ABSTRACT
Hepatitis E virus infections have been continuously reported in Indian subcontinent, Africa, southeast and central Asia, posing great health threats to the public, especially to pregnant women. Hecolin® is the only licensed HEV vaccine developed by Xiamen Innovax Biotech Co., Ltd. Extensive characterizations on antigenicity, physicochemical properties, efficacy in clinical trials, and manufacturing capability have made Hecolin® a promising vaccine for HEV control. However, there are many obstacles in large scale application of Hecolin®. Efforts are needed to further evaluate safety and efficacy in HEV risk populations, and to complement HEV standards for quality control. Passing World Health Organization prequalification and licensing outside China are priorities as these are also hindering Hecolin® promotion. Multilateral cooperation among Chinese vaccine manufacturers, Chinese National Regulatory Authorization (NRA) and WHO will expedite the entrance of Hecolin® into international market, so that Hecolin® could play its due role in global hepatitis E control.
KEYWORDS: epidemiology, Hepatitis E virus, Hecolin®, quality control, vaccine
Introduction
Hepatitis E virus is a nonenveloped virus with a positive-sense, single-stranded RNA genome and belongs to genus Hepevirus of the Hepeviridae family.1,2 HEV infections are responsible for over 50% of acute viral hepatitis cases in areas like India and mainland China.3-6 An estimated 35 million HEV infections occur annually worldwide, resulting in more than 70,000 deaths.7,8 Average mortality rate is between 0.2%–4%, while it can reach to 10%–25% in pregnant women who are at higher risk for HEV.9 Africa and Indian subcontinent, including Kenya, Sudan, India, Pakistan, Bangladesh and Nepal are the most commonly affected areas.10 In 2004 and 2013, large HEV outbreaks were documented among refugees camps in Sudan and Kenya during humanitarian emergencies caused by internal conflicts, and pregnant women suffered disproportionately high mortality from hepatitis E.11-15
HEV can be divided into four genotypes I, II, III, IV.16 Genome homology among four genotypes varies between 70–90%, and above 90% in the same genotype.16 HEV genotypes I, II are restricted to humans, while genotypes III, IV primarily infect mammalian animals with occasional cross-species transmission to humans.17 Despite of the genotypic differences, all known HEVs belong to the same serotype.18 HEV RNA genome contains 3 open reading frames (ORF1–3), and ORF2 codes for the viral capsid protein which is the target of HEV neutralizing antibodies.19
Many HEV vaccine candidates have been under investigation for years, and most of them are based on antigenic HEV ORF2 protein: one recombinant HEV vaccine developed by GlaxoSmithKline achieved good safety and efficacy in volunteers from the Nepalese Army reported in a Phase II clinical trial study.20 Unfortunately, following development of this vaccine was ceased. Another HEV vaccine with trade name as Hecolin®, a recombinant Virus-like particles (VLP) vaccine developed by Xiamen Innovax Biotech Co., Ltd (China), also showed good safety and efficacy in clinical trials and got successfully licensed in China in 2012.21 To date, Hecolin® is the only known HEV vaccine on market. However, Hecolin® is now facing the dilemma in global marketing, and so far it is not able to play a fundamental role in global hepatitis E outbreaks and pandemics control. This review introduced the current epidemiology and vaccine development of HEV, with particular emphasis on obstacles in global application of Hecolin® and possible strategies to cope with these challenges.
Current epidemiology of HEV
Although HEV was first recognized during a waterborne hepatitis epidemic in Kashmir Valley of India in 1978,22 the first documented hepatitis E pandemic could be traced back to New Delhi, India in 1955–1956 after the first direct experimental evidence of the existence of HEV had been founded in patients with hepatitis A-like disease in Tashkent, Uzbekistan in 1983.23-25 Africa, Indian subcontinent and mainland China have been historically reported as areas with high HEV prevalence.25-30,8,31 Geographic distribution of the 4 genotypes of HEV are distinct: genotype I,II are mainly circulating in developing countries and transmitted through contaminated food or water in younger adults, infections in pregnant women usually associated with high incidence of severe complications and mortality;32 genotype III, IV are often founded in developed countries and adapt to a zoonotic transmission way, such as through consumption of uncooked or undercooked meat,33 sporadic infections are often reported in older individuals or those with comorbidities such as chronic liver disease.34 Transfusion transmission and vertical (maternal-fetal) transmission also occur and are well documented.35,36
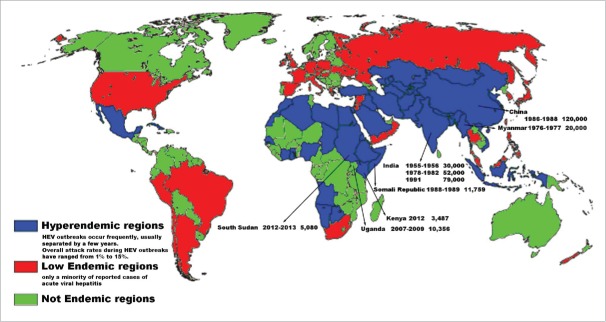
From 1950 to 2000, Hepatitis E outbreaks have been reported in Indian subcontinent, Africa, Mexico, Southeast Asia and Central Asia.25-30 Major documented Hepatitis E outbreaks are listed here chronologically (Fig. 1): New Delhi, India in 1955–1956 (30,000 cases);23,25 Myanmar (20,000 cases) in 1976–1977;37 Kashmir, India (52,000 cases) in 1978–1982;22,38 Xinjiang, China (120,000 cases) in 1986–1988;4 Kanpur, India (79,000 cases) in 1991;28 Kitgum, Uganda(10,356 cases) in 2007–2009.31,39 Since 2010, Africa and India Subcontinent are still highly endemic areas, though the numbers of outbreaks are reduced. Several outbreaks in refugees camps with high mortality rate in pregnant women have drawn the attention of the public.40,41 In mainland China, hepatitis E occurs mainly as sporadic cases and occasional food-borne outbreaks in nursing homes.5,42 In developed countries and areas, sporadic case or small scale outbreaks of HEV infection have been recorded in the USA, European countries (UK, France, the Netherlands, Austria, Spain, Greece and Germany), and developed Asian–Pacific countries (Japan, Korea, Australia and New Zealand).43 The most recent outbreak reported was in France in 2013 caused by the consumption of undercooked piglet's liver and 12 were asymptomatic among the 17 cases infected with HEV genotype III.44
Figure 1.
Global distribution of HEV infection 43.
Indian subcontinent
The persistent high prevalence of HEV in Indian subcontinent has been a major issue for public health. Major hepatitis E outbreaks had been reported in Pakistan, Bangladesh, Sri Lanka, Nepal, etc.8 More than 26 outbreaks in India had been reported in 1955–2012,3 and at least 4 outbreaks were with more than 10,000 cases.25,37,45 In 21st century, Indian subcontinent has still been HEV epidemic area: Pakistan (2005, 2006);46 Nepal (2006, 2009, 2014);47,48 Bangladesh (2010);49,50 India (2012).
