ABSTRACT
Our previous studies showed that mycobacterial L-forms persist in the blood of BCG vaccinated people and that BCG vaccine is able to produce, under appropriate conditions, filterable, self-replicating L-bodies with virus-like size. Because filterability is one of the characteristics of L-forms, considerable interest has been shown in their capacity to cross the maternal-fetal barrier. The current study demonstrated isolation of mycobacterial L-form cultures from umbilical cord blood of 5 healthy newborns of healthy mothers vaccinated previously with BCG. The isolated cultures showed distinctive growth characteristics of cell wall deficient L-form bacteria. Transmission electron microscopy demonstrated presence of L-bodies with extremely small size of 100 nm and revealed morphological transformations, typical for L-forms. IS6110 Real Time PCR assay confirmed that all L-form isolates were of mycobacterial origin and belonged to Mycobacterium tuberculosis complex which includes vaccinal BCG substrains. In conclusion, we could suggest that reproductive filterable L-bodies of BCG origin are able to fall in blood circulation of the fetus by vertical transmitted pathway and colonize newborns.
KEYWORDS: BCG, cord blood, L-forms, mycobacteria, newborns
Introduction
BCG is the only licensed TB vaccine in use at present, with an estimated 100 million children receiving BCG every year globally.1,2 Although the ability of BCG to provide an immune protection has been debated since the 1930s, BCG was introduced into the Expanded Program of Immunization (EPI) in 1974.3 However, randomized controlled trials have shown efficacies ranging from 0 to 80%.4,5 Like many other currently used vaccines, BCG was developed empirically, without understanding the mechanisms of its immune protection.6 In addition, the development of new TB vaccines continued, with no understanding of their protective immune mechanisms, and their immunogenicity is tested against BCG itself.6-10 The prophylactic vaccine strategy relies on replicating BCG bacilli in order to obtain long-lasting immune response.10 The maintenance of specific immunity necessarily needs BCG bacilli to produce immunogenic substances, associated mainly with cell wall compounds.12
Our last study confirmed that live mycobacterial L-forms persist in the blood of BCG vaccinated people. Forty five of 97 genetically tested blood cultures were identified as L-forms of mycobacteria belonging to Mycobacterium tuberculosis complex.13 We also found that BCG bacilli can convert to cell wall deficient forms (L-forms) inside macrophages during infection in guinea pigs and that the L-conversion phenomenon significantly enhanced their survival and persistence ability.14 As far as cell wall deficiency is considered as a natural phenomenon facilitating the bacterial adaptation under unfavorable circumstances, many authors believe that mycobacterial L-forms are able to persist within the hosts for a long period and that they are of clinical significance for latent, chronic and relapsing infections.15-20 Under stress conditions mycobacteria can transform into L-forms, which undergo through various morphological stages and replicate by unusual modes.15 Although not yet proven, we hypothesize that when BCG bacilli transform into L-forms, they probably cease to be immunogenic. They turn into strongly modified population of live microbial forms with new biological properties, which are able to persist in blood of BCG vaccinated people.13
In fact, the pleomorphic L-forms represent various stages in the life cycle of stressed bacteria. They exist as unusual morphological units, such as large and elementary bodies, vesicles, granules, filterable forms and others.15-20 We found that BCG vaccine is able to produce under appropriate conditions filterable self-replicating L-form elements with virus-like size smaller than the size of bacterial filter pores of 0.2 μm.20
Because filterability is one of the characteristics of BCG L-forms, considerable interest is shown in their capacity to cross the maternal-fetal barrier. In this respect, the aim of our study was to isolate mycobacterial L-form cultures from cord blood of newborns whose mothers were previously vaccinated with BCG.
Results
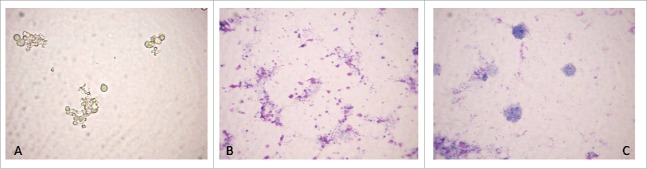
Bacterial cultures were isolated from cord blood samples of all 5 investigated newborns. Light microscopy examinations of isolates revealed step-phase morphological L-form transformations during primary broth and subsequent agar growth phases. Native unstained smear from primary broth phase culture showed spherical L-form cells of different size in their natural image, demonstrating that they were live and replicating (Fig. 1A). Giemsa stained smears revealed granular forms, most of them aggregated in clusters (Fig. 1B) and large spherical bodies (Fig. 1C).
