ABSTRACT
Cancer immunotherapy has recently generated much excitement after the continuing success of the immunomodulating anti-CTLA-4 and anti-PD-1 antibodies against various types of cancers. Aside from these immunomodulating antibodies, bispecific antibodies, chimeric antigen receptor T cells, and other technologies are being actively studied. Among the various approaches to cancer immunotherapy, 2 bispecific antibodies are currently approved for patient care. Many more bispecific antibodies are now in various phases of clinical development and will become the next generation of antibody-based therapies. Further understanding of immunology and advances in protein engineering will help to generate a greater variety of bispecific antibodies to fight cancer. Here, we focus on bispecific antibodies that recruit immune cells to engage and kill tumor cells.
KEYWORDS: bispecific antibody, cancer, heterodimerization, immunotherapy, NK cells, scFv, single domain antibody, T cells
Introduction
Cancer is the second leading cause of mortality in developed countries, and the third leading cause of death in developing countries. After several decades of intensive research and development, the survival rate of cancer patients has been dramatically improved for most cancers. For example, chronic myeloid leukemia (CML), which used to be a fatal disease caused by the fusion oncogene BCR-ABL, is now a manageable condition due to the introduction of tyrosine kinase inhibitors.1 For many other cancers, however, there is still a lack of effective treatments, especially treatments that can result in long-term cancer-free survival.
Cancer immunotherapy was proposed decades ago but has only recently been realized as a promising approach to revolutionize cancer treatment. Cancer immunotherapy, which harnesses the body's immune system to fight cancer, was named “2013's Breakthrough of the Year” by Science.2 The promise of cancer immunotherapy comes from the success of immune checkpoint antibodies, such as the anti-CTLA-4 monoclonal antibody (ipilimumab) and 2 anti-PD-1 monoclonal antibodies (nivolumab and pembrolizumab), which are efficacious against a variety of advanced solid tumors including melanoma, non-small cell lung carcinoma, and renal carcinoma.3 These studies showed that modulation of the immune system is a viable way to combat cancer. In contrast to traditional chemotherapy and targeted therapy, which mainly focus on cancer cells, cancer immunotherapy helps activate the immune system in patients to recognize and fight cancer cells.4
In addition to immunomodulating antibodies, bispecific antibodies are another promising strategy to combat cancer by directly engaging immune cells in the fight against tumor cells. A bispecific antibody is based on a conventional monoclonal antibody. However, while a conventional antibody only binds one specific target, a bispecific antibody can recognize and bind 2 different antigens simultaneously. In light of this advantage, bispecific antibodies can be designed to inhibit 2 cell surface receptors or ligands. This effect, admittedly, can also be achieved with a monoclonal antibody strategy by combining 2 or even multiple antibodies together; however, a bispecific strategy offers an opportunity to reduce cost in terms of development, production clinical trials, and regulatory reviews, compared to the single antibody-based agents developed in combination therapies.5,6 Another advantage of a bispecific antibody is that it can redirect immune effector cells to the proximity of tumor cells, which is not achievable with a combination monoclonal antibody strategy. Currently, 2 bispecific antibodies, catumaxomab (anti-EpCAM and anti-CD3) and blinatumomab (anti-CD19 and anti-CD3), have been approved for patient care and have made a substantial impact on both research and the development of biologics.7-9
Many more diverse formats for bispecific antibodies are now in transit from the bench to bedside (Table 1). This review is focused on bispecific antibodies that recruit immune cells for cancer therapy, which was first demonstrated in vitro 30 y ago.10 Most of the bispecific antibodies are specific to CD3 to recruit T cells to tumor cells, which in turn are targeted by a variety of tumor antigen-specific antibodies. The recruited T cells then exert potent cytotoxicity toward the tumor cells. In addition to T cells, natural killer (NK) cells and dendritic cells (DCs) have also been targeted by bispecific antibodies (Fig. 1).
Table 1.
Bispecific antibodies in clinical development.
| Name | Format | MW(kDa) | Targets | Immune cells engaged | Indication | Development status |
|---|---|---|---|---|---|---|
| Catumaxomab | TrioMab | 150 | EPCAM + CD3 | T cell | Malignant ascites | Approved |
| Ovary cancer | Phase II | |||||
| Gastric cancer | Phase II | |||||
| Epithelial cancer | Phase I | |||||
| Lymphomum (FBTA05) | TrioMab | 150 | CD20 + CD3 | T cell | BCL | Phase I |
| Ertumaxomab | TrioMab | 150 | HER2 + CD3 | T cell | Metastatic breast cancer | Phase I |
| Blinatumomab (AMG 103) | BiTE | 50 | CD19 + CD3 | T cell | B cell ALL | approved |
| ALL relapsed refractory | Phase II | |||||
| DLBCL | Phase II | |||||
| NHL | Phase I | |||||
| Solitomab (AMG 110) | BiTE | 50 | EPCAM + CD3 | T cell | Colorectal cancer | Phase I |
| Lung and gastrointestinal cancer | Phase I | |||||
| AMG 211 (MEDI-565) | BiTE | 50 | CEA + CD3 | T cell | Gastrointestinal cancers | Phase I |
| MT 112 (BAY2010112) | BiTE | 50 | PSMA + CD3 | T cell | Prostate cancer | Phase I |
| MGD006 | DART | 50 | CD123 + CD3 | T cell | AML | Phase I |
| MGD007 | DART + Fc | 100 | gpA33 + CD3 | T cell | Colorectal cancer | Phase I |
| AFM11 | TandAb | 100 | CD19 + CD3 | T cell | NonHodgkin's lymphoma | Phase I |
| AFM13 | TandAb | 100 | CD30 + CD16 | NK cell | Hodgkin's lymphoma | Phase I |
| IMCgp100 | ImmTAC | 75 | Gp100 + TCR | T cell | Malignant melanoma | Phase II |
| melanoma | Phase I | |||||
| rM28 | Tandem scFv | 50 | MAPG + CD28 | T cell | Metastatic melanoma | Phase II |
Figure 1.
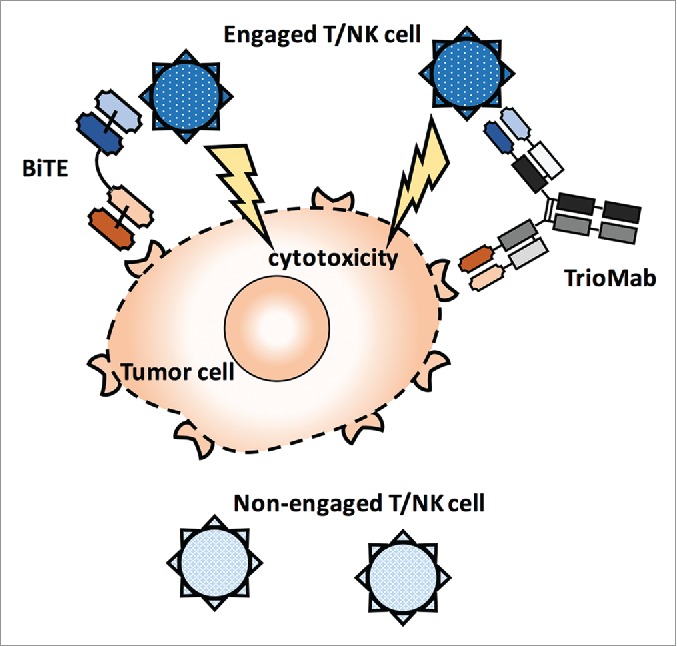
Mechanisms of action of bispecific antibodies. BiTE and TrioMab are shown here to demonstrate the tumor cell killing induced by bispecific antibodies. T cells or NK cells are recruited to the proximity of tumor cells by bispecific antibodies. Engaged T or NK cells will then attack tumor cells and lead to cytotoxicity, while non-engaged T cells or NK cells remain inactive toward the tumor cells.