Africa
Hepatitis E outbreaks caused 11,759 cases and 346 deaths in Somali Republic in 1988–1989, while over 10,356 cases and 160 deaths in Uganda in 2007–2009.31 The prevalence rate has remained high in these areas since 2010. Hepatitis outbreaks had been reported in Sudan (2010–2011),12,13,51 South Sudan (5,080 cases, 2012–2013),12 and Kenya (3,232 and 255 cases in 2 outbreaks, 2012).11,13 Case fatality rate (CFR) in these outbreaks was astonishingly high: In Darfur of Sudan, reported CFR was 33%;52 in South Sudan, CFR was 10% among pregnant women.15 HEV genotype I and II have been the major circulating types,1,53-57 while genotype III has also been reported in some cases.58,59
Mainland China
There were 9 hepatitis E outbreaks reported in 1982–1986,4 and one hepatitis E pandemic of HEV genotype I happened in Xinjiang in 1986–1988 with over 120,000 persons infected.60 After that, pandemics of HEV have been replaced with sporadic infections and small scale outbreaks. Since 1990, increasing cases in older population have been noticed as several outbreaks in nursing homes have been reported lately.61-66 Along with economic growth and improvement in public health, HEV has been observed to adopt zoonotic transmission pattern with sporadic infections instead of previous waterborne or foodborne pattern in China.67 Epidemiology surveillance based on a community of 400,000 population found that HEV genotype IV was the dominant type and occasional genotype I infections were also revealed, indicating that both genotype I, IV were both circulating in mainland China.67
Licensing and availability of HEV vaccine
HEV vaccine development
For the lack of in vitro culture system, HEV vaccine design strategies using inactivation or attenuation are not feasible.68 So HEV vaccine development has been focused on HEV capsid protein ORF2 (Open reading frame2). ORF2 is 660 aa in length. X-ray crystal structure analysis shows that ORF2 protein monomer contains 3 distinct domains: the shell(S), middle (M), and protruding (P) domains.4,69 Recombinant antigens, DNA vaccine or other new recombinant vaccines candidates have been proposed: various subunit vaccines based on truncated ORF2 purified in E.coli expression system;70-74 VLP vaccines expressed either in E.coli, insect cells (Sf9) or Chinese hamster ovary cell (CHO) expression systems;75-78 DNA vaccine consisting expression vector encoding full length ORF3 or (and) ORF2.79
HEV vaccines that have undergone clinical trials
Currently, there are 3 vaccines have entered into clinical trials. One developed by GSK have finished phase II clinical trial; another developed by Changchun Institute of Biological Products Co., Ltd (China) is now in phase I clinical trial (Clinical trial NO. CXSL1000041); the third one is the previously mentioned Hecolin® which has finished phase III clinical trial and been licensed in China. Hecolin® is based on recombinant HEV ORF2 truncated protein HEV 239 (aa368-aa606) expressed in E.coli expression system. After purification and refolding, HEV 239 can efficiently self-assemble into VLPs particles, which have high structural similarity with the capsid of mature HEV virions.80-82 Notably, HEV 239 has good immunoreactivity with patient serum samples from acute and convalescent phase of hepatitis E patients.75,83 Hecolin® has shown good protection of rhesus monkey from HEV infection in animal challenge studies. In completed phase III clinical trial, the efficacy of Hecolin® has reached to 96%, and the geometric mean antibody titer levels induced by the 3 dose regimen is 15.9 World Health Organization units (Wu)/ml (95% CI:13.8–18.2).84,85 Efficacy after 4.5 y is 86.8% and seropositivity is over 50% in a follow-up study.86 Clinical data have proven good short-term and long-term protection efficacy of Hecolin® in 16–65 y old healthy Chinese people.
Availability of Hecolin®
Hecolin® is a VLP vaccine developed by genetic engineering. The encoding gene is from the ORF2 of a HEV genotype I strain. Innovax Biotech has optimized the manufacturing technique of 50 L fermentation tank for fermentation cultivation. Recombinant proteins are refolded and self-assembled into VLP particles.87 Finished products are available as a pre-filled syringe containing aluminum hydroxide-adsorbed antigen suspended in buffered saline. The complete vaccination series consists of 3 doses (30μg/0.5ml/dose) on a schedule of 0, 1, and 6 months. This manufacturing technique can be easily to scale up and has advantages in quality control.88 Although actual manufacturing output of Hecolin® in Innovax Biotech is about 200,000 doses/year currently, Innovax Biotech has great potential to produce 5000,000 doses/year according to manufacturing capacity planning, so Hecolin® will have the availability to meet HE vaccination need worldwide in the future.
Bottlenecks in the application of HEV vaccine
Bottlenecks of Hecolin® application in mainland China
Although China is the first country that has licensed HEV vaccine, the reported cases of hepatitis E since 2012 have only declined by 7.58% (29,202 cases in 2011, 26,988 cases in 2014).89 According to data released by China Notifiable Disease Reporting System (CNDRS) in 2014, hepatitis E still consisted about 50% of the reported acute hepatitis cases, and the ratio had been increasing annually, the number of HE cases reported after Hecolin® licensing had remained at similar high level. Hecolin® has not been fully exploited its protective potential in hepatitis E outbreaks control in China. Inadequate clinical data and insufficient public awareness of HEV related diseases are the main reasons on account for this difficult situation. Current clinical trial data on efficacy and safety are limited to 16–65 y old healthy people, and no data are available in children under 16 y old, adults over 65 y old, or HEV high risk groups (pregnant women and persons combined with other diseases). Moreover, for the lack of studies in disease burden and cost-benefit of HEV related diseases, the public are currently unaware of the importance of the HEV vaccine and show poor acceptance to this rather new vaccine.
Bottlenecks in the worldwide application of Hecolin®
Hepatitis E has been recognized as a public health problem in many developing countries and Hecolin® is considered as a promising HEV vaccine.90-92 However, worldwide application of Hecolin® is also hindered and HEV risk groups are in the dilemma.