Figure 1.
Light microscopy of primary broth phase culture from a cord blood sample of newborn F4: native unstained smear - spherical L-form cells of different size in their natural image (A); Giemsa stained smears – granular and spherical L-bodies (B, C). Magnification: 1000 x.
TEM of broth-growing cultures demonstrated the presence of small granular L-bodies of different size but also as small as 0.2 µm in diameter, indicating that they were able to pass through bacterial filters (Fig. 2A, B, C). It should be noted that most of them were of extremely small size – around and under 100 nm (Fig. 2A, B). They were observed mostly as heavily clustered granules (Fig. 2A, B). Round single bodies with well-defined outlines (Fig. 2C) and such with irregular shape and granular internal structure were also seen (Fig. 2D). It is interesting to note that fusion of individual elements within the clusters occurred, as shown at greater magnification in electron micrographs (Fig. 2B).
Figure 2.
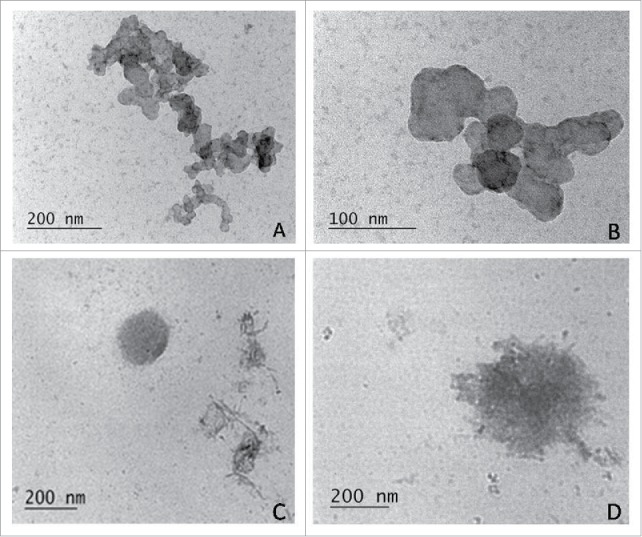
TEM of small granular L-bodies in broth-growing cultures, isolated from cord blood samples of newborns F3 and F4: densely clustered granules with extremely small size - around and under 100 nm (A, B); round single L- body with well-defined outlines (C); L-body with irregular shape and granular internal structure (D).
Electron microscopic examinations in keeping with the observations made with light microscopy, demonstrated that broth-growing L-phase variants developed to medium-sized bodies of 0.4- 2.0 µm in diameter (Fig. 3A, B, D) or large bodies reaching diameters of 5 µm or greater (Fig. 3C). Generally, the ultrathin sections of L-bodies from broth cultures showed lack of bacterial cell walls. The shape of L-form variants was primarily spherical, but they varied strongly in size (Fig. 3). Different modes of L-form replication as binary and asymmetric fission, budding or segmentation resulting in release of elementary bodies were ubiquitously noticed (Fig. 3). It is interesting to note that some of cells underwent disorganization and lysis (Fig 3E, F, G) which resulted in releasing of electron dense L-bodies. Such were seen among fibrillar material in the external milieu (Fig. 3H).
Figure 3.