Strategies to generate a bispecific antibody
Bispecific antibodies can be generated in multiple ways. Chemical conjugation of 2 different purified monoclonal antibodies was used to make bispecific antibodies by oxidative recombination more than 50 y ago11 and yielded 2 bispecific antibodies that are currently in clinical development.12,13 More recently, genetic engineering has been used with increasing frequency to create various types of bispecific antibodies. During the past 2 decades, more than 50 formats have been proposed, and some are in different clinical trials phases for the treatment of cancer or autoimmune diseases.4-6,14,15 Genetic engineering also allows for greater flexibility in the design of a bispecific antibody in terms of size, valence, specificity, half-life, and biodistribution.16 These bispecific antibodies represent many formats or technologies, including TrioMab,17-19 bispecific T-cell engager (BiTE),15,20 tandem antibodies (TandAbs),21 immune-cell-mobilizing monoclonal TCRs against cancer (ImmTACs),22,23 dual-action Fab (DAF),24 IgG single-chain Fv fragments (scFv),25 CrossMab,26 “dock and lock” (DNL) antibodies,27 dual variable domain IgG (DVD-Ig),28-30 and nanobodies.31
On the basis of format, bispecific antibodies can be subdivided into 2 groups: IgG-like or bispecific fragment molecules (Fig. 2). IgG-like bispecific antibodies retain the structure of an IgG molecule with a functional Fc region. The Fc region facilitates purification and improves solubility and stability. Furthermore, the Fc region can induce antibody-dependent cellular cytotoxicity or complement-dependent cytotoxicity and can increase the serum half-life due to the large molecular size and FcRn-mediated recycling mechanism.32 These features can be useful for certain therapeutic applications. In contrast, bispecific fragment molecules lacking Fc regions rely solely on their antigen-binding capacity for execution of their therapeutic activities. The smaller size of bispecific fragment molecules, however, can potentially enable better tumor tissue penetration and ensure stronger therapeutic effects.
Figure 2.
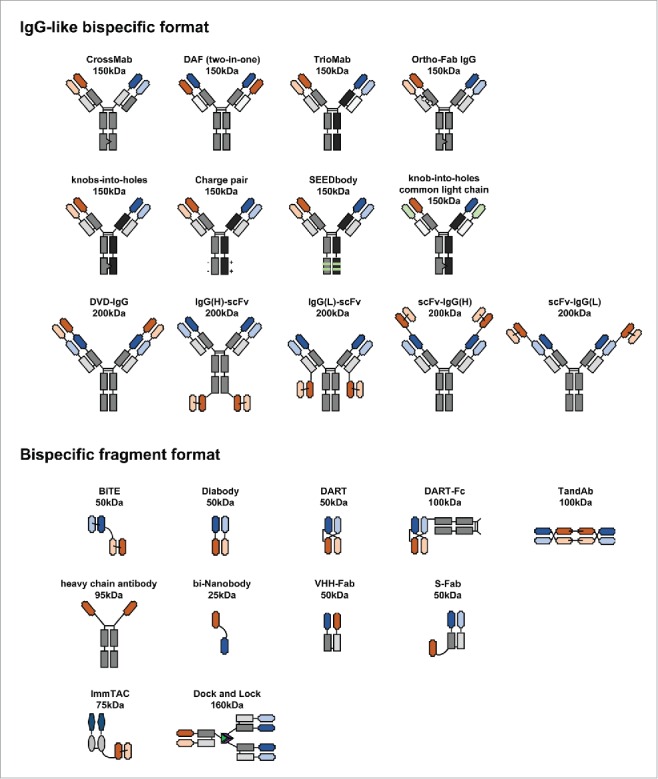
Diverse formats of bispecific antibodies. Heavy chains are shown in dark shades of black, gray, orange or green, while corresponding light chains are in lighter shades of the same colors. Peptide linkers are shown in thin black lines and engineered disulfide bonds by thin green lines. Heavy chain mutations are shown with dark gray triangles. Approximate molecular weights are estimated assuming ∼12.5 kDa per immunoglobulin domains.
The IgG-like bispecific format
This is a commonly used format of bispecific antibody. A bispecific antibody of the hybrid IgG format is monovalent for each antigen. Nevertheless, bispecificity can be achieved via fusion of either the amino or carboxy termini of either light or heavy chains with additional antigen-binding units (Fig. 2). The first approved bispecific antibody is catumaxomab (in 2009), which was developed for the treatment of malignant ascites in patients with EpCAM-positive tumors.7,33 It is currently also being tested in phase I clinical trials against gastric cancer and phase I/II trials against epithelial cancer. Catumaxomab is produced by the fusion of a mouse hybridoma and rat hybridoma; this procedure results in a hybrid antibody of a rat IgG2b and murine IgG2a with anti-CD3 and anti-EpCAM binding capacity, respectively.17 The anti-EpCAM Fab fragment of catumaxomab can bind to tumor cells that express EpCAM, and anti-CD3 Fab of catumaxomab can recruit T lymphocytes to tumor cells. The Fc region of catumaxomab can bind to and activate Fcγ receptor-positive accessory cells, such as monocytes, macrophages, DCs, and NK cells.34 Catumaxomab shows potent cytotoxicity toward ovarian carcinoma cells, preventing or reducing the accumulation of ascites.7,18,33 Because catumaxomab is a mouse-rat hybrid IgG molecule, human anti-mouse or anti-rat antibody responses are observed in most patients.6
Several other bispecific antibodies have been created using a similar quadroma technology. For example, FTBA0535 (which was designed to redirect T lymphocytes to B-cell lymphoma cells by targeting CD20) and ertumaxomab36 (which was designed against tumor cells expressing HER2) are now in different phases of clinical development for patients with relapsed or refractory B-cell lymphoma or metastatic breast cancer, respectively.37
The manufacture of hybrid bispecific antibodies is a serious challenge because typical antibody populations that are secreted are heterogeneous. Additionally, light chains can mispair with a noncognate heavy chain. As a consequence, the desired bispecific antibody can be coexpressed with up to 9 unwanted, mispaired species.38,39 In addition to the problems with the pairing of heavy chains and light chains, human anti-mouse antibody (HAMA) responses are another potential problem with this bispecific format.16
Homodimerization of the 2 heavy chains of IgG is mediated by the interaction between CH3 domains. To overcome the problem of unwanted heavy-chain paring, the “knobs-into-holes” strategy was developed.40,41 The knobs-into-holes strategy utilizes a “knob” mutation (T366W) and pairing “hole” mutations (T336S, L368A, and Y407V) in the CH3 domains. The mutated CH3 domains favor heterodimerization of heavy chains over homodimer formation.40,41 On the basis of the knobs-into-holes method, a variety of other strategies to increase heavy chain heterodimerization were proposed, such as rational design of electrostatic steering mutations,42,43 the use of alternative mutations,44 and hybrid CH3 domains derived from IgG and IgA.45 Although most bispecific antibodies are based on human IgG1 to generate IgG-like bispecific antibodies, some are based on other immunoglobulin isotypes including IgG2 and IgG4.43,46
In addition to heavy-chain pairing, another issue with bispecific antibodies in the IgG format is the problem with light-chain pairing. One way to circumvent this problem is to use a common light chain for both antibodies, which can be obtained by screening phage display libraries.47 More recently, antibodies with a common light chain have been derived from transgenic mice with a single light chain.48 Another way to overcome the problem with light-chain pairing is to express and purify the knob- or hole-containing half-antibodies separately in 2 types of host cells, followed by in vitro assembly into full bispecific antibodies.43,49,50 A major advantage of the in vitro assembly strategy is its wide applicability to pre-existing antibodies, thereby reducing research and development costs. Additionally, the 2 different light chains usually enhance antigen-binding affinity and specificity of the resulting antibody.48,51
Another solution to the issues with heavy-chain and light-chain pairing is to fuse a second antigen-binding unit to the N or C terminus of either the heavy or light chains of the first parental monoclonal antibody to achieve both multivalence and bispecificity. In this case, antigen-binding units can be either single-chain Fc fragments (scFv) or single-domain antibodies (VL or VH).51,52 A higher specific binding capacity can be attained due to the simultaneous binding to antigens with all variable domains.30 DVD-IgGs are generated using this strategy. To create a DVD-IgG, the variable heavy chain domain (VH) and variable light chain domain (VL) from one parental monoclonal antibody are fused to the VH and VL, respectively, of another parental monoclonal antibody.28,30 DVD-IgGs are bispecific and bivalent toward each antigen, with the potential of an extended range of valence and specificity.