As the only available data are from clinical trials conducted in China where overall hepatitis E incidence is low and genotype IV is the major type, efficacy of Hecolin® in worldwide areas needs further verification, especially in HEV endemic areas where genotype I, II are the prevailing types, like Africa, India subcontinent. Also, the current recommended Immunization Schedule of 3 doses at 0, 1 and 6 months is not suitable for controlling hepatitis E outbreaks in emergencies, especially in refugees camps. Limited data showed that 2 doses (at 0 and 6 months or at 0 and 1 month) could also lead to a high rate of seroconversion and conveyed good protection.20,84,93 In a Phase IIa study of Hecolin® conducted in healthy seronegative persons aged 16–55 y old, seroconversion rates were 98% in the 2-dose group (at 0 and 6 month) compared with 100% in the 3-dose group (at 0, 1 and 6 month) and 8% in the control group; however, the geometric mean concentrations (GMCs) of antibody in 3-dose group were 2-fold higher than those in 2-dose group.93 In a Phase III study of Hecolin®, Vaccine efficacy after first 2 doses (at 0 and 1 months) was 100% (95% CI 9.1–100.0).84 It is absolutely necessary to establish an accelerated vaccination schedule for those who face imminent HEV exposure. Additionally, Hecolin® is a white suspension with each dose of vaccine supplied in a non auto-disable pre-filled syringe (one per package, with a package volume of 100 cm3, 13.2×3.7×2.15 cm); so the package of Hecolin® needs to meet some general requisites for transportation and storage to facilitate centralized procurement of vaccines; improvements on multiple doses, large package, suitable preservative(s) addition are also necessary. The last but the most important reason is that Hecolin® have not received WHO prequalification (PQ) or licenses outside of China yet, so it is not available to HEV endemic countries receiving vaccine through United Nations International Children's Emergency Fund supply division or other United Nations procurement agencies, especially to the refugees at high risk.94
Current status and bottlenecks in commercial scale production and quality control of HEV vaccine
Efforts have been made to implement reliable production and quality control of HEV vaccine. First of all, production and quality control of Hecolin® have met the general requirements of WHO and Chinese Pharmacopoeia (3rd edition). Then, by establishing a series of in-house references and utilizing a toolbox combining biophysical, biochemical, and immunochemical methods, Innovax Biotech have showed that antigen characteristics was comparable from a bench scale to a manufacturing scale, ensuring process reproducibility and product consistency in the vaccine production process.88 National Institutes for Food and Drug Control, China (NIFDC) have analyzed the potency consistency among 14 released batches of Hecolin® in animal models; the average potency was 0.05 and all analyzed batches showed consistent potency (unpublished data). As the size of released batches of Hecolin® is still small, the quality consistency of mass production still needs further assessment.
Although WHO guideline on Hecolin® production and quality control has not been issued, some HEV related standards have already been established: the first WHO HEV IgG standard was established in 2002 (NIBSC code: 95/584);95 the first WHO HEV RNA standard was established in 2013 (NIBSC code: 6329/10, HEV genotype IIIa) and can be used in quality control of diagnostic reagents and quantification of HEV RNA in serum; Chinese national anti-HEV IgG linear standard calibrated against WHO anti-HEV IgG reference was established by NIFDC in 2008, and it has been used for quantification of anti-HEV IgG in serum samples from clinical trials. But both WHO and Chinese national standards for antigen quality control and potency evaluation of HEV vaccine are still lacking.
Strategies to overcome the bottleneck on HEV vaccine application
WHO, NIFDC and Innovax Biotech have carried out the work from different aspects to promote the worldwide application of HEV vaccine for HEV control.
WHO have issued several guidelines for the prevention and control hepatitis E epidemics. Technique report “Waterborne Outbreaks of Hepatitis E: Recognition, Investigation and Control” issued by WHO (Jun, 2014) providing knowledge on epidemiology, diagnosis, clinical symptoms of hepatitis E, assisting public health authorities to cope with hepatitis E outbreaks;96 in the same year, recommendations of HEV working group on the use of hepatitis E vaccine was released by WHO(Dec, 2014).97 In May, 2015, WHO issued a regularly position paper on HEV vaccine;98 in this paper, WHO asked for further investigation on safety and efficacy of Hecolin® in expanded populations (including the elderly, children, pregnant women, etc.) and when co-administered with another vaccines, as well as the cross-protection efficacy against different genotypes other than genotype I. These issued documents and related initiatives by WHO have laid the groundwork for global promotion and application of HEV vaccine.
NIFDC has been conducting the research to establish HEV standards for vaccine evaluation. NIFDC has recently launched the development of national HEV antigen standard and vaccine potency standard. Besides, for the lack of HEV infection mouse model, a competitive ELISA method has been developed to evaluate vaccine potency in vitro. 8G12, a well-characterized mouse broad neutralizing monoclonal antibody developed by Innovax Biotech, can recognize the dominant neutralizing epitope on HEV capsid protein. Previous studies have shown that 8G12 can strongly compete the binding of human and Macaques rhesus HEV convalescent sera as well as Hecolin® vaccinated sera to HEV ORF2 protein,99 indicating that most neutralizing antibodies elicited by HEV natural infection or Hecolin® vaccination might recognize similar epitope as 8G12. 99-101 Thus, the feasibility to use 8G12 as a probe to analyze the correlation between protection efficacy and 8G12-like antibodies elicited by HEV vaccine immunization was explored. Preliminary data have suggested that 8G12 can be used as substitute standard for serum samples from preclinical animal studies and clinical trials in vaccine evaluation (unpublished data). This would greatly facilitate the preclinical evaluation of other new HEV vaccines and post-license surveillance of Hecolin®.
Innovax Biotech has been further evaluating Hecolin® in clinical trials, as well as stability assessment in Hecolin® large-scale production. After Phase III clinical study for Hecolin®, Innovax Biotech has continued a 4.5-year follow-up study in the original cohorts of vaccine group (56302 subjects) and control group (56302 subjects). 60 cases of hepatitis E were identified: 7 cases were confirmed in the vaccine group (0.3 cases/10,000 person-years), and 53 cases in the control group (2.1 cases/10,000 person-years), representing a vaccine efficacy of 86.8% (95% CI: 71–94%). 87% of those who received 3 doses of Hecolin® maintained anti-HEV antibodies positive for at least 4.5 y compared with 9% in control group.86 Another phase IV clinical study have been conducting to determine the safety and immunogenicity of Hecolin® in people older than 65 years, and evaluate the efficacy of hepatitis E vaccine in this population(Clinical trial: NCT02189603). For scale-up production, stability and product consistency of Hecolin®, multiple methods including differential scanning calorimetry (DSC), surface plasma resonance (SPR), analytical ultracentrifugation (AUC), mAb binding profiling were applied in the comprehensive characterization of the recombinant hepatitis E VLP in Hecolin®. Data showed consistency of vaccine products from different batches in particle size, thermo stability, and immunogenicity, proving that antigen production process was robust and scalable during the manufacturing of Hecolin®. Furthermore, effects of aluminum containing adjuvant on structural characteristics, biological activity and stability of Hecolin® have also been analyzed. Both vaccine bulk and product with aluminum adjuvant showed good thermo stability measured by DSC (Tm = 70–72°C).88 As antigenicity or the binding activity to various antibodies is a critical attribute, Innovax Biotech have produced a panel of mouse monoclonal antibodies with different epitopes, like 8C11, 8H3, 13D8,12A10 and 16D7. Base on these mAbs, one-site binding and label-free assay (SPR) and 2-site binding assay (sandwich ELISA) have been carried out to evaluate the antibody binding activity of Hecolin®. These mAbs provides a convenient toolbox for HEV vaccine antigenicity control of different batches.88
Summary
In 2013, Chinese NRA passed the assessment in 2010 and the reassessment in 2014 by WHO. NIFDC became the seventh WHO Collaborating Center for standardization and evaluation of biologics in 2013. Since then, Chinese vaccines are added to the WHO international vaccine purchase list. This paves the road for vaccines produced by Chinese manufacturers to enter into international market.102 So far, Chinese live attenuated Japanese encephalitis vaccine and influenza vaccine have already passed WHO prequalification, showing that China is contributing to worldwide disease prevention and control. For Hecolin®, top priorities need to be done, including further verification on the safety and efficacy on HEV risk groups, optimization of immunization schedule, passing WHO prequalification, licensing in HEV endemic countries, and development of vaccine reference materials for quality control. Collaborative efforts from vaccine manufacturers, Chinese NRA and WHO will bring Hecolin® to the world and confer benefits to people with high HEV infection risk.
