Abstract
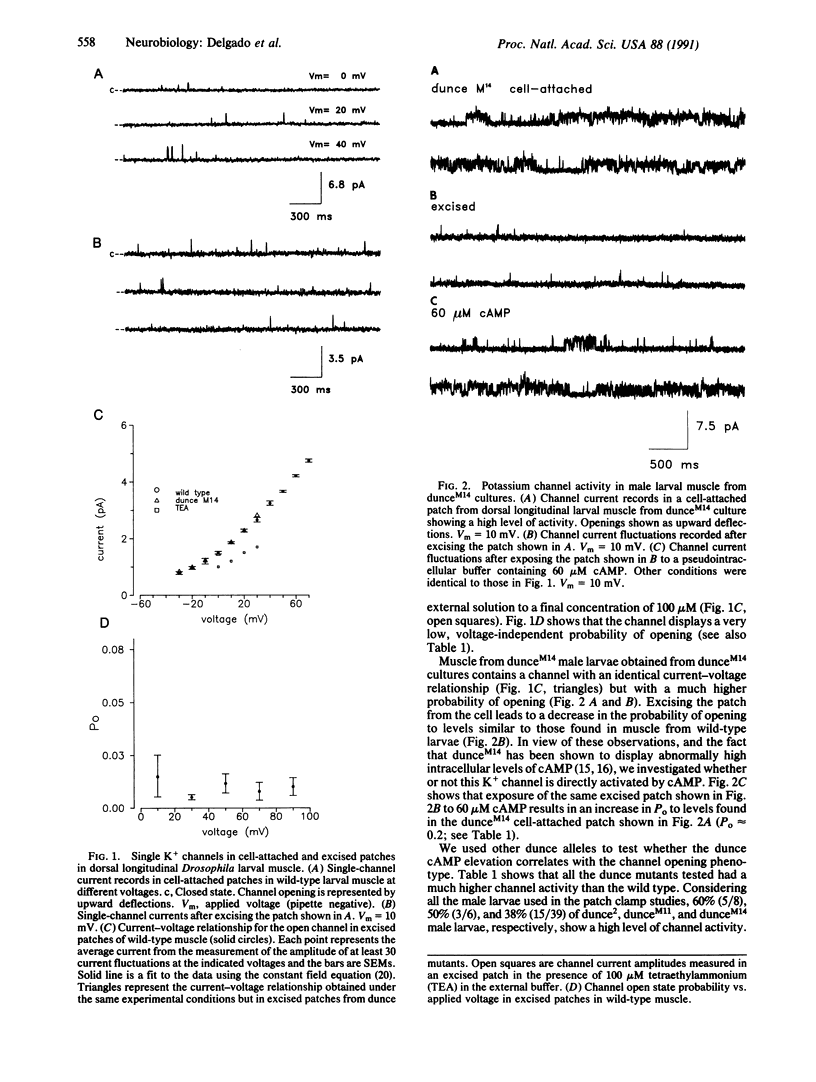
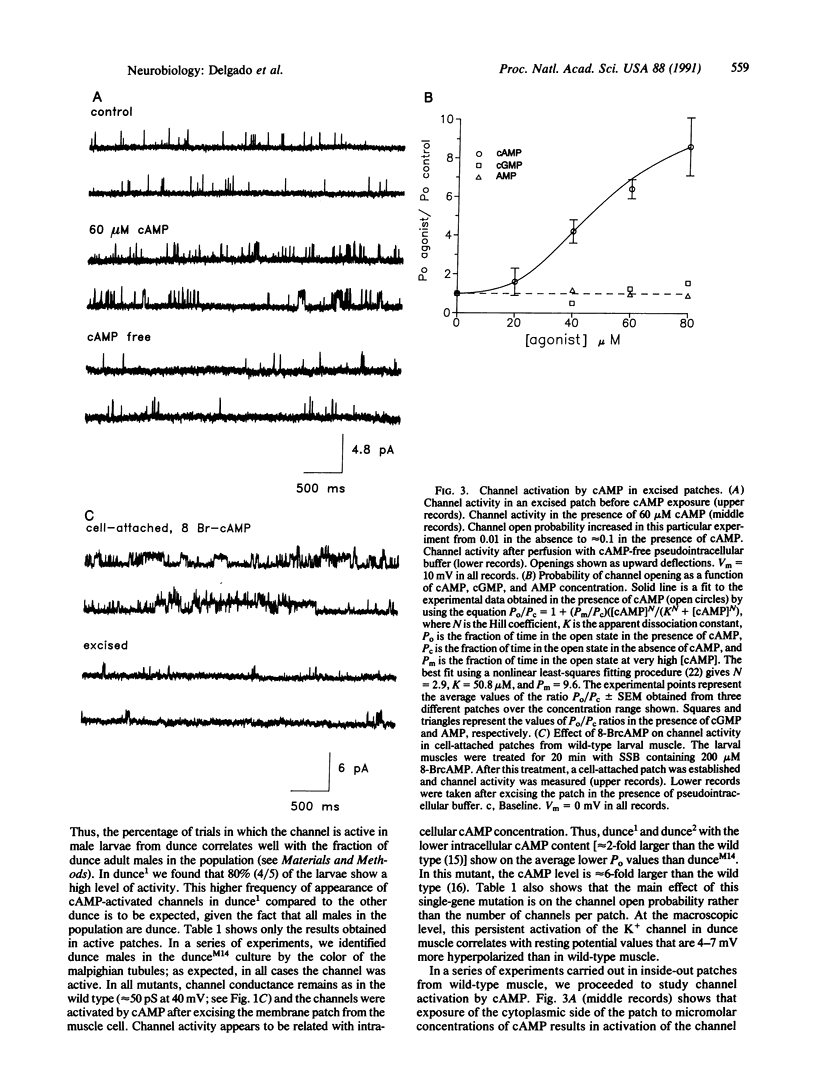
Single-channel recording from longitudinal ventrolateral Drosophila larval muscle reveals the presence of a potassium-selective channel that is directly and reversibly activated by cAMP in a dose-dependent fashion. Activation is specific and it cannot be mimicked by a series of agents that include AMP, cGMP, ATP, inositol trisphosphate, and Ca2+. Channel current records obtained from larval muscle in different dunce mutants possessing abnormally high levels of cAMP show that, in the mutants, the channel displays an increased probability of opening.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byers D., Davis R. L., Kiger J. A., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981 Jan 1;289(5793):79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- Chen C. N., Denome S., Davis R. L. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Davidson N. Isolation of the Drosophila melanogaster dunce chromosomal region and recombinational mapping of dunce sequences with restriction site polymorphisms as genetic markers. Mol Cell Biol. 1984 Feb;4(2):358–367. doi: 10.1128/mcb.4.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Kiger J. A., Jr Dunce mutants of Drosophila melanogaster: mutants defective in the cyclic AMP phosphodiesterase enzyme system. J Cell Biol. 1981 Jul;90(1):101–107. doi: 10.1083/jcb.90.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R., Barla R., Latorre R., Labarca P. L-glutamate activates excitatory and inhibitory channels in Drosophila larval muscle. FEBS Lett. 1989 Jan 30;243(2):337–342. doi: 10.1016/0014-5793(89)80157-7. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Neurogenetic dissection of learning and short-term memory in Drosophila. Annu Rev Neurosci. 1988;11:537–563. doi: 10.1146/annurev.ne.11.030188.002541. [DOI] [PubMed] [Google Scholar]