Another efficient technical solution that simultaneously resolves the problem with light- and heavy-chain pairing in one host cell is the CrossMab method,26 where correct pairings of the light chains are achieved via domain crossover with a heterodimerized heavy chain using the knobs-into-holes strategy.47 The CH1 domain of one heavy chain is exchanged with the constant domain of the corresponding light chain (CL). For the domain crossovers, either the variable domains or the constant domains are swapped between light and heavy chains to create 2 asymmetric Fab arms.53
More recently, a combination of computational design and X-ray crystallography was used to introduce mutations into both the CH1-CL and VH-VL interface of the Fab fragments, resulting in orthogonal Fab interfaces, thus enforcing correct heavy chain–light chain pairing.54 This design was used in combination with a heavy-chain heterodimerization strategy to facilitate efficient IgG production in a single host cell.54
Another alternative solution to the chain pairing problems is to use a single heavy or light chain and to engineer the variable domains to recognize 2 unrelated antigens.51 The two-in-one or DAF platform takes advantage of the differential yet overlapping complementarity-determining regions (CDRs) as main contacts for each antigen.24,55,56 The tetraspecific antibody FL518 combines 2 different DAF antibodies in CrossMab format and binds to HER2, VEGF, EGFR, and HER3 simultaneously,57 showing in vivo antitumor activity superior to that of the 2 parental DAF antibodies.57
The bispecific-fragment format
Bispecific antibodies can be constructed without some or all of the constant domains of an antibody. The smaller size of such antibodies offers a possible advantage for better tumor tissue penetration over IgG-like format antibodies. Yet, the smaller size also shortens the serum half-life. A rapidly growing repertoire of bispecific fragment formats has been proposed and studied (Fig. 2). One common approach is to fuse 2 different scFv molecules in either the VH-VL or VL-VH orientation, with a short linker (1–10 amino acid residues) between the 2 scFvs51 (although sometimes a certain orientation inhibits antigen binding58). Recombinant DNA technology allows for construction of a bispecific antibody in a variety of formats with great flexibility. A number of formats have been proposed, including BiTE,15,20 TandAbs,21 Diabody,59 dual-affinity-retargeting format (DART),60 and ImmTacs.22,23
A BiTE antibody is a 55-kDa recombinant protein composed of 2 scFvs, linked via a glycine-serine 5-amino-acid nonimmunogenic linker.61,62 BiTE was one of the first proposed formats of bispecific antibodies and is currently the most advanced in terms of clinical development. There are 2 scFvs; one is designed to bind to CD3 on T cells and the other to a tumor-associated antigen on tumor cells in a tandem scFv format,63 which can redirect T cells to kill tumor cells directly. The BiTE strategy can be expanded via fusion of a third scFv fragment, resulting in a trivalent and/or trispecific antibody.64,65 BiTEs are produced as recombinant proteins in CHO cells.61 Although BiTEs have a short half-life in serum (because they lack the Fc region and have a relatively small molecular size), they are effective and can exert specific tumor cell cytotoxicity at picomolar concentrations in vitro.66 BiTEs can induce antigen-dependent polyclonal T-cell activation, potent T-cell mediated cytotoxicity, and T-cell proliferation,61,62,67 which are likely related to the efficient activation of the T-cell receptor (TCR) complex.62
The BiTE antibody blinatumomab (AMG 103, MT103) is approved by the US Food and Drug Administration for treatment of patients with Philadelphia chromosome-negative precursor B-cell acute lymphoblastic leukemia (B-ALL).8,9 Blinatumomab is composed of 2 scFvs, one targeting CD19 and one against CD3, with equilibrium dissociation constants (KD) of 10−9 and 10−7 M, respectively.66 After engagement of T cells via the anti-CD3 domain, blinatumomab creates a structural immune synapse with CD19+ cells,68 leading to T-cell activation and cytotoxicity toward CD19+ cells.69,70 Both CD4+ and CD8+ T cells can be induced to proliferate and engage in cytotoxicity without previous stimulation.71 Perforin and granzyme expression is increased after engagement with the complex, which leads to cell lysis of the CD19+ cells.71 Blinatumomab has been shown to be effective, as patients with non-Hodgkin's lymphoma who receive blinatumomab as a single agent show apoptosis of CD19+ cells70 at a much lower dose compared with the anti-CD20 mAb rituximab.72 However, blinatumomab requires continuous intravenous infusion for several weeks by means of a portable pump to ensure continuous activation of T cells against target cells due to the rapid clearance of blinatumomab. Other BiTEs that are in various stages of development, targeting tumor-associated antigens such as EGFR, EpCAM, fibroblast-activating protein α, prostate stem cell antigen (PSCA), HER2, carcinoembryonic antigen (CEA), ephrin A2 (EphA2), MET, and CD33.6,62
A similar approach, called diabody, involves coexpression of 2 different scFv fragments by means of a short peptide linker that allows for the heterodimerization of the 2 complementary fragments.59 Mutations can be introduced into the VL–VH interface to favor heterodimerization over homodimerization to improve the yield of the desired format.73,74 The introduction of an engineered disulfide bond into a diabody can improve stability. The same approach to improve stability is used in the DART format60 as well as a dimeric tetravalent tandem diabody called “tandAb,” which is produced from 2 pairs of VH and VL domains connected via a polypeptide linker.75,76
DARTs are diabody-like molecules where the VH region of the first antibody is linked to the VL of the second antibody, and the VH of the second antibody is linked to the VL of the first antibody.6 As noted above, DARTs are further stabilized via introduction of disulfide bonds.77 A CD19-CD3 DART (MG011), in comparison with a bispecific antibody of identical specificity and similar structure (blinatumomab), was found to be significantly more potent in redirected CD19+ cell killing assays in vitro.77 As with blinatumomab, no activation of T cells by the DART was observed in the absence of engagement with targeted CD19+ cells. The greater affinity and higher association rate for CD19 and CD3 of a DART, as well as the fixed orientation of the 2 binding domains, may contribute to the superior clinical performance of MGD011.78 The fixed orientation of the 2 binding domains may also increase the number of productive engagements between the effector and target cells.78
MGD006, a DART that binds to cell surface protein CD123 and CD3 simultaneously,79 showed potent activity in preclinical evaluation toward myeloid leukemia79 and is now in a phase I clinical trial for patients with relapsed or refractory acute myeloid leukemia.6 Another DART, MGD007, was designed to retarget T cells to GpA33+ gastrointestinal tumor cells. Unlike MGD006, MGD007 is fused to an Fc fragment, a conventional protein engineering strategy that prolongs serum half-life.80,81,6
Another bispecific strategy is a DNL. A DNL construct involves the natural interaction between the dimerization and docking domain (DDD) of cAMP-dependent protein kinase A and the anchoring domain (AD) of A-kinase anchor protein (AKAP).47,54 Two types of modules, the first one, a homodimer containing the DDD (fused with an Fab directed against the first antigen) and the second one containing the AD (fused to another Fab to the second antigen) are generated and then combined to form noncovalent complexes. Disulfide bonds are then formed between cysteine residues incorporated into the DDD and AD sequences to form a trivalent molecule.27,78 Therefore, a DNL construct is composed of one Fab-AD and 2 Fab-DDD moieties.82
ImmTACs
ImmTACs, i.e., immune-cell-mobilizing monoclonal TCRs against cancer, are soluble bispecific agents that comprise an anti-CD3 scFv linked to an affinity-matured TCR that recognizes target MHC-peptide complexes.22,23 The ImmTAC technology offers an opportunity to retarget T cells to intracellular tumor proteins presented as MHC-peptide complexes.51
Like other bispecific retargeting strategies, ImmTACs also utilize an anti-CD3 scFv arm of nanomolar affinity to target effector T cells. Unlike the other approaches, ImmTACs recognize tumor cells through an engineered high-affinity TCR (usually a monoclonal TCR) rather than through an antibody fragment. ImmTACs trigger T-cell activation through a natural steric and biological immune synapse.22,23 The monoclonal TCR on ImmTACs can target intracellular tumor-associated antigens via recognition of MHC-peptide complexes (on the cell surface) that are rarely accessible to antibodies.83 This strategy expands the spectrum of tumor-specific targets, with the potential to reduce off-target toxicity.84 When in contact with tumor cells, ImmTACs specifically bind to a defined MHC-peptide complex displayed on the cell surface via high-affinity TCR-based recognition. Then, the CD3 arm recruits polyclonal T cells, thus, leading to the formation of an immune synapse and eventual killing of the tumor cells.22
Many ImmTACs have been developed, including those targeting gp100 (a melanocyte differentiation antigen), MAGE-A3 (a cancer neoantigen expressed in a wide array of tumors), Melan-A/MART-1 (a lineage-specific antigen expressed by metastatic melanomas), and NY-ESO-1 (a cancer neoantigen expressed in multiple myeloma).83 Currently, the most advanced ImmTACs program, IMCgp100, is in phase II trials in patients with malignant melanoma.