Abbreviations
- AUC
Analytical ultracentrifugation
- CFR
Case fatality rate
- CNDRS
China Notifiable Disease Reporting System
- DSC
differential scanning calorimetry
- GMCs
geometric mean concentrations
- HEV
hepatitis E virus;
- NIFDC
National Institutes for Food and Drug Control, China
- NRA
Chinese National Regulatory Authorization;
- PQ
prequalification
- SPR
surface plasma resonance
- VLP
Virus-like particles
- WHO
World Health Organization
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Zhiyi Jing from National Institute of Biological Sciences, Beijing for plotting global prevalence diagram of HEV.
Funding
This work was supported by 863 Project (No. 2012AA02A402) from the Ministry of Science and Technology, China.
References
- [1].Buisson Y, Grandadam M, Nicand E, Cheval P, van Cuyck-Gandre H, Innis B, Rehel P, Coursaget P, Teyssou R, Tsarev S. Identification of a novel hepatitis E virus in Nigeria. J Gen Virol 2000; 81:903-9; PMID:10725415; http://dx.doi.org/ 10.1099/0022-1317-81-4-903 [DOI] [PubMed] [Google Scholar]
- [2].Ahmad I, Holla RP, Jameel S. Molecular virology of hepatitis E virus. Virus Res 2011; 161:47-58; PMID:21345356; http://dx.doi.org/ 10.1016/j.virusres.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kmush B, Wierzba T, Krain L, Nelson K, Labrique AB. Epidemiology of hepatitis E in low- and middle-income countries of Asia and Africa. Semin Liver Dis 2013; 33:15-29; PMID:23564386; http://dx.doi.org/ 10.1055/s-0033-1338111 [DOI] [PubMed] [Google Scholar]
- [4].Zhuang H, Cao XY, Liu CB, Wang GM. Epidemiology of hepatitis E in China. Gastroenterologia Japonica 1991; 26 Suppl 3:135-8; PMID:1909252 [DOI] [PubMed] [Google Scholar]
- [5].Jia Z, Yi Y, Liu J, Cao J, Zhang Y, Tian R, Yu T, Wang H, Wang X, Su Q, et al.. Epidemiology of hepatitis E virus in China: results from the Third National Viral Hepatitis Prevalence Survey, 2005-2006. PloS One 2014; 9:e110837; PMID:25360522; http://dx.doi.org/ 10.1371/journal.pone.0110837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Khuroo MS, Rustgi VK, Dawson GJ, Mushahwar IK, Yattoo GN, Kamili S, Khan BA. Spectrum of hepatitis E virus infection in India. J Med Virol 1994; 43:281-6; PMID:7931190; http://dx.doi.org/ 10.1002/jmv.1890430316 [DOI] [PubMed] [Google Scholar]
- [7].Sridhar S, Lau SK, Woo PC. Hepatitis E: A disease of reemerging importance. J Formosan Med Assoc = Taiwan yi zhi 2015; 114:681-90; PMID:25773541; http://dx.doi.org/ 10.1016/j.jfma.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aggarwal RGS. The global prevalence of hepatitis E virus infection and susceptibility: a systematic review Geneva, Switz, World Health Organization; 2010 [Google Scholar]
- [9].Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012; 55:988-97; PMID:22121109; http://dx.doi.org/ 10.1002/hep.25505 [DOI] [PubMed] [Google Scholar]
- [10].Lanini S, Garbuglia AR, Lapa D, Puro V, Navarra A, Pergola C, Ippolito G, Capobianchi MR. Epidemiology of HEV in the Mediterranean basin: 10-year prevalence in Italy. BMJ Open 2015; 5:e007110; PMID:26173715; http://dx.doi.org/ 10.1136/bmjopen-2014-007110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guthmann JP, Klovstad H, Boccia D, Hamid N, Pinoges L, Nizou JY, Tatay M, Diaz F, Moren A, Grais RF, et al.. A large outbreak of hepatitis E among a displaced population in Darfur, Sudan, 2004: the role of water treatment methods. Clin Infect Dis: Off Pub Infect Dis Soc Am 2006; 42:1685-91; PMID:16705572; http://dx.doi.org/ 10.1086/504321 [DOI] [PubMed] [Google Scholar]
- [12].Investigation of hepatitis E outbreak among refugees - Upper Nile, South Sudan, 2012–2013. MMWR Morbid Mortal Weekly Rep 2013; 62:581-6; PMID:23884344 [PMC free article] [PubMed] [Google Scholar]
- [13].Ahmed JA, Moturi E, Spiegel P, Schilperoord M, Burton W, Kassim NH, Mohamed A, Ochieng M, Nderitu L, Navarro-Colorado C, et al.. Hepatitis E outbreak, Dadaab refugee camp, Kenya, 2012. Emerg Infect Dis 2013; 19:1010-2; PMID:23735820; http://dx.doi.org/ 10.3201/eid1906.130275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Browne LB, Menkir Z, Kahi V, Maina G, Asnakew S, Tubman M, Elyas HZ, Nigatu A, Dak D, Maung UA, et al.. Notes from the field: hepatitis E outbreak among refugees from South Sudan - Gambella, Ethiopia, April 2014-January 2015. MMWR Morbidity Mortality Weekly Rep 2015; 64:537; PMID:25996097 [PMC free article] [PubMed] [Google Scholar]
- [15].Centers for Disease C, Prevention. Investigation of hepatitis E outbreak among refugees - Upper Nile, South Sudan, 2012–2013. MMWR Morbidity Mortality Weekly Report 2013; 62:581-6; PMID:23884344 [PMC free article] [PubMed] [Google Scholar]
- [16].Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol 2006; 16:5-36; PMID:16175650; http://dx.doi.org/ 10.1002/rmv.482 [DOI] [PubMed] [Google Scholar]
- [17].Perez-Gracia MT, Suay B, Mateos-Lindemann ML. Hepatitis E: an emerging disease. Infect, Genet Evolution: J Mol EpidemiolEvolutionary Genet Infect Dis 2014; 22:40-59; PMID:24434240; http://dx.doi.org/ 10.1016/j.meegid.2014.01.002 [DOI] [PubMed] [Google Scholar]
- [18].Zhang J, Shih JW, Wu T, Li SW, Xia NS. Development of the hepatitis E vaccine: from bench to field. Semin Liver Dis 2013; 33:79-88; PMID:23564392; http://dx.doi.org/ 10.1055/s-0033-1338116 [DOI] [PubMed] [Google Scholar]