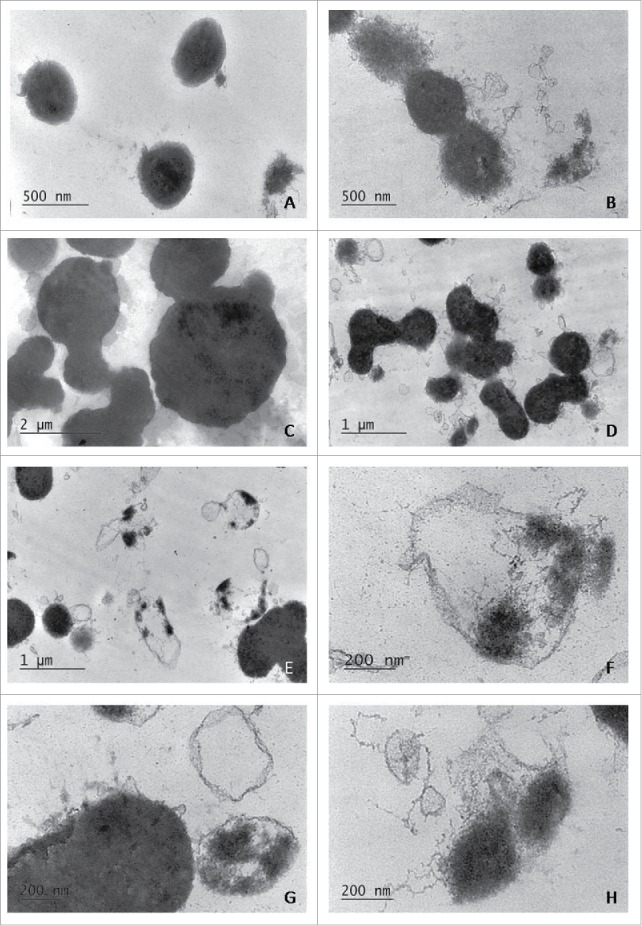
TEM of broth-growing L-phase variants, isolated from cord blood samples of newborns F3 and F4: spherical cell wall deficient L-bodies of different size, replicating by binary and asymmetric fission or budding (A, B, C, D); L-form cells, which underwent segmentation and lysis resulting in release of elementary bodies (E, F, G); small electron dense L-bodies, seen among fibrillar material in the external milieu (H).
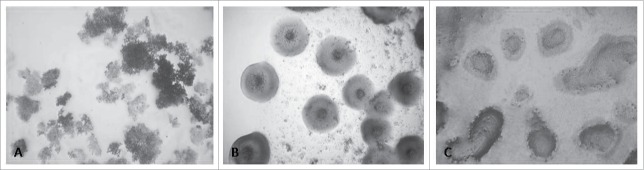
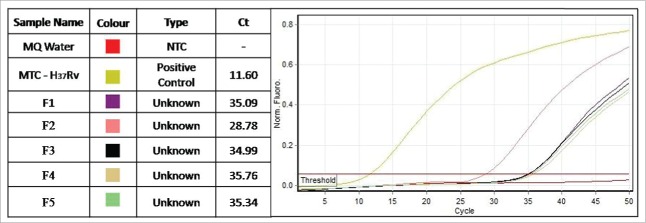
Subsequent sub-culturing from broth onto semisolid medium resulted in fine grained growth (Fig. 4A) or formation of L-type colonies with so called “fried egg appearance” (Fig. 4B), which are distinctive for L-forms. Some of the L-type colonies were ingrown into the agar (Fig. 4C). The cultures isolated from the all 5 cord blood samples were tested by IS6110 Real Time PCR, which is specific for detection of Mycobacterium tuberculosis complex (MTC), and they were all positive. Figure 5 presents the Cycle threshold (Ct) values of the 5 positive cultures. It should be noted that, excepting the pathogenic species, MTC also includes the vaccinal strain of Mycobacterium bovis BCG.
Figure 4.
Growths on semisolid medium after sub-culturing from primary broth isolates of newborns F3 and F4: fine grained growth (A); typical L-form colonies with “fried egg appearance” (B); L-type colonies, ingrown into the agar (C) Magnification: 400x.
Figure 5.
IS6110 Real Time PCR of L-form cultures from cord blood of 5 newborns (F1, F2, F3, F4, F5). Legend: MQ-molecular grate water; NTC – non-template control; MTC- M. tuberculosis H37Rv.
Discussion
The umbilical cord blood composition is similar to peripheral blood composition of fetus during the last stage of gestation.22 The fetus is anatomically and physiologically protected from the systemic circulation of mother by the placental barrier. However, in cases of intercurrent disease during pregnancy and despite the integrity of placental barrier, there are a number of bacterial and viral pathogens capable of crossing it and causing subsequent fetal infections, known as vertically transmitted infections.23 The mechanisms by which microbial agents initially invade this barrier are not well known.