Retargeting of NK cells
Aside from T cells, NK cells also play a crucial role in the recognition and eradication of tumors. NK cells have also been explored as targets for bispecific antibodies. To engage NK cells, the CD16 marker on NK cells is frequently used. AFM13, a tetravalent bispecific TandAb (CD30/CD16A), was developed recently.85 AFM13 has 2 binding sites for the NK cell marker CD16A and 2 for CD30, which is a cell surface marker of Hodgkin's lymphoma cells. AFM13 was shown to induce stronger cytotoxicity toward tumor cells than an optimized anti-CD30 IgG or a bivalent bispecific CD16A/CD30 diabody.85 Because of its larger molecular size (100 kDa), AFM13 exhibited a significant increase in serum half-life over BiTEs. AFM13 is now in phase II clinical trials for patients with Hodgkin's lymphoma, and in a phase I clinical trial for CD30+ lymphoma. An alternative NK cell-retargeting bispecific antibody was constructed in the format of bispecific killer engagers (BiKEs) and showed potent cytotoxicity.86,87 In a BiKE, the anti-CD3 arm in a BiTE construct is replaced with an anti-CD16 humanized scFv arm to recruit NK cells. Recently, to take advantage of the stimulation and proliferation capacity of IL-15,80 a trispecific killer engager (TriKE) construct was proposed using IL-15 to link the 2 antibody arms for the purpose of boosting anti-tumor activity.88
Heavy-chain-only antibodies
To reduce the heavy chain–light chain problem, the VH or VL alone can be engineered to bind an antigen.90-92 Such a VH or VL can be used as a building block for bispecific antibodies. However, a better strategy is to use single-domain antibodies. A single-domain antibody is derived from natural camel heavy-chain-only antibodies (HCAbs).93 The variable domain of the HCAbs is referred to as the VHH, also known as a single-domain antibody or nanobody. The VHH is the structural and functional equivalent of the Fab fragment of conventional antibodies94 and the smallest fully functional, naturally derived antigen-binding fragment. Nanobodies have been shown to have several properties that makes them promising building blocks for antibody engineering, including small molecular size (15 kDa), high expression level, and high stability and solubility in vitro.28,95,96 The CDR3 of camel VHHs forms an extended loop covering the lipophilic site; this property results in improved solubility.97,98 Moreover, nanobodies were demonstrated to be as specific as regular antibodies.99
The major concern with nanobodies is their immunogenicity in humans. However, as nanobodies are small, undergo rapid renal clearance, and their amino acid sequences are highly similar to that of human VH, no significant immunogenicity has been observed in mice or humans injected with nanobody-containing constructs.100-102 A humanization strategy has also been developed to further decrease the immunogenicity, which involves humanization of 12 out of the 14 amino acid residues that differ from the corresponding sequence in human IgG.103
The small size of nanobodies makes them amendable to bulk production in bacterial cells.99 In general, nanobodies are cloned behind a secretion signal for bacterial expression. The proteins are produced in the periplasm where formation of disulfide bonds is possible. A yield of several milligrams per liter of culture can be achieved in the lab with a simple culture flask.
The relatively low molecular weight generally results in a better tissue bio-distribution, as well as access to epitopes on some targets that are difficult to access with antibodies of the IgG format.104 The low molecular weight, however, may also cause rapid renal clearance, which could hamper therapeutic success.
Bispecific antibodies can be constructed by linking 2 nanobodies (specific to 2 antigens) in tandem via a peptide linker.105 Apart from tandem VHHs, one alternative to achieve bispecificity is to fuse one VHH single-domain antibody with the CH1 and another with the CL, in a single Fab fragment.106 One research group combined an anti-CD16 VHH in this Fab format to recruit NK cells with an anti-HER2 VHH to target HER2+ tumor cells.107 This Fab-like bispecific antibody showed increased potency against HER2+ tumor cells in comparison with trastuzumab.107 S-Fab, a hybrid of human Fab with a VHH, was constructed by linking a single-domain anti-CEA VHH to a human anti-CD3 Fab.108 S-Fab can be efficiently expressed and purified from bacteria and showed excellent stability in serum and potent antitumor activity in vitro and in vivo.108 Another NK cell-retargeting bispecific antibody called BiSS was generated in the binanobody format recently.89 BiSS can be efficiently expressed and purified from bacteria. It utilizes an anti-CD16 VHH arm to recruit NK cells and an anti-CEA VHH arm to target CEA-expressing tumor cells. These studies suggest that the flexibility of VHHs will enable their broad use in bispecific antibodies.
Concluding remarks
As the next generation of antitumor strategies, bispecific antibodies have received much attention because of their unique mechanism of action and potent tumoricidal effect. The success of blinatumomab as monotherapy for B-ALL added a great deal of excitement to this field. Numerous bispecific retargeting antibody candidates are now being tested in clinical trials,6,51 with a plethora of others in preclinical studies.
Future advances in bispecific antibody technology will be focused on the development of new antibody formats to accommodate the complexity of tumor biology. Beyond bispecific antibodies, more extensive research into multispecific and multivalent antibodies is also anticipated. Because the majority of current bispecific antibodies target known tumor cell surface antigens, discovery of new targets is also urgently needed, including novel tumor cell surface antigens and intracellular tumor antigen-associated peptide-MHC complexes, to increase efficacy and reduce adverse effects of bispecific antibodies. Of equal interest is the rise of a new class of bispecific antibody targeting tumor-associated antigens and checkpoints to tumor-site specifically reverse immune suppression and release effector cells in-check. It should be noted that tumor biology is complex and diverse. Genome sequencing has revealed the heterogeneous nature of tumors, even within the same patient, and the wide variety of mutations that may be present in cancer cells.109 It is likely that most bispecific retargeting antibodies alone may not be as efficacious as blinatumomab is against B-ALL, which is considered less complex than solid tumors. A combination of different therapeutics to mount a multifaceted attack on a tumor is expected to result in long-lasting cancer-free survival.
Abbreviations
- AD
anchoring domain
- B-ALL
B-cell acute lymphoblastic leukemia
- BiKE
bispecific killer engager
- BiTE
bispecific T-cell engager
- CML
chronic myeloid leukemia
- CDR
complementarity-determining region
- DC
dendritic cell
- DDD
dimerization and docking domain
- DNL
“dock and lock”
- DAF
dual-action Fab
- DART
dual-affinity-retargeting format
- DVD-IgG
dual variable domain IgG
- ImmTACs
immune-cell-mobilizing monoclonal TCRs against cancer
- CL
light chain
- NK
natural killer
- scFv
single-chain Fv fragment
- TCR
T-cell receptor
- TandAb
tandem antibody
- VH
variable heavy chain domain
- VL
variable light chain domain
Disclosure of potential conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
This work was financially supported by the Introduced Innovative R&D Team Leadership of Guangdong Province (PR China) (2011Y038).