- [19].Bradley DW. Hepatitis E virus: a brief review of the biology, molecular virology, and immunology of a novel virus. J Hepatol 1995; 22:140-5; PMID:7602068 [PubMed] [Google Scholar]
- [20].Shrestha MP, Scott RM, Joshi DM, Mammen MP Jr, Thapa GB, Thapa N, Myint KS, Fourneau M, Kuschner RA, Shrestha SK, et al.. Safety and efficacy of a recombinant hepatitis E vaccine. New Engl J Med 2007; 356:895-903; PMID:17329696; http://dx.doi.org/ 10.1056/NEJMoa061847 [DOI] [PubMed] [Google Scholar]
- [21].Haffar S, Bazerbachi F, Lake JR. Making the case for the development of a vaccination against hepatitis E virus. Liver Int: Off J Int Assoc Study Liver 2015; 35:311-6; PMID:24836400; http://dx.doi.org/ 10.1111/liv.12590 [DOI] [PubMed] [Google Scholar]
- [22].Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med 1980; 68:818-24; PMID:6770682; http://dx.doi.org/ 10.1016/0002-9343(80)90200-4 [DOI] [PubMed] [Google Scholar]
- [23].Gupta DN, Smetana HF. The histopathology of viral hepatitis as seen in the Delhi epidemic (1955–56). Indian J Med Res 1957; 45:101-13; PMID:13438544 [PubMed] [Google Scholar]
- [24].Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, Poleschuk VF. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 1983; 20:23-31; PMID:6409836; http://dx.doi.org/ 10.1159/000149370 [DOI] [PubMed] [Google Scholar]
- [25].Wong DC, Purcell RH, Sreenivasan MA, Prasad SR, Pavri KM. Epidemic and endemic hepatitis in India: evidence for a non-A, non-B hepatitis virus aetiology. Lancet 1980; 2:876-9; PMID:6107544; http://dx.doi.org/ 10.1016/S0140-6736(80)92045-0 [DOI] [PubMed] [Google Scholar]
- [26].Khuroo MS. Chronic liver disease after non-A, non-B hepatitis. Lancet 1980; 2:860-1; PMID:6107528 [PubMed] [Google Scholar]
- [27].Velazquez O, Stetler HC, Avila C, Ornelas G, Alvarez C, Hadler SC, Bradley DW, Sepulveda J. Epidemic transmission of enterically transmitted non-A, non-B hepatitis in Mexico, 1986–1987. Jama 1990; 263:3281-5; PMID:2112204; http://dx.doi.org/ 10.1001/jama.1990.03440240071018 [DOI] [PubMed] [Google Scholar]
- [28].Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organization 1992; 70:597-604; PMID:1464145 [PMC free article] [PubMed] [Google Scholar]
- [29].Corwin AL, Khiem HB, Clayson ET, Pham KS, Vo TT, Vu TY, Cao TT, Vaughn D, Merven J, Richie TL, et al.. A waterborne outbreak of hepatitis E virus transmission in southwestern Vietnam. Am J Trop Med Hygiene 1996; 54:559-62; PMID:8686771 [DOI] [PubMed] [Google Scholar]
- [30].Utsumi T, Hayashi Y, Lusida MI, Amin M, Soetjipto, Hendra A, Soetjiningsih, Yano Y, Hotta H. Prevalence of hepatitis E virus among swine and humans in two different ethnic communities in Indonesia. Arch Virol 2011; 156:689-93; PMID:21191625; http://dx.doi.org/ 10.1007/s00705-010-0883-x [DOI] [PubMed] [Google Scholar]
- [31].Kim JH, Nelson KE, Panzner U, Kasture Y, Labrique AB, Wierzba TF. A systematic review of the epidemiology of hepatitis E virus in Africa. BMC Infect Dis 2014; 14:308; PMID:24902967; http://dx.doi.org/ 10.1186/1471-2334-14-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khuroo MS. Discovery of hepatitis E: the epidemic non-A, non-B hepatitis 30 years down the memory lane. Virus Res 2011; 161:3-14; PMID:21320558; http://dx.doi.org/ 10.1016/j.virusres.2011.02.007 [DOI] [PubMed] [Google Scholar]
- [33].Purcell RH, Emerson SU. Hidden danger: the raw facts about hepatitis E virus. J Infect Dis 2010; 202:819-21; PMID:20695795; http://dx.doi.org/ 10.1086/655900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ramachandran J, Eapen CE, Kang G, Abraham P, Hubert DD, Kurian G, Hephzibah J, Mukhopadhya A, Chandy GM. Hepatitis E superinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol 2004; 19:134-8; PMID:14731121; http://dx.doi.org/ 10.1111/j.1440-1746.2004.03188.x [DOI] [PubMed] [Google Scholar]
- [35].Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J, et al.. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet 2014; 384:1766-73; PMID:25078306; http://dx.doi.org/ 10.1016/S0140-6736(14)61034-5 [DOI] [PubMed] [Google Scholar]
- [36].Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepatitis 2003; 10:61-9; PMID:12558914; http://dx.doi.org/ 10.1046/j.1365-2893.2003.00398.x [DOI] [PubMed] [Google Scholar]
- [37].Khuroo MS. Hepatitis E: the enterically transmitted non-A, non-B hepatitis. Indian J Gastroenterol: Off J Indian Soc Gastroenterol 1991; 10:96-100; PMID:1916971 [PubMed] [Google Scholar]
- [38].Chakraborty S, Datta M, Pasha ST, Kumar S. Non-A non-B viral hepatitis: a common-source outbreak traced to sewage contamination of drinking water. J Commun Dis 1982; 14:41-6; PMID:6815259 [PubMed] [Google Scholar]
- [39].Teshale EH, Howard CM, Grytdal SP, Handzel TR, Barry V, Kamili S, Drobeniuc J, Okware S, Downing R, Tappero JW, et al.. Hepatitis E epidemic, Uganda. Emerg Infect Dis 2010; 16:126-9; PMID:20031058; http://dx.doi.org/ 10.3201/eid1601.090764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kerry Thomson JLDJLRL. Investigation of Hepatitis E Outbreak Among Refugees — Upper Nile, South Sudan, 2012–2013. Morbidity and Mortality Weekly Report (MMWR), CDC 2013; 62:581-6 [PMC free article] [PubMed] [Google Scholar]