Today, the central dogma of human microbiota states that the blood of healthy people is sterile; respectively the blood circulation of fetus in healthy pregnant women is also a sterile environment.24 However, some authors suggest that term fetuses are not completely sterile and that a prenatal mother-to-child efflux of commensal bacteria may exist.25
The current study demonstrates successful isolation of bacterial cultures from umbilical cord blood of 5 healthy newborns, all born of healthy mothers after normal pregnancy. It became evident that the isolated cultures showed the distinctive characteristics of cell wall deficient L-form bacteria. Formation of colonies with unique shape of “fried eggs” was a convincing criterion for L-form growth.26 Significantly, IS6110 Real Time PCR assay confirmed that all L-form isolates were of mycobacterial origin. The insertion sequence 6110 is a unique conservative marker of mycobacteria belonging to MTC.27 Moreover, IS6110 is present in the genome as multiple copies which significantly increase the sensitivity of PCR amplification.28
Here, TEM observations revealed typical for L-forms morphological transformations and developmental stages, as well as unusual modes of replication such as budding, asymmetric division or segmentation and breaking of large L-bodies up into small elements. Cell wall-free mode of proliferation, involving membrane blebbing, tubulation and vesiculation, was found to be dependent on an altered rate of membrane synthesis.29
Of great interest was the observation of L-bodies with extremely small sizes of under 100 nm which was clearly demonstrated by TEM. Remarkably, processes of fusion of these densely clustered small bodies were noted. Klieneberger described firstly processes of regeneration initiated by the fusion of the smallest L-bodies. She believed that these bodies are elementary living units consisting mainly of DNA granules with narrow fringe of protoplasm.30
Keeping in mind that L-form cultures were isolated after filtration of inoculated with cord blood broth through bacterial filters with 0.2 µm size of pores, we suggest that these very small L-bodies were able to germinate again and to launch a new life L-cycle. In fact, we show here that the fetal blood circulation of all investigated neonates was colonized by mycobacterial L-forms. In our previous study, we found that mycobacterial L-forms were persisting in blood of vaccinated with BCG people.13 If the investigated cord blood from babies born to mothers vaccinated with BCG was a carrier of extremely small (filterable) elements, we can assume that live vaccinal bacilli existing as cell wall free L-bodies in pregnant women, might be transmitted vertically from mother to fetus.
It is considered that the molecular transport mechanism across the maternal-embryonic barrier depends on the stage of embryogenesis and physiological changes during pregnancy.31 Much of the current knowledge about placental barrier comes from the understanding of its impermeability to many large molecules and circulating pathogens.32 Silica nanoparticles smaller than 300 nm have been shown to penetrate the placental barrier in mice.33 Nanosized vesicles of 30–100 nm in diameter (exosomes) and their trafficking within the placental micro-environment have been recognized to play a role in mediating embryo-maternal interactions.34,35 It has been shown that exosomes, released by BCG-infected macrophages contain mycobacterial components.35 In our previous study we found that in BCG treated guinea pigs (i.p.), the macrophages uptake BCG bacilli and that at the late intervals of infection, L-form formations inside the macrophages have been detected. The well packed BCG L forms could be released to the extracellular space probably exploiting apoptotic-like pathway for subsequent rounds of new entry and uptake by macrophages. 14
The key question in this study is how the observed mycobacterial L-bodies in cord blood of newborns were able to cross the maternal-fetal barrier during normal pregnancy and how this may result in colonization of fetal blood circulation. It is known that some viruses enter the fetal tissues during the first trimester,36 when the embryo is separated from mother blood circulation through not fully matured barrier and that in this period trophoblast cells are invading into uterus tissue and entering maternal spiral arteries.37 Therefore, there is a possibility for the filterable L-bodies to pass in blood circulation of the fetus. We could suggest routes like hematogenous transmission across the placenta by direct infection of trophoblasts or through intercellular communication and/or placental transfer of infected macrophages or their products from the maternal blood supply. Nevertheless, the most important finding is that these small forms were reproductive and they developed typical L-form culture.
It should be noted that the current study design is limited within the country of Bulgaria, where the BCG vaccination is obligatory and practically the entire population in the country is vaccinated, including the mothers of investigated newborns. Unvaccinated persons are absent or difficult to find.
The essence of the problem here is how to interpret the phenomenon of vertically transmitted BCG L-forms and the effects of their colonization in newborns. We should put a question for reflection whether the unusual blood colonization in face of mycobacterial L-forms could bring to unpredictable harmful side effects of allergic and autoimmune character. Furthermore, if the vaccination with BCG was introduced since 1921, whether its vertical transmission from generation to generation in humans has made the vaccine immortal and what is the benefit or harm of this?