References
- [1].Apperley JF. Chronic myeloid leukaemia. Lancet 2015; 385:1447-59; PMID:25484026; http://dx.doi.org/ 10.1016/S0140-6736(13)62120-0 [DOI] [PubMed] [Google Scholar]
- [2].Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013; 342:1432-3; PMID:24357284; http://dx.doi.org/ 10.1126/science.342.6165.1432 [DOI] [PubMed] [Google Scholar]
- [3].Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the generation Z of negative checkpoint regulators. Front Immunol 2015; 6:418; PMID:26347741; http://dx.doi.org/ 10.3389/fimmu.2015.00418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest 2015; 125:3335-7; PMID:26325031; http://dx.doi.org/ 10.1172/JCI83871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kontermann R. Dual targeting strategies with bispecific antibodies. MAbs 2012; 4:182-97; PMID:22453100; http://dx.doi.org/ 10.4161/mabs.4.2.19000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today 2015; 20:838-47; PMID:25728220; http://dx.doi.org/ 10.1016/j.drudis.2015.02.008 [DOI] [PubMed] [Google Scholar]
- [7].Heiss MM. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized Phase II/III trial. Int J Cancer 2010; 127:2209-21; PMID:20473913; http://dx.doi.org/ 10.1002/ijc.25423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Przepiorka D, Ko CW, Deisseroth A, Yancey CL, Candau-Chacon R, Chiu HJ, Gehrke BJ, Gomez-Broughton C, Kane RC, Kirshner S, et al.. FDA Approval: Blinatumomab. Clin Cancer Res 2015; 21:4035-9; PMID:26374073; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-0612 [DOI] [PubMed] [Google Scholar]
- [9].Newman MJ, Benani DJ. A review of blinatumomab, a novel immunotherapy. J Oncol Pharm Pract 2015; PMID:26607163 [DOI] [PubMed] [Google Scholar]
- [10].Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature 1985; 314:628-31; PMID:2859527; http://dx.doi.org/ 10.1038/314628a0 [DOI] [PubMed] [Google Scholar]
- [11].Nisonoff A, Rivers MM. Recombination of a mixture of univalent antibody fragments of different specificity. Arch Biochem Biophys 1961; 93:460-2; PMID:13729244; http://dx.doi.org/ 10.1016/0003-9861(61)90296-X [DOI] [PubMed] [Google Scholar]
- [12].Yankelevich M, Kondadasula SV, Thakur A, Buck S, Cheung NK, Lum LG. Anti-CD3 x anti-GD2 bispecific antibody redirects T-cell cytolytic activity to neuroblastoma targets. Pediatr Blood Cancer 2012; 59:1198-205; PMID:22707078; http://dx.doi.org/ 10.1002/pbc.24237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sen M, Wankowski DM, Garlie NK, Siebenlist RE, Van Epps D, LeFever AV, Lum LG. Use of anti-CD3 x anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu+ tumors. J Hematother Stem Cell Res 2001; 10:247-60; PMID:11359672; http://dx.doi.org/ 10.1089/15258160151134944 [DOI] [PubMed] [Google Scholar]
- [14].Weiner GJ. Building better monoclonal antibody-based therapeutics. Nature reviews Cancer 2015; 15:361-70; PMID:25998715; http://dx.doi.org/ 10.1038/nrc3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Batlevi CL, Matsuki E, Brentjens RJ, Younes A. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol 2016; 13:25-40; PMID:26525683; http://dx.doi.org/ 10.1038/nrclinonc.2015.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Byrne H, Conroy PJ, Whisstock JC, O'Kennedy RJ. A tale of two specificities: bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol 2013; 31:621-32; PMID:24094861; http://dx.doi.org/ 10.1016/j.tibtech.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chelius D, Ruf P, Gruber P, Ploscher M, Liedtke R, Gansberger E, Hess J, Wasiliu M, Lindhofer H. Structural and functional characterization of the trifunctional antibody catumaxomab. MAbs 2010; 2:309-19; PMID:20418662; http://dx.doi.org/ 10.4161/mabs.2.3.11791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Berek JS, Edwards RP, Parker LP, DeMars LR, Herzog TJ, Lentz SS, Morris RT, Akerley WL, Holloway RW, Method MW, et al.. Catumaxomab for the treatment of malignant ascites in patients with chemotherapy-refractory ovarian cancer: a phase II study. Int J Gynecol Cancer 2014; 24:1583-9; PMID:25254563; http://dx.doi.org/ 10.1097/IGC.0000000000000286 [DOI] [PubMed] [Google Scholar]
- [19].Mau-Sorensen M, Dittrich C, Dienstmann R, Lassen U, Buchler W, Martinius H, Tabernero J. A phase I trial of intravenous catumaxomab: a bispecific monoclonal antibody targeting EpCAM and the T cell coreceptor CD3. Cancer Chemother Pharmacol 2015; 75:1065-73; PMID:25814216; http://dx.doi.org/ 10.1007/s00280-015-2728-5 [DOI] [PubMed] [Google Scholar]
- [20].Frankel SR, Baeuerle PA. Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol 2013; 17:385-92; PMID:23623807; http://dx.doi.org/ 10.1016/j.cbpa.2013.03.029 [DOI] [PubMed] [Google Scholar]
- [21].Cochlovius B. Cure of Burkitt's lymphoma in severe combined immunodeficiency mice by T cells, tetravalent CD3 x CD19 tandem diabody, and CD28 costimulation. Cancer Res 2000; 60:4336-41; PMID:10969772 [PubMed] [Google Scholar]
- [22].Oates J, Hassan NJ, Jakobsen BK. ImmTACs for targeted cancer therapy: Why, what, how, and which. Mol Immunol 2015; 67:67-74; PMID:25708206; http://dx.doi.org/ 10.1016/j.molimm.2015.01.024 [DOI] [PubMed] [Google Scholar]
- [23].Oates J, Jakobsen BK. ImmTACs: Novel bi-specific agents for targeted cancer therapy. Oncoimmunology 2013; 2:e22891; PMID:23525668; http://dx.doi.org/ 10.4161/onci.22891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, Man W, Peale F, Ross S, Wiesmann C, et al.. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science 2009; 323:1610-4; PMID:19299620; http://dx.doi.org/ 10.1126/science.1165480 [DOI] [PubMed] [Google Scholar]
- [25].Orcutt KD, Ackerman ME, Cieslewicz M, Quiroz E, Slusarczyk AL, Frangioni JV, Wittrup KD. A modular IgG-scFv bispecific antibody topology. Protein Eng Des Sel 2010; 23:221-8; PMID:20019028; http://dx.doi.org/ 10.1093/protein/gzp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schaefer W, Regula JT, Bahner M, Schanzer J, Croasdale R, Durr H, Gassner C, Georges G, Kettenberger H, Imhof-Jung S, et al.. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A 2011; 108:11187-92; PMID:21690412; http://dx.doi.org/ 10.1073/pnas.1019002108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rossi EA, Goldenberg DM, Cardillo TM, McBride WJ, Sharkey RM, Chang CH. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A 2006; 103:6841-6; PMID:16636283; http://dx.doi.org/ 10.1073/pnas.0600982103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu RR, Santora L, et al.. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol 2007; 25:1290-7; PMID:17934452; http://dx.doi.org/ 10.1038/nbt1345 [DOI] [PubMed] [Google Scholar]
- [29].Gu J, Ghayur T. Generation of dual-variable-domain immunoglobulin molecules for dual-specific targeting. Methods Enzymol 2012; 502:25-41; PMID:22208980; http://dx.doi.org/ 10.1016/B978-0-12-416039-2.00002-1 [DOI] [PubMed] [Google Scholar]