- [41].H. LBBZMVKGMSAMTHZEANDDUAMJ Notes from the Field: Hepatitis E Outbreak Among Refugees from South Sudan — Gambella, Ethiopia, April 2014–January 2015. Morbidity and Mortality Weekly Report (MMWR), CDC 2015; 64:537. [PMC free article] [PubMed] [Google Scholar]
- [42].XU Ke WA-p, ZHANG Jun, TIAN Hua, YAN Qiang, WU Zhi-ming, ZHANG Jun, ZHANG Xue-feng, TANG Fen-yang. Outbreak of hepatitis E among aged people in a nursing home. Chinese J Zoonoses 2012; 28:115-9 [Google Scholar]
- [43].Aggarwal R. The Global Prevalence of Hepatitis E Virus Infection and Susceptibility: A Systematic Review. In: The Department of Immunization VaB ed.: World Health Organization, 2010 [Google Scholar]
- [44].Guillois Y, Abravanel F, Miura T, Pavio N, Vaillant V, Lhomme S, Le Guyader FS, Rose N, Le Saux JC, King LA, et al.. High Proportion of Asymptomatic Infections in an Outbreak of Hepatitis E Associated With a Spit-Roasted Piglet, France, 2013. Clin Infect Dis: Off Pub Infect Dis Soc Am 2016; 62:351-7; PMID:26429341; http://dx.doi.org/ 10.1093/cid/civ862 [DOI] [PubMed] [Google Scholar]
- [45].Arankalle VA, Chadha MS, Tsarev SA, Emerson SU, Risbud AR, Banerjee K, Purcell RH. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc Nat Acad Sci U S A 1994; 91:3428-32; PMID:8159764; http://dx.doi.org/ 10.1073/pnas.91.8.3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bashir K, Hussain N, Hasnain S, Elahi S. Seroprevalence of hepatitis E virus immunoglobulin G and M antibodies in adults: a hospital-based study. Indian J Med Microbiol 2009; 27:139-41; PMID:19384037; http://dx.doi.org/ 10.4103/0255-0857.49427 [DOI] [PubMed] [Google Scholar]
- [47].Gurley ES, Hossain MJ, Paul RC, Sazzad HM, Islam MS, Parveen S, Faruque LI, Husain M, Ara K, Jahan Y, et al.. Outbreak of hepatitis E in urban bangladesh resulting in maternal and perinatal mortality. Clin Infect Dis: Off Pub Infect Dis Soc Am 2014; 59:658-65; PMID:24855146; http://dx.doi.org/ 10.1093/cid/ciu383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sharma SP. Hepatitis E and cholera outbreak in Kathmandu. CMAJ 2006; 175:860; PMID:17030933; http://dx.doi.org/ 10.1503/cmaj.061166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Labrique AB, Zaman K, Hossain Z, Saha P, Yunus M, Hossain A, Ticehurst JR, Nelson KE. Epidemiology and risk factors of incident hepatitis E virus infections in rural Bangladesh. Am J Epidemiol 2010; 172:952-61; PMID:20801864; http://dx.doi.org/ 10.1093/aje/kwq225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].icddr b Health and Science Bulletin. 2009; 7:14-20 [Google Scholar]
- [51].Rayis DA, Jumaa AM, Gasim GI, Karsany MS, Adam I. An outbreak of hepatitis E and high maternal mortality at Port Sudan, Eastern Sudan. PathogensGlobal Health 2013; 107:66-8; PMID:23683332; http://dx.doi.org/ 10.1179/2047773213Y.0000000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Boccia D, Guthmann JP, Klovstad H, Hamid N, Tatay M, Ciglenecki I, Nizou JY, Nicand E, Guerin PJ. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin Infect Dis 2006; 42:1679-84; PMID:16705571; http://dx.doi.org/ 10.1086/504322 [DOI] [PubMed] [Google Scholar]
- [53].Escriba JM, Nakoune E, Recio C, Massamba PM, Matsika-Claquin MD, Goumba C, Rose AM, Nicand E, Garcia E, Leklegban C, et al.. Hepatitis E, Central African Republic. Emerg Infect Dis 2008; 14:681-3; PMID:18394300; http://dx.doi.org/ 10.3201/eid1404.070833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].He J, Binn LN, Tsarev SA, Hayes CG, Frean JA, Isaacson M, Innis BL. Molecular characterization of a hepatitis E virus isolate from Namibia. J Biomed Sci 2000; 7:334-8; PMID:10895057; http://dx.doi.org/ 10.1007/BF02253253 [DOI] [PubMed] [Google Scholar]
- [55].Nicand E, Armstrong GL, Enouf V, Guthmann JP, Guerin JP, Caron M, Nizou JY, Andraghetti R. Genetic heterogeneity of hepatitis E virus in Darfur, Sudan, and neighboring Chad. J Med Virol 2005; 77:519-21; PMID:16254969; http://dx.doi.org/ 10.1002/jmv.20487 [DOI] [PubMed] [Google Scholar]
- [56].Delarocque-Astagneau E, Abravanel F, Moshen A, Le Fouler L, Gad RR, El-Daly M, Ibrahim EM, El-Aidy S, Lashin T, El-Hoseiny M, et al.. Epidemiological and virological characteristics of symptomatic acute hepatitis E in Greater Cairo, Egypt. Clin Microbiol Infect 2012; 18:982-8; PMID:22264267; http://dx.doi.org/ 10.1111/j.1469-0691.2011.03727.x [DOI] [PubMed] [Google Scholar]
- [57].Maila HT, Bowyer SM, Swanepoel R. Identification of a new strain of hepatitis E virus from an outbreak in Namibia in 1995. J Gen Virol 2004; 85:89-95 [DOI] [PubMed] [Google Scholar]
- [58].Epelboin L, Nicand E, Roussin C, Lernout T, Pettinelli ME, Tesse S, Ali R, Aubry P. A sporadic case of genotype 3f acute hepatitis E in Mayotte. Med et Maladies Infect 2011; 41:392-4; PMID:21493025; http://dx.doi.org/ 10.1016/j.medmal.2011.02.013 [DOI] [PubMed] [Google Scholar]
- [59].Kamel AH, Ali MA, El-Nady HG, Deraz A, Aho S, Pothier P, Belliot G. Presence of enteric hepatitis viruses in the sewage and population of Greater Cairo. Clin Microbiol Infect 2011; 17:1182-5; PMID:21375654; http://dx.doi.org/ 10.1111/j.1469-0691.2011.03461.x [DOI] [PubMed] [Google Scholar]
- [60].Wang HY. [Investigation of an outbreak of HNANB[E] in Shufu County, Xinjiang]. Zhonghua liu xing bing xue za zhi=Zhonghua liuxingbingxue zazhi 1989; 10:270-3; PMID:2515002 [PubMed] [Google Scholar]