- Elkins T., Ganetzky B., Wu C. F. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8415–8419. doi: 10.1073/pnas.83.21.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Oberhauser A., Alvarez O., Latorre R. Activation by divalent cations of a Ca2+-activated K+ channel from skeletal muscle membrane. J Gen Physiol. 1988 Jul;92(1):67–86. doi: 10.1085/jgp.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian D. M., Schwarz T. L., Tempel B. L., Timpe L. C., Jan L. Y. Ion channels in Drosophila. Annu Rev Physiol. 1988;50:379–394. doi: 10.1146/annurev.ph.50.030188.002115. [DOI] [PubMed] [Google Scholar]
- Sachs F., Neil J., Barkakati N. The automated analysis of data from single ionic channels. Pflugers Arch. 1982 Dec;395(4):331–340. doi: 10.1007/BF00580798. [DOI] [PubMed] [Google Scholar]
- Salkoff L. B., Tanouye M. A. Genetics of ion channels. Physiol Rev. 1986 Apr;66(2):301–329. doi: 10.1152/physrev.1986.66.2.301. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Wyman R. Genetic modification of potassium channels in Drosophila Shaker mutants. Nature. 1981 Sep 17;293(5829):228–230. doi: 10.1038/293228a0. [DOI] [PubMed] [Google Scholar]
- Solc C. K., Aldrich R. W. Voltage-gated potassium channels in larval CNS neurons of Drosophila. J Neurosci. 1988 Jul;8(7):2556–2570. doi: 10.1523/JNEUROSCI.08-07-02556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solc C. K., Zagotta W. N., Aldrich R. W. Single-channel and genetic analyses reveal two distinct A-type potassium channels in Drosophila. Science. 1987 May 29;236(4805):1094–1098. doi: 10.1126/science.2437657. [DOI] [PubMed] [Google Scholar]
- Tanouye M. A., Kamb C. A., Iverson L. E., Salkoff L. Genetics and molecular biology of ionic channels in Drosophila. Annu Rev Neurosci. 1986;9:255–276. doi: 10.1146/annurev.ne.09.030186.001351. [DOI] [PubMed] [Google Scholar]
- Wu C. F., Ganetzky B. Genetic alteration of nerve membrane excitability in temperature-sensitive paralytic mutants of Drosophila melanogaster. Nature. 1980 Aug 21;286(5775):814–816. doi: 10.1038/286814a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Brainard M. S., Aldrich R. W. Single-channel analysis of four distinct classes of potassium channels in Drosophila muscle. J Neurosci. 1988 Dec;8(12):4765–4779. doi: 10.1523/JNEUROSCI.08-12-04765.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