- [30].Jakob CG, Edalji R, Judge RA, DiGiammarino E, Li Y, Gu J, Ghayur T. Structure reveals function of the dual variable domain immunoglobulin (DVD-Ig) molecule. MAbs 2013; 5:358-63; PMID:23549062; http://dx.doi.org/ 10.4161/mabs.23977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Els Conrath K, Lauwereys M, Wyns L, Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem 2001; 276:7346-50; PMID:11053416; http://dx.doi.org/ 10.1074/jbc.M007734200 [DOI] [PubMed] [Google Scholar]
- [32].Lazar GA. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA 2006; 103:4005-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev 2010; 36:458-67; PMID:20347527; http://dx.doi.org/ 10.1016/j.ctrv.2010.03.001 [DOI] [PubMed] [Google Scholar]
- [34].Zeidler R, Mysliwietz J, Csanady M, Walz A, Ziegler I, Schmitt B, Wollenberg B, Lindhofer H. The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer 2000; 83:261-6; PMID:10901380; http://dx.doi.org/ 10.1054/bjoc.2000.1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stanglmaier M, Faltin M, Ruf P, Bodenhausen A, Schroder P, Lindhofer H. Bi20 (fBTA05), a novel trifunctional bispecific antibody (anti-CD20 x anti-CD3), mediates efficient killing of B-cell lymphoma cells even with very low CD20 expression levels. Int J Cancer 2008; 123:1181-9; PMID:18546289; http://dx.doi.org/ 10.1002/ijc.23626 [DOI] [PubMed] [Google Scholar]
- [36].Jager M, Schoberth A, Ruf P, Hess J, Lindhofer H. The trifunctional antibody ertumaxomab destroys tumor cells that express low levels of human epidermal growth factor receptor 2. Cancer Res 2009; 69:4270-6; PMID:19435924; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2861 [DOI] [PubMed] [Google Scholar]
- [37].Buhmann R, Michael S, Juergen H, Horst L, Peschel C, Kolb HJ. Immunotherapy with FBTA05 (Bi20), a trifunctional bispecific anti-CD3 x anti-CD20 antibody and donor lymphocyte infusion (DLI) in relapsed or refractory B-cell lymphoma after allogeneic stem cell transplantation: study protocol of an investigator-driven, open-label, non-randomized, uncontrolled, dose-escalating Phase I/II-trial. J Transl Med 2013; 11:160; PMID:23815981; http://dx.doi.org/ 10.1186/1479-5876-11-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature 1983; 305:537-40; PMID:6137772; http://dx.doi.org/ 10.1038/305537a0 [DOI] [PubMed] [Google Scholar]
- [39].Suresh MR, Cuello AC, Milstein C. Advantages of bispecific hybridomas in one-step immunocytochemistry and immunoassays. Proc Natl Acad Sci U S A 1986; 83:7989-93; PMID:2429324; http://dx.doi.org/ 10.1073/pnas.83.20.7989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Atwell S, Ridgway JB, Wells JA, Carter P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J Mol Biol 1997; 270:26-35; PMID:9231898; http://dx.doi.org/ 10.1006/jmbi.1997.1116 [DOI] [PubMed] [Google Scholar]
- [41].Ridgway JB, Presta LG, Carter P. 'Knobs-into-holes' engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng 1996; 9:617-21; PMID:8844834; http://dx.doi.org/ 10.1093/protein/9.7.617 [DOI] [PubMed] [Google Scholar]
- [42].Gunasekaran K, Pentony M, Shen M, Garrett L, Forte C, Woodward A, Ng SB, Born T, Retter M, Manchulenko K, et al.. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J Biol Chem 2010; 285:19637-46; PMID:20400508; http://dx.doi.org/ 10.1074/jbc.M110.117382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Strop P, Ho WH, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, Rickert M, Appah CT, Pascua E, Radcliffe T, et al.. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol 2012; 420:204-19; PMID:22543237; http://dx.doi.org/ 10.1016/j.jmb.2012.04.020 [DOI] [PubMed] [Google Scholar]
- [44].Von Kreudenstein TS, Escobar-Carbrera E, Lario PI, D'Angelo I, Brault K, Kelly J, Durocher Y, Baardsnes J, Woods RJ, Xie MH, et al.. Improving biophysical properties of a bispecific antibody scaffold to aid developability: Quality by molecular design. mAbs 2013; 5:646-54; PMID:23924797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Davis JH, Aperlo C, Li Y, Kurosawa E, Lan Y, Lo KM, Huston JS. SEEDbodies: fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng Des Sel 2010; 23:195-202; PMID:20299542; http://dx.doi.org/ 10.1093/protein/gzp094 [DOI] [PubMed] [Google Scholar]
- [46].Spiess C, Bevers J 3rd, Jackman J, Chiang N, Nakamura G, Dillon M, Liu H, Molina P, Elliott JM, Shatz W, et al.. Development of a human IgG4 bispecific antibody for dual targeting of interleukin-4 (IL-4) and interleukin-13 (IL-13) cytokines. J Biol Chem 2013; 288:26583-93; PMID:23880771; http://dx.doi.org/ 10.1074/jbc.M113.480483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, Carter P. An efficient route to human bispecific IgG. Nat Biotechnol 1998; 16:677-81; PMID:9661204; http://dx.doi.org/ 10.1038/nbt0798-677 [DOI] [PubMed] [Google Scholar]
- [48].Dhimolea E, Reichert JM. World Bispecific Antibody Summit, September 27-28, 2011, Boston, MA: MAbs; 2012; 4:4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Spiess C, Merchant M, Huang A, Zheng Z, Yang NY, Peng J, Ellerman D, Shatz W, Reilly D, Yansura DG, et al.. Bispecific antibodies with natural architecture produced by co-culture of bacteria expressing two distinct half-antibodies. Nat Biotechnol 2013; PMID:23831709 [DOI] [PubMed] [Google Scholar]
- [50].Jackman J, Chen Y, Huang A, Moffat B, Scheer JM, Leong SR, Lee WP, Zhang J, Sharma N, Lu Y, et al.. Development of a two-part strategy to identify a therapeutic human bispecific antibody that inhibits IgE receptor signaling. J Biol Chem 2010; 285:20850-9; PMID:20444694; http://dx.doi.org/ 10.1074/jbc.M110.113910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol 2015; 67:95-106; PMID:25637431; http://dx.doi.org/ 10.1016/j.molimm.2015.01.003 [DOI] [PubMed] [Google Scholar]
- [52].LaFleur DW, Abramyan D, Kanakaraj P, Smith RG, Shah RR, Wang G, Yao XT, Kankanala S, Boyd E, Zaritskaya L, et al.. Monoclonal antibody therapeutics with up to five specificities: functional enhancement through fusion of target-specific peptides. MAbs 2013; 5:208-18; PMID:23575268; http://dx.doi.org/ 10.4161/mabs.23043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fenn S, Schiller CB, Griese JJ, Duerr H, Imhof-Jung S, Gassner C, Moelleken J, Regula JT, Schaefer W, Thomas M, et al.. Crystal structure of an anti-Ang2 CrossFab demonstrates complete structural and functional integrity of the variable domain. PLoS One 2013; 8:e61953; PMID:23613981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lewis SM, Wu X, Pustilnik A, Sereno A, Huang F, Rick HL, Guntas G, Leaver-Fay A, Smith EM, Ho C, et al.. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol 2014; 32:191-8; PMID:24463572; http://dx.doi.org/ 10.1038/nbt.2797 [DOI] [PubMed] [Google Scholar]
- [55].Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, Totpal K, Wong A, Lee CV, Stawicki S, et al.. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 2011; 20:472-86; PMID:22014573; http://dx.doi.org/ 10.1016/j.ccr.2011.09.003 [DOI] [PubMed] [Google Scholar]
- [56].Eigenbrot C, Fuh G. Two-in-One antibodies with dual action Fabs. Curr Opin Chem Biol 2013; 17:400-5; PMID:23683347; http://dx.doi.org/ 10.1016/j.cbpa.2013.04.015 [DOI] [PubMed] [Google Scholar]
- [57].Hu S, Fu W, Xu W, Yang Y, Cruz M, Berezov SD, Jorissen D, Takeda H, Zhu W. Four-in-one antibodies have superior cancer inhibitory activity against EGFR, HER2, HER3, and VEGF through disruption of HER/MET crosstalk. Cancer Res 2015; 75:159-70; PMID:25371409; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-1670 [DOI] [PubMed] [Google Scholar]
- [58].Albrecht H, Denardo GL, Denardo SJ. Monospecific bivalent scFv-SH: effects of linker length and location of an engineered cysteine on production, antigen binding activity and free SH accessibility. J Immunol Methods 2006; 310:100-16; PMID:16499921; http://dx.doi.org/ 10.1016/j.jim.2005.12.012 [DOI] [PubMed] [Google Scholar]
- [59].Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A 1993; 90:6444-8; PMID:8341653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Johnson S, Burke S, Huang L, Gorlatov S, Li H, Wang W, Zhang W, Tuaillon N, Rainey J, Barat B, et al.. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol 2010; 399:436-49; PMID:20382161; http://dx.doi.org/ 10.1016/j.jmb.2010.04.001 [DOI] [PubMed] [Google Scholar]
- [61].Baeuerle PA, Kufer P, Bargou R. BiTE: Teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther 2009; 11:22-30; PMID:19169956 [PubMed] [Google Scholar]
- [62].Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res 2009; 69:4941-4; PMID:19509221; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-0547 [DOI] [PubMed] [Google Scholar]
- [63].Hayden MS, Linsley PS, Gayle MA, Bajorath J, Brady WA, Norris NA, Fell HP, Ledbetter JA, Gilliland LK. Single-chain mono- and bispecific antibody derivatives with novel biological properties and antitumour activity from a COS cell transient expression system. Ther Immunol 1994; 1:3-15; PMID:7584477 [PubMed] [Google Scholar]
- [64].Muller D, Kontermann RE. Bispecific antibodies for cancer immunotherapy: Current perspectives. Biodrugs 2010; 24:89-98; PMID:20199124; http://dx.doi.org/ 10.2165/11530960-000000000-00000 [DOI] [PubMed] [Google Scholar]
- [65].Kellner C, Bruenke J, Stieglmaier J, Schwemmlein M, Schwenkert M, Singer H, Mentz K, Peipp M, Lang P, Oduncu F, et al.. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J Immunother 2008; 31:871-84; PMID:18833000; http://dx.doi.org/ 10.1097/CJI.0b013e318186c8b4 [DOI] [PubMed] [Google Scholar]
- [66].Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, Kufer P, Riethmuller G, Bargou R, Baeuerle PA. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer 2002; 100:690-7; PMID:12209608; http://dx.doi.org/ 10.1002/ijc.10557 [DOI] [PubMed] [Google Scholar]
- [67].Wolf E, Hofmeister R, Kufer P, Schlereth B, Baeuerle PA. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today 2005; 10:1237-44; PMID:16213416; http://dx.doi.org/ 10.1016/S1359-6446(05)03554-3 [DOI] [PubMed] [Google Scholar]
- [68].Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol 2006; 43:763-71; PMID:16360021; http://dx.doi.org/ 10.1016/j.molimm.2005.03.007 [DOI] [PubMed] [Google Scholar]
- [69].Schlereth B, Quadt C, Dreier T, Kufer P, Lorenczewski G, Prang N, Brandl C, Lippold S, Cobb K, Brasky K, et al.. T-cell activation and B-cell depletion in chimpanzees treated with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Cancer Immunol Immunother 2006; 55:503-14; PMID:16032400; http://dx.doi.org/ 10.1007/s00262-005-0001-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, et al.. Tumor Regression in Cancer Patients by Very Low Doses of a T Cell–Engaging Antibody. Science 2008; 321:974-7; PMID:18703743; http://dx.doi.org/ 10.1126/science.1158545 [DOI] [PubMed] [Google Scholar]
- [71].Haas C, Krinner E, Brischwein K, Hoffmann P, Lutterbuse R, Schlereth B, Kufer P, Baeuerle PA. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology 2009; 214:441-53; PMID:19157637; http://dx.doi.org/ 10.1016/j.imbio.2008.11.014 [DOI] [PubMed] [Google Scholar]
- [72].Maloney DG, Grillo-Lopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, Varns C, Rosenberg J, Levy R. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol 1997; 15:3266-74; PMID:9336364 [DOI] [PubMed] [Google Scholar]
- [73].Igawa T, Tsunoda H, Kikuchi Y, Yoshida M, Tanaka M, Koga A, Sekimori Y, Orita T, Aso Y, Hattori K, et al.. VH/VL interface engineering to promote selective expression and inhibit conformational isomerization of thrombopoietin receptor agonist single-chain diabody. Protein Eng Des Sel 2010; 23:667-77; PMID:20576629; http://dx.doi.org/ 10.1093/protein/gzq034 [DOI] [PubMed] [Google Scholar]
- [74].Tan PH, Sandmaier BM, Stayton PS. Contributions of a highly conserved VH/VL hydrogen bonding interaction to scFv folding stability and refolding efficiency. Biophys J 1998; 75:1473-82; PMID:9726949; http://dx.doi.org/ 10.1016/S0006-3495(98)74066-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kipriyanov SM, Moldenhauer G, Schuhmacher J, Cochlovius B, Von der Lieth CW, Matys ER, Little M. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol 1999; 293:41-56; PMID:10512714; http://dx.doi.org/ 10.1006/jmbi.1999.3156 [DOI] [PubMed] [Google Scholar]
- [76].Arndt MA, Krauss J, Kipriyanov SM, Pfreundschuh M, Little M. A bispecific diabody that mediates natural killer cell cytotoxicity against xenotransplantated human Hodgkin's tumors. Blood 1999; 94:2562-8; PMID:10515858 [PubMed] [Google Scholar]
- [77].Moore PA, Zhang W, Rainey GJ, Burke S, Li H, Huang L, Gorlatov S, Veri MC, Aggarwal S, Yang Y, et al.. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 2011; 117:4542-51; PMID:21300981; http://dx.doi.org/ 10.1182/blood-2010-09-306449 [DOI] [PubMed] [Google Scholar]
- [78].May C, Sapra P, Gerber HP. Advances in bispecific biotherapeutics for the treatment of cancer. Biochem Pharmacol 2012; 84:1105-12; PMID:22858161; http://dx.doi.org/ 10.1016/j.bcp.2012.07.011 [DOI] [PubMed] [Google Scholar]
- [79].Chichili GR, Huang L, Li H, Burke S, He L, Tang Q, Jin L, Gorlatov S, Ciccarone V, Chen F, et al.. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates. Sci Transl Med 2015; 7:289ra82; PMID:26019218; http://dx.doi.org/ 10.1126/scitranslmed.aaa5693 [DOI] [PubMed] [Google Scholar]
- [80].Chen S, Huang Q, Liu J, Xing J, Zhang N, Liu Y, Wang Z, Li Q. A targeted IL-15 fusion protein with potent anti-tumor activity. Cancer Biol Ther 2015; 16:1415-21; PMID:26176990; http://dx.doi.org/ 10.1080/15384047.2015.1071739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715-25; PMID:17703228; http://dx.doi.org/ 10.1038/nri2155 [DOI] [PubMed] [Google Scholar]
- [82].Gold DV, Goldenberg DM, Karacay H, Rossi EA, Chang CH, Cardillo TM, McBride WJ, Sharkey RM. A novel bispecific, trivalent antibody construct for targeting pancreatic carcinoma. Cancer Res 2008; 68:4819-26; PMID:18559529; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-0232 [DOI] [PubMed] [Google Scholar]
- [83].Liddy N, Bossi G, Adams KJ, Lissina A, Mahon TM, Hassan NJ, Gavarret J, Bianchi FC, Pumphrey NJ, Ladell K, et al.. Monoclonal TCR-redirected tumor cell killing. Nat Med 2012; 18:980-7; PMID:22561687; http://dx.doi.org/ 10.1038/nm.2764 [DOI] [PubMed] [Google Scholar]
- [84].Dahan R, Reiter Y. T-cell-receptor-like antibodies - generation, function and applications. Expert Rev Mol Med 2012; 14:e6; PMID:22361332 [DOI] [PubMed] [Google Scholar]
- [85].Reusch U, Burkhardt C, Fucek I, Le Gall F, Le Gall M, Hoffmann K, Knackmuss SH, Kiprijanov S, Little M, Zhukovsky EA. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. MAbs 2014; 6:728-39; PMID:24670809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Schmohl JU, Gleason MK, Dougherty PR, Miller JS, Vallera DA. Heterodimeric Bispecific Single Chain Variable Fragments (scFv) Killer Engagers (BiKEs) Enhance NK-cell Activity Against CD133+ Colorectal Cancer Cells. Target Oncol 2015; 1-9; PMID:24590691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 2014; 123:3016-26; PMID:24652987; http://dx.doi.org/ 10.1182/blood-2013-10-533398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Vallera DA, Felices M, McElmurry RT, McCullar V, Zhou X, Schmohl J, Zhang B, Lenvik A, Panoskaltsis-Mortari A, Verneris MR. IL-15 trispecific killer engagers (TriKEs) make natural killer cells specific to CD33+ targets while also inducing in vivo expansion, and enhanced function. Clin Cancer Res 2016; clincanres. 2710.015; PMID:26847056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dong B, Zhou C, He P, Li J, Chen S, Miao J, Li Q, Wang Z. A novel bispecific antibody, BiSS, with potent anti-cancer activities. Cancer Biol Ther 2016; 17:364-70; PMID:26828900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Davies J, Riechmann L. Antibody VH domains as small recognition units. Biotechnology (N Y) 1995; 13:475-9; PMID:9634788 [DOI] [PubMed] [Google Scholar]
- [91].van den Beucken T, van Neer N, Sablon E, Desmet J, Celis L, Hoogenboom HR, Hufton SE. Building novel binding ligands to B7.1 and B7.2 based on human antibody single variable light chain domains. J Mol Biol 2001; 310:591-601; PMID:11439026; http://dx.doi.org/ 10.1006/jmbi.2001.4703 [DOI] [PubMed] [Google Scholar]
- [92].Ward ES, Gussow D, Griffiths AD, Jones PT, Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature 1989; 341:544-6; PMID:2677748; http://dx.doi.org/ 10.1038/341544a0 [DOI] [PubMed] [Google Scholar]
- [93].Pain C, Dumont J, Dumoulin M. Camelid single-domain antibody fragments: Uses and prospects to investigate protein misfolding and aggregation, and to treat diseases associated with these phenomena. Biochimie 2015; 111:82-106; PMID:25656912; http://dx.doi.org/ 10.1016/j.biochi.2015.01.012 [DOI] [PubMed] [Google Scholar]
- [94].Serge M, Padlan EA. Nanobodies: Natural Single-Domain Antibodies. Annu Rev Biochem 2013; 82:775-97; PMID:23495938; http://dx.doi.org/ 10.1146/annurev-biochem-063011-092449 [DOI] [PubMed] [Google Scholar]
- [95].van der Linden RHJ, Frenken LGJ, de Geus B, Harmsen MM, Ruuls RC, Stok W, de Ron L, Wilson S, Davis P, Verrips CT. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim Biophys Acta 1999; 1431:37-46 [DOI] [PubMed] [Google Scholar]
- [96].Dumoulin M, Conrath K, Van Meirhaeghe A, Meersman F, Heremans K, Frenken LGJ, Muyldermans S, Wyns L, Matagne A. Single-domain antibody fragments with high conformational stability. Protein Sci 2002; 11:500-15; PMID:11847273; http://dx.doi.org/ 10.1110/ps.34602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Dolk E, van der Vaart M, Lutje Hulsik D, Vriend G, de Haard H, Spinelli S, Cambillau C, Frenken L, Verrips T. Isolation of llama antibody fragments for prevention of dandruff by phage display in shampoo. Appl Environ Microbiol 2005; 71:442-50; PMID:15640220; http://dx.doi.org/ 10.1128/AEM.71.1.442-450.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Stanfield RL, Dooley H, Flajnik MF, Wilson IA. Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science 2004; 305:1770-3; PMID:15319492; http://dx.doi.org/ 10.1126/science.1101148 [DOI] [PubMed] [Google Scholar]
- [99].Ghannam A, Kumari S, Muyldermans S, Abbady AQ. Camelid nanobodies with high affinity for broad bean mottle virus: a possible promising tool to immunomodulate plant resistance against viruses. Plant Mol Biol 2015; 87:355-69; PMID:25648551; http://dx.doi.org/ 10.1007/s11103-015-0282-5 [DOI] [PubMed] [Google Scholar]
- [100].Cortez-Retamozo V, Backmann N, Senter PD, Wernery U, De Baetselier P. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res 2004; 64:2853; PMID:15087403; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-3935 [DOI] [PubMed] [Google Scholar]
- [101].Baral TN, Magez S, Stijlemans B, Conrath K, Vanhollebeke B. Experimental therapy of African trypanosomiasis with a nanobody-conjugated human trypanolytic factor. Nat Med 2006; 12:580; PMID:16604085; http://dx.doi.org/ 10.1038/nm1395 [DOI] [PubMed] [Google Scholar]
- [102].Kijanka M, Warnders FJ, El Khattabi M, Lub-de Hooge M, van Dam GM, Ntziachristos V, de Vries L, Oliveira S, van Bergen En Henegouwen PM. Rapid optical imaging of human breast tumour xenografts using anti-HER2 VHHs site-directly conjugated to IRDye 800CW for image-guided surgery. Eur J Nucl Med Mol Imaging 2013; 40:1718-29; PMID:23778558; http://dx.doi.org/ 10.1007/s00259-013-2471-2 [DOI] [PubMed] [Google Scholar]
- [103].Vincke C, Loris R, Saerens D, Martinez-Rodriguez S, Muyldermans S, Conrath K. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J Biol Chem 2009; 284:3273; PMID:19010777; http://dx.doi.org/ 10.1074/jbc.M806889200 [DOI] [PubMed] [Google Scholar]
- [104].Desmyter A, Transue TR, Ghahroudi MA, Thi MH, Poortmans F, Hamers R, Muyldermans S, Wyns L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat Struct Biol 1996; 3:803-11; PMID:8784355; http://dx.doi.org/ 10.1038/nsb0996-803 [DOI] [PubMed] [Google Scholar]
- [105].Conrath KE, Lauwereys M, Wyns L, Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem 2001; 276:7346; PMID:11053416; http://dx.doi.org/ 10.1074/jbc.M007734200 [DOI] [PubMed] [Google Scholar]
- [106].Rozan C, Cornillon A, Petiard C, Chartier M, Behar G, Boix C, Kerfelec B, Robert B, Pelegrin A, Chames P, et al.. Single-domain antibody-based and linker-free bispecific antibodies targeting FcgammaRIII induce potent antitumor activity without recruiting regulatory T cells. Mol Cancer Ther 2013; 12:1481-91; PMID:23757164; http://dx.doi.org/ 10.1158/1535-7163.MCT-12-1012 [DOI] [PubMed] [Google Scholar]
- [107].Turini M, Chames P, Bruhns P, Baty D, Kerfelec B. A FcgammaRIII-engaging bispecific antibody expands the range of HER2-expressing breast tumors eligible to antibody therapy. Oncotarget 2014; 5:5304-19; PMID:24979648; http://dx.doi.org/ 10.18632/oncotarget.2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Li L, He P, Zhou C, Jing L, Dong B, Chen S, Zhang N, Liu Y, Miao J, Wang Z, et al.. A novel bispecific antibody, S-Fab, induces potent cancer cell killing. J Immunother 2015; 38:350-6; PMID:26448579; http://dx.doi.org/ 10.1097/CJI.0000000000000099 [DOI] [PubMed] [Google Scholar]
- [109].Watson IR, Takahashi K, Futreal PA, Chin L. Emerging patterns of somatic mutations in cancer. Nat Rev Genet 2013; 14:703-18; PMID:24022702; http://dx.doi.org/ 10.1038/nrg3539 [DOI] [PMC free article] [PubMed] [Google Scholar]