- [61].Li J. Clinical analysis of 70 elderly patients with viral hepatitis E China. Modern Med 2013; 20:41-3 [Google Scholar]
- [62].Wang JQ, Liu JH, Guan CP, Zheng XY. Epidemiological characteristics of viral hepatitis in Fuzhou in 2007–2011. China Prev Med 2013; 14:43-6 [Google Scholar]
- [63].Li FR, Ding H, Yao J, Wang XL, Tang YF, Bao ZF, Wang ZF, Gan WQ, Mao HY, Fontaine RE, Zeng G. An Outbreak of HEV Among Aged People in a Nursing Home. Chinese J Vaccines Immun 2007; 13:473-6 [Google Scholar]
- [64].Tang QR, Zhu DD Epidemiology of hepatitis E in Ningbo, 1999 - 2008. Dis Surveillance 2010; 25:258-60 [Google Scholar]
- [65].Yu WH. Molecular epidemiology of swine HEV in a village of Yunnan. Chinese J Zoonoses 2012; 28:274-7 [Google Scholar]
- [66].Wang YP, Zhao WH, Li AM, Li B, Ding L Epidemiology of Hepatitis E in Jiangdu city. Jiangsu Health Care 2011; 13:2–3 [Google Scholar]
- [67].Zhu FC, Huang SJ, Wu T, Zhang XF, Wang ZZ, Ai X, Yan Q, Yang CL, Cai JP, Jiang HM, et al.. Epidemiology of zoonotic hepatitis E: a community-based surveillance study in a rural population in China. PloS One 2014; 9:e87154; PMID:24498033; http://dx.doi.org/ 10.1371/journal.pone.0087154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Okamoto H. Culture systems for hepatitis E virus. J Gastroenterol 2013; 48:147-58; PMID:23104469; http://dx.doi.org/ 10.1007/s00535-012-0682-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Guu TS, Liu Z, Ye Q, Mata DA, Li K, Yin C, Zhang J, Tao YJ. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc Natl Acad Sci U S A 2009; 106:12992-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Purdy MA, McCaustland KA, Krawczynski K, Spelbring J, Reyes GR, Bradley DW. Preliminary evidence that a trpE-HEV fusion protein protects cynomolgus macaques against challenge with wild-type hepatitis E virus (HEV). J M Virol 1993; 41:90-4; PMID:8228944; http://dx.doi.org/ 10.1002/jmv.1890410118 [DOI] [PubMed] [Google Scholar]
- [71].Zhang JZ, Ng MH, Xia NS, Lau SH, Che XY, Chau TN, Lai ST, Im SW. Conformational antigenic determinants generated by interactions between a bacterially expressed recombinant peptide of the hepatitis E virus structural protein. J Med Virol 2001; 64:125-32; PMID:11360244; http://dx.doi.org/ 10.1002/jmv.1027 [DOI] [PubMed] [Google Scholar]
- [72].Im SW, Zhang JZ, Zhuang H, Che XY, Zhu WF, Xu GM, Li K, Xia NS, Ng MH. A bacterially expressed peptide prevents experimental infection of primates by the hepatitis E virus. Vaccine 2001; 19:3726-32; PMID:11395207; http://dx.doi.org/ 10.1016/S0264-410X(01)00100-1 [DOI] [PubMed] [Google Scholar]
- [73].Yarbough PO. Hepatitis E virus. Advances in HEV biology and HEV vaccine approaches. Intervirology 1999; 42:179-84; PMID:10516473; http://dx.doi.org/ 10.1159/000024978 [DOI] [PubMed] [Google Scholar]
- [74].Arankalle VA, Lole KS, Deshmukh TM, Srivastava S, Shaligram US. Challenge studies in Rhesus monkeys immunized with candidate hepatitis E vaccines: DNA, DNA-prime-protein-boost and DNA-protein encapsulated in liposomes. Vaccine 2009; 27:1032-9; PMID:19095027; http://dx.doi.org/ 10.1016/j.vaccine.2008.11.097 [DOI] [PubMed] [Google Scholar]
- [75].Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, Ge SX, Xian YL, Pang SQ, Ng MH, et al.. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine 2005; 23:2893-901; PMID:15780738; http://dx.doi.org/ 10.1016/j.vaccine.2004.11.064 [DOI] [PubMed] [Google Scholar]
- [76].Purcell RH, Nguyen H, Shapiro M, Engle RE, Govindarajan S, Blackwelder WC, Wong DC, Prieels JP, Emerson SU. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine 2003; 21:2607-15; PMID:12744897; http://dx.doi.org/ 10.1016/S0264-410X(03)00100-2 [DOI] [PubMed] [Google Scholar]
- [77].Huang WJ, Zhang HY, Harrison TJ, Lan HY, Huang GY, Wang YC. Immunogenicity and protective efficacy in rhesus monkeys of a recombinant ORF2 protein from hepatitis E virus genotype 4. Archives Virol 2009; 154:481-8; PMID:19240977; http://dx.doi.org/ 10.1007/s00705-009-0335-7 [DOI] [PubMed] [Google Scholar]
- [78].Zhang M, Emerson SU, Nguyen H, Engle R, Govindarajan S, Blackwelder WC, Gerin J, Purcell RH. Recombinant vaccine against hepatitis E: duration of protective immunity in rhesus macaques. Vaccine 2002; 20:3285-91; PMID:12213398; http://dx.doi.org/ 10.1016/S0264-410X(02)00314-6 [DOI] [PubMed] [Google Scholar]
- [79].Kamili S, Spelbring J, Carson D, Krawczynski K. Protective efficacy of hepatitis E virus DNA vaccine administered by gene gun in the cynomolgus macaque model of infection. J Infect Dis 2004; 189:258-64; PMID:14722891; http://dx.doi.org/ 10.1086/380801 [DOI] [PubMed] [Google Scholar]
- [80].Yang C, Pan H, Wei M, Zhang X, Wang N, Gu Y, Du H, Zhang J, Li S, Xia N. Hepatitis E virus capsid protein assembles in 4 M urea in the presence of salts. Protein Sci: Pub Protein Soc 2013; 22:314-26; PMID:23281113; http://dx.doi.org/ 10.1002/pro.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Li SW, Zhang J, He ZQ, Gu Y, Liu RS, Lin J, Chen YX, Ng MH, Xia NS. Mutational analysis of essential interactions involved in the assembly of hepatitis E virus capsid. J Biol Chem 2005; 280:3400-6; PMID:15557331; http://dx.doi.org/ 10.1074/jbc.M410361200 [DOI] [PubMed] [Google Scholar]
- [82].Li S, Tang X, Seetharaman J, Yang C, Gu Y, Zhang J, Du H, Shih JW, Hew CL, Sivaraman J, et al.. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus-host interaction. PLoS Pathogens 2009; 5:e1000537; PMID:19662165; http://dx.doi.org/ 10.1371/journal.ppat.1000537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Li F, Torresi J, Locarnini SA, Zhuang H, Zhu W, Guo X, Anderson DA. Amino-terminal epitopes are exposed when full-length open reading frame 2 of hepatitis E virus is expressed in Escherichia coli, but carboxy-terminal epitopes are masked. J Med Virol 1997; 52:289-300; PMID:9210039; http://dx.doi.org/ 10.1002/(SICI)1096-9071(199707)52:3%3c289::AID-JMV10%3e3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- [84].Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, et al.. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010; 376:895-902; PMID:20728932; http://dx.doi.org/ 10.1016/S0140-6736(10)61030-6 [DOI] [PubMed] [Google Scholar]
- [85].Zhao Q, Zhang J, Wu T, Li SW, Ng MH, Xia NS, Shih JW. Antigenic determinants of hepatitis E virus and vaccine-induced immunogenicity and efficacy. J Gastroenterol 2013; 48:159-68; PMID:23149436; http://dx.doi.org/ 10.1007/s00535-012-0701-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, Wang H, Jiang HM, Wang YJ, Yan Q, et al.. Long-term efficacy of a hepatitis E vaccine. New Eng J Med 2015; 372:914-22; PMID:25738667; http://dx.doi.org/ 10.1056/NEJMoa1406011 [DOI] [PubMed] [Google Scholar]
- [87].Wang X, Li M, Li S, Wu T, Zhang J, Xia N, Zhao Q. Prophylaxis against hepatitis E: at risk populations and human vaccines. Exp Rev Vacc 2016:1-13; PMID:26775537; http://dx.doi.org/ 10.1586/14760584.2016.1143365 [DOI] [PubMed] [Google Scholar]
- [88].Zhang X, Wei M, Pan H, Lin Z, Wang K, Weng Z, Zhu Y, Xin L, Zhang J, Li S, et al.. Robust manufacturing and comprehensive characterization of recombinant hepatitis E virus-like particles in Hecolin®. Vaccine 2014; 32:4039-50; PMID:24892250; http://dx.doi.org/ 10.1016/j.vaccine.2014.05.064 [DOI] [PubMed] [Google Scholar]
- [89].Reports on Notifiable Infectious Disease in China. Chinese Center for Disease Control and Prevention. Available online at: http://www.chinacdc.cn/tjsj/fdcrbbg/
- [90].Joshi RM. Hepatitis E virus: a renewed hope with Hecolin. Clin Microbiol 2014; 4; PMID:2511071925110719 [Google Scholar]
- [91].Labrique AB, Sikder SS, Krain LJ, West KP Jr, Christian P, Rashid M, Nelson KE. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis 2012; 18:1401-4; PMID:22931753; http://dx.doi.org/ 10.3201/eid1809.120241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Park SB. Hepatitis E vaccine debuts. Nature 2012; 491:21-2; PMID:23128204; http://dx.doi.org/ 10.1038/491021a [DOI] [PubMed] [Google Scholar]
- [93].Zhang J, Liu CB, Li RC, Li YM, Zheng YJ, Li YP, Luo D, Pan BB, Nong Y, Ge SX, et al.. Randomized-controlled phase II clinical trial of a bacterially expressed recombinant hepatitis E vaccine. Vaccine 2009; 27:1869-74; PMID:19168109; http://dx.doi.org/ 10.1016/j.vaccine.2008.12.061 [DOI] [PubMed] [Google Scholar]
- [94].Lam E, McCarthy A, Brennan M. Vaccine-preventable diseases in humanitarian emergencies among refugee and internally-displaced populations. Hum Vacc Immunotherap 2015; 11:2627-36; PMID:26406333; http://dx.doi.org/ 10.1080/21645515.2015.1096457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ferguson M, Walker D, Mast E, Fields H. Report of a collaborative study to assess the suitability of a reference reagent for antibodies to hepatitis E virus. Biolog: J Int Assoc Biolog Standardization 2002; 30:43-8; PMID:11846429; http://dx.doi.org/ 10.1006/biol.2001.0315 [DOI] [PubMed] [Google Scholar]
- [96].WHO Technical report. Waterborne Outbreaks of Hepatitis E: recognition, investigation and control. 2014. Available online at: http://www.who.int/hiv/pub/hepatitis/HepE-manual/en/ [Google Scholar]
- [97].WHO. Recommendations of HEV Working Group on the use of hepatitis E vaccine. 2014. Available online at: http://www.who.int/immunization/sage/meetings/2014/october/3_Hep_E_vacc_WG_SAGE_Recs_final_1Oct2014.pdf [Google Scholar]
- [98].Who . Hepatitis E vaccine: WHO position paper, May 2015 - Recommendations. Vaccine 2016; 34:304-5; PMID:26232546; http://dx.doi.org/ 10.1016/j.vaccine.2015.07.056 [DOI] [PubMed] [Google Scholar]
- [99].Gu Y, Tang X, Zhang X, Song C, Zheng M, Wang K, Zhang J, Ng MH, Hew CL, Li S, et al.. Structural basis for the neutralization of hepatitis E virus by a cross-genotype antibody. Cell Res 2015; 25:604-20; PMID:25793314; http://dx.doi.org/ 10.1038/cr.2015.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zhao Q, Li S, Yu H, Xia N, Modis Y. Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol 2013; 31:654-63; PMID:24125746; http://dx.doi.org/ 10.1016/j.tibtech.2013.09.002 [DOI] [PubMed] [Google Scholar]
- [101].Zhang X, Xin L, Li S, Fang M, Zhang J, Xia N, Zhao Q. Lessons learned from successful human vaccines: Delineating key epitopes by dissecting the capsid proteins. Hum Vacc Immunotherap 2015; 11:1277-92; PMID:25751641; http://dx.doi.org/ 10.1080/21645515.2015.1016675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Xu M, Liang Z, Xu Y, Wang J. Chinese vaccine products go global: vaccine development and quality control. Exp Rev Vacc 2015; 14:763-73; PMID:25697690; http://dx.doi.org/ 10.1586/14760584.2015.1012503 [DOI] [PubMed] [Google Scholar]