Materials and methods
Blood samples
Blood samples were recovered from umbilical vein of 5 newborns (F1, F2, F3, F4 and F5). A total of 5 subjects were studied, which met the following criteria : healthy mothers vaccinated with BCG; without history of exposure to tuberculosis (TB); negative for TB by PPD skin test (Mantoux test); normal pregnancy and childbirth (without pathological complications); newborns without pathological deviations. The investigated 5 subjects were born to healthy mothers after normal pregnancies and childbirths without pathological complications. The investigated newborns were without pathological deviations.
The gestational age was between 37 and 40 gestational weeks, the birth weight – between 2930 and 3710 g, and the length – between 47 and 52 cm.
The mothers of the investigated newborns had been vaccinated with BCG and none of them had a history of exposure to tuberculosis. The mothers of investigated newborns were tested negative for tuberculosis, according to the reading of PPD skin test (Mantoux test). Since 1952, the BCG vaccine has been given routinely and obligatory to babies in Bulgaria at 48 h after birth by intradermal application. The strain used for vaccinating mothers was Mycobacterium bovis Sofia SL222.
Blood samples were aseptically collected using standard technique with a needle that was connected to K2E-EDTA Vacutainer tubes (BD Vacutainer, Plymouth, UK) and a cord blood flowed through the needle into the tube . All specimens were handled and anonymized, according to the ethical and legal guidelines. The procedures were approved by the Ethics Committee of Human Experimentation in Bulgaria.
Isolation of L-form cultures
Isolation of L-form cultures from cord blood of newborns was performed according to the protocol described in our previous study.13 In brief, blood lysis was done with sterile distilled water at strictly fixed v/v ratio and after 30 min exposure to room temperature. The lysed blood samples were inoculated in tubes with Tryptic Soy Broth (TSB, Becton Dickinson), which were then filtered through a bacterial filter with 0.2µm size of pores and incubated at 37°C for 72 hours. Then, strictly fixed aliquots from broth were plated with special technique on Petri dishes with semisolid medium. The semisolid medium was prepared from TSB solidified with 0.8% (w/v) Agar (Fluca) in Petri dishes of 94×15 mm. At least 3 Petri dishes per one broth culture were plated. The Petri dishes were enveloped with parafilm and incubated at 37°C for one week. Induction of visible growth, respectively formation of L-form colonies or biofilm was achieved by specific technique of the so called “blind passage.” The “blind passage” was performed by flooding the surface of 3 Petri dishes with TSB, after that aspirating, merging and transferring the lavage fluid to the surface of a new Petri dish with semisolid agar, which was then incubated again for a period of one week at 37°C. Colonial or biofilm growth occurred by the end of the second week. The plates were examined daily macroscopically and microscopically for appearance of growth. Direct light microscopic observations of cultures were combined with Ziehl–Neelsen stained preparations.
Transmission electron microscopy (TEM)
Observations of cord blood L-form isolates were performed by electron microscopy. Selected samples from L-form colonies and biofilm that developed during the second phase/week of cultivation in semisolid agar, were fixed with 4% (v/v) glutaraldehyde in 0.1 M cacodylate buffer with 4.5% w/v sucrose, pH 7.2 and post-fixed in 1% (w/v) osmium tetroxide in the same buffer at room temperature for 2 h and dehydrated in serial ascending ethanol concentrations. After dehydration in ethanol and propylene- oxide series, cell pellets were embedded in epoxy resin Epon-Araldite (Serva, Heidelberg, Germany). Resin blocks polymerised at 56°C for 48 h. Ultrathin cell sections were made with crystal glass knives on a Reichert-Jung Ultracut Microtome and were stained with 5% (w/v) uranyl acetate in 70% (v/v) methanol and 0.4% (w/v) lead citrate. Observations were made with electron microscope JEOL JEM -1011 SAP10 (Japan) at 40–100 kV.
Real time PCR detection of specific for MTB complex IS6110 in L-form cultures
Single L-form colonies were picked up for genetic testing. Several precautions were taken to avoid contamination during the extraction procedure and in the PCR reactions. The DNA extraction, PCR and post-PCR analyses were conducted in separate laminar flow biosafety cabinet and rooms. Sterile aerosol protection filter tips were used to avoid cross-contamination. Two extraction blanks were always included in the same procedure and an additional PCR blank was included in each PCR reaction, containing no DNA template.
Chromosomal DNA was isolated as described by Van Embden et al.38 Real Time PCR mixtures containing a final concentration of 1X PCR buffer (Tris.Cl; KCