(13/85) | 28.9(13/45) |
| Education level | |||
| Middle school or below | 60.8(31/51)* | 9.8(10/102)** | 25.5(12/47) |
| Colledge or above | 77.5(155/200) | 29.8(76/255) | 33.5(63/188) |
| Citizenships | |||
| In Beijing | 75.8(157/207) | 27.3(72/264)* | 33.8(67/198) |
| Not in Beijing | 63.6(28/44) | 14.(13/93) | 21.6(8/37) |
| Knew “HeaB event” or not | |||
| Yes | 73.8(186/252) | 31.3(79/252)** | 31.8(75/236) |
| No | — | 6.6(7/106) | — |
| Investigation stage | |||
| First | 64.7(11/17) | 13.3(10/75)* | —# |
| Second | 76.7(89/116) | 29.1(44/151) | 42.7(50/117)** |
| Third | 72.3(86/119) | 24.2(32/132) | 21.0(25/119) |
P < 0.05,
P < 0.001, #We didn't ask participants whether or not they preferred to delay or reject HepB vaccination in the first investigation.
Table 2.
Logistic regression analysis for participants' attitude and intention of vaccination during the “HepB event” from December 2013 to January 2014.
| Variable | Parameter estimation | Standard error | Wald-Chi -Square | P | OR | 95%CI | |
|---|---|---|---|---|---|---|---|
| Participants who knew the event and were sure or suspected that the children' death was related to HepB | Gender | −0.215 | 0.316 | 0.461 | 0.497 | 0.807 | 0.434 –1.500 |
| Age groups | 0.259 | 0.401 | 0.417 | 0.518 | 1.296 | 0.590–2.844 | |
| Education level | −0.162 | 0.104 | 2.406 | 0.121 | 0.851 | 0.693–1.044 | |
| Citizenships | 0.232 | 0.429 | 0.292 | 0.589 | 1.261 | 0.544–2.926 | |
| Investigation stage | 0.256 | 0.313 | 0.668 | 0.414 | 1.291 | 0.699–2.385 | |
| Participants who believed that the HepB was unsafe | Gender | 0.557 | 0.303 | 3.382 | 0.066 | 1.745 | 0.964–3.157 |
| Age groups | 0.241 | 0.413 | 0.340 | 0.560 | 1.272 | 0.566–2.860 | |
| Education level | 0.378 | 0.130 | 8.448 | 0.004 | 1.459 | 1.131–1.882 | |
| Citizenships | 0.155 | 0.443 | 0.123 | 0.726 | 1.168 | 0.490–2.780 | |
| Investigation stage | −0.395 | 0.291 | 1.842 | 0.175 | 0.674 | 0.381–1.192 | |
| Knew event or not | −2.070 | 0.757 | 7.473 | 0.006 | 0.126 | 0.029–0.557 | |
| Proportion of participants who knew the event and prefered to delay or reject HepB vaccination | Gender | 0.133 | 0.304 | 0.191 | 0.662 | 1.142 | 0.630–2.072 |
| Age groups | 0.122 | 0.419 | 0.085 | 0.771 | 1.130 | 0.497–2.568 | |
| Education level | 0.052 | 0.114 | 0.208 | 0.648 | 1.053 | 0.843–1.316 | |
| Citizenships | −0.587 | 0.468 | 1.573 | 0.210 | 0.556 | 0.222–1.392 | |
| Investigation stage | −1.111 | 0.301 | 13.626 | 0.000 | 0.329 | 0.183–0.594 |
The proportion of participants with a college degree or above who believed the HepB unsafe (30%, 76/255) was higher than that participants with middle school education background or below (10%, 10/102) (χ2 = 16, P < 0.001). The proportion of participants who didn't know about the event and believe that the HepB unsafe (7%, 7/106) was much lower than participants who knew about the event (31%, 79/252) (χ2 = 25, P < 0.001). The proportion of participants who didn't know about the event and believe the HepB unsafe was 8% (5/59), 3% (1/34) and 8% (1/13) in the 3 investigations respectively, compared with 31% (5/16), 37% (43/117) and 26% (31/119) of participants who knew the event (Table 1, Table 2, Table 3).
Table 3.
Participants' attitude toward the HepB safety during the “HepB event” from December 2013 to January 2014.
| Proportion of participants who knew the event and believed that the HepB was unsafe (%) | Proportion of participants who didn't know the event and believed that the HepB was unsafe (%) | χ2 | P | |
|---|---|---|---|---|
| First investigation | 31.3 (5/16) | 8.5 (5/59) | – | 0.031* |
| Second investigation | 36.8 (43/117) | 2.9 (1/34) | 15 | <0.001 |
| Third investigation | 26.1 (31/119) | 7.7 (1/13) | – | 0.19* |
Fisher precise probability calculation method
In the second investigation, after the media reported the suspension on the use of the KTC vaccine, 43% (50/117) of participants preferred to delay or reject HepB vaccination, while in the third investigation, after announcing that the infant death was unrelated with the HepB, the proportion decreased to 21% (25/119) (χ2 = 13, P < 0.001) (Table 1, Table 2).
During the last stage, 63% (83/132) of participants knew that China Food and Drug Administration and National Health and Family Planning Commission announced that the infant death was unrelated to the HepB, but still as high as 78% (65/83) of participants reported that they did not believe or were not sure of the information. Among 33 participants who clearly didn't believe the information, 28 (85%) were sure or suspected that the child death was related to HepB vaccination and 64% (21/33) believed that the HepB was not safe.
Discussion
Public decision toward vaccination is not only influenced by scientific evidence alone, but also influenced by a mix of psychological, sociocultural, personal experience and values etc.5,6 For example, media release incorrect information which negative impacted vaccine acceptance,7,8 and sometimes the public may misunderstand the knowledge related vaccination reported by media, that caused parental vaccine hesitancy.9 And vaccine safety issues are focus of media reports so that ongoing monitoring of news on vaccine safety may help authority departments communicate with media and the public more effectively.10,11
In order to know the influence of “HepB event” on immunization program in China, from December 2013 to January 2014, we performed this quick evaluation on the attitudes of the participants toward the HepB safety and their intention of vaccination during the initiation, peak and ending stages of the “HepB event” reported by Chinese media. In the 3 stages of the “HepB event,” the awareness rate of participants on the event was rapidly rising, showing that the public paid great attention to the event, and that information is spread quickly. The investigation also showed that the awareness rate was high among young or highly educated participants, indicating that these groups should be the priority when conducting public education and risk communication if similar events might occur in the future. If these groups can obtain proper information on time, the negative impact of the event can be minimized.
In addition, the investigation showed that the attitude of the public did not change significantly in such a short period of time during the progress of the event. In the 3 stages of the event, the proportion of participants who were sure or suspected that the child death was related to the HepB vaccination consistently remained as high as 65%, 77% and 72% respectively. As a result, when similar events occur in the future, it is still necessary for relevant authority departments to promptly provide more positive information and event interpretation, so that avoiding vaccine hesitancy among the public. In addition, the Department of Health should continuously monitor the potential long-term impact of the “HepB event” on vaccination, stress the important role of vaccines on infectious disease control and prevention, conduct health education among the public, and improve the vaccine confidence among the public.
This investigation showed that negative information reported by media could impact vaccine confidence. Vaccine is for healthy people to prevent disease, and therefore the risk tolerance level of vaccine was far lower than that of general drugs among the public.12 For example, Xiong Changhui et al.13 found that 6.8% participants believed that vaccine was unsafe in Nanchang of Jiangxi; Alimu·Mamuti et al.14 found that 15% participants believed that vaccination was unsafe or were not sure whether the vaccine was safe in Hotan of Xinjiang; Qin Xin et al.15 found that 14% of the public believed that the H1N1 influenza vaccine was unsafe in an investigation in Beijing. Results showed that the “HepB event” had a negative impact on the confidence of the participants in the HepB safety. In this “HepB event,” the proportion of the participants believing the vaccine unsafe, was lower among who didn't know about the event than the participants who knew the event. The causes of the differences include: a. Determination of the association of an adverse event with HepB vaccination requires profound scientific knowledge and it is very difficult for the general public to understand this; b. A large number of medias used eye-catching titles, such as “3 infants in Hunan inoculated with HepB occurred adverse event, and 2 died,” “8 people inoculated HepB died” etc.,1,16 which misled the public; c. Some participants didn't trust the authorities. The results of this investigation showed that even if the government announced that the infant death was unrelated with the HepB vaccination, still 78% of participants didn't believe or were not sure about the information. Therefore, when similar events might take place in the future, it is necessary for relevant government departments to effectively communicate with the media and the public, and promptly issue positive information and conclusions, thereby reducing the negative impact of the event.
In addition, the intention of the participants to HepB vaccination was also influenced. In the peak stage of the media report, the proportion of participants who preferred to delay or reject vaccination was 43%. Timely HepB vaccination among the newborn is the most effective way to prevent HepB disease in children. Through more than 20 y of hard efforts, HepB disease among children in China is significantly reduced. The event has serious influence on vaccination, its long-term effect still needs further assessment and follow-up, and health professionals need to take actions to prevent the vaccination rate from declining.
This investigation used convenience sampling method, the advantages of this method are fast, easy, readily available, and cost effective. This method of sampling can gain a quick understanding of certain trends, but does not allow for a more complete representation of the entire population.17 This investigation quickly evaluated the influence of this event on the participants' intention of vaccination so that we could provide evidence for authority departments to make intervention decision. This investigation was continuous done at different stages of this event in the same place, so that it could dynamically reflected the whole change process of the influences of the event among the public. In the future, the method could be more frequently used for investigation among different locations and different populations for similar events.
Methods
Target population
Parents or grandparents with preschool children on the investigation site, the age of parents or grandparents ranged from 20 to 75 y old.
Investigation sites
Convenience sampling method was used in this investigation. Finally, we decided to conduct the 3 investigations in Beijing Zoo where children and participants would like to visit.
Investigation dates and methods
Quick field evaluation including 3 investigations respectively was carried out during the 3 stages of HepB event on December 19, 2013, December 29, 2013 and January 12, 2014. The investigations were completed in accordance with the pre-established questionnaire in the form of face-to-face interview.
Investigation content
The questions included general demographic information, participants' awareness of the HepB event, attitudes toward HepB safety and impact on the follow-up vaccination. Questions involved in 3 questionnaires remained relatively stable. A few questions were added in the second and third investigation questionnaire according to the result of the first investigation and the new progress, such as whether or not participants who knew the event prefer to delay or reject HepB vaccination etc.
Statistical analysis
We established a database using Statistical software of epidemiology data 3.1 (EPI Data 3.1), and the software of statistical product and service solutions (SPSS version 17.0) was used for data analysis. Chi square and logistic regression analyses were used to test qualitative data with 95% confidence interval (95%CI) calculated.
Ethical Issues
We obtained oral informed consent from all participants before the investigation, and 2 investigators interviewed one participant together. We would continue the investigation only when the participant gave a consent. We didn't collect any private information of participants during the interview, such as the name, work place, phone number etc. The investigation didn't pose any risk for the participants and their children.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully acknowledge support for the surveys provided by Lichun Guo, Zengpin Hao, Jinhua Zhao, Zhezhe Cui, Lin Xiao, Xiaolei Zhu, Xianghuan Mo.
References
- [1].Chen Y, Guan TF. Three Infants Had Adverse Effects after Hepatitis B Vaccine Inoculation, 2 died. 2013. http://pic.people.com.cn/n/2013/-1214/c1016-23840697-4.html. Accessed 20October2014 [Google Scholar]
- [2].China Food and Drug Administration Notification of Suspending the Use of Some Batches of the Vaccines Produced by a Shenzhen-based BioKangtai Company. 2013. http://www.sda.gov.cn/WS01/CL-0844/95068.html. Accessed 10August2014 [Google Scholar]
- [3].China Food and Drug Administration National Health and Family Planning Commission of the People'Republic of China. Notification of Suspending the Use of the Recombinant Hepatitis B Vaccines Produced by BioKangtai company 2013. http://www.sda.gov.cn/WS01./CL0051/95316.html. Accessed 15July2014 [Google Scholar]
- [4].China News Hepatitis B vaccine event survey result was published, and there was no problem of vaccine quality. 2014. http://www.chinanews.com/gn/2014/01-03/5694134.shtml. Accessed 14April2016 [Google Scholar]
- [5].Larson HJ, Cooper LZ, Eskola J, Katz SL, Ratzan S. Addressing the vaccine confidence gap. Lancet 2011; 378:526-35; PMID:21664679; http://dx.doi.org/ 10.1016/S0140-6736(11)60678-8 [DOI] [PubMed] [Google Scholar]
- [6].Leask J, Chapman S, Hawe P, Burgess M. What maintains parental support for vaccination when challenged by anti-vaccination messages? A qualitative study. Vaccine 2006; 24:7238-45; PMID:17052810; http://dx.doi.org/ 10.1016/j.vaccine.2006.05.010 [DOI] [PubMed] [Google Scholar]
- [7].Offit PA1, Coffin SE. Communicating science to the public: MMR vaccine and autism. Vaccine 2003; 22:1-6; PMID:14604564; http://dx.doi.org/ 10.1016/S0264-410X(03)00532-2 [DOI] [PubMed] [Google Scholar]
- [8].Chen Y. The Status of Vaccine Safety Surveillance Evaluation System. Zhejiang Prev Med 2007; 19:56-58 [Google Scholar]
- [9].Dixon G, Clarke C. The effect of falsely balanced reporting of the autism–vaccine controversy on vaccine safety perceptions and behavioral intentions. Health Education Research 2013; 28:352-359; PMID:23193194; http://dx.doi.org/ 10.1093/her/cys110 [DOI] [PubMed] [Google Scholar]
- [10].Chenoa S, Robbins C. Australian newspaper coverage of human papillomavirus vaccination, October 2006–December 2009. Journal of Health Communication 2012; 17:149-159; PMID:22136302; http://dx.doi.org/ 10.1080/10810730.2011.585700 [DOI] [PubMed] [Google Scholar]
- [11].Hussain H1, Omer SB, Manganello JA, Kromm EE, Carter TC, Kan L, Stokley S, Halsey NA, Salmon DA. Immunization Safety in US Print Media, 1995–2005. Pediatrics 2011; 127:100-106; http://dx.doi.org/ 10.1542/peds.2010-1722O [DOI] [PubMed] [Google Scholar]
- [12].Chinese Ministry of Health Guidelines for the Ethical Review of Biomedical Research Involving HumanSubjects. 2007. http://www.nhfpc.gov.cn/qjjys/s3581/200804/b9f1bfee4ab344ec892e68097296e2a8.shtml. Accessed 15February. 2016 [Google Scholar]
- [13].Xiong CH, Liao Z, Wen HR, Zhang YX, Peng SH, Chen SH, Li J, Wan GF. Investigation on attitude about measles vaccine strengthen Immunization again among children's guardian in Nanchang. Journal of Medical Pest Control 2011; 27:20-21, 25 [Google Scholar]
- [14].Alimu MMT, Zhou YQ, Sun XN. Survey on the Knowledge of Expanded Immunization Program Among Children's Caregivers in Hotan Prefecture. Chinese Journal of Vaccines and Immunization 2013; 19:62-64 [Google Scholar]
- [15].Qin X, Niu C, Huang ZL, Xu MY et al.. The Famil- iarity of Influenza A (H1N1) Perception of Vaccine Safety, Vaccination Behaviour and Their Influential Mechanism. Acta Psychologica Sinica 2011; 43:684–695 [Google Scholar]
- [16].CCTV News Following Eight People Died after Hepatitis B Vaccine Inoculation Event. 2013. http://v.ifeng.com/vblog/news/201312/5f2e56f7-86cb-4d15-973f-98a0b8253241.sht. Accessed 20October2014 [Google Scholar]
- [17].When is Convenience Sampling Used over Random?. 2016. http://www.conveniencesampling.net/Convenience-Sampling-Pros-and-Cons.html. Accessed 13April2016 [Google Scholar]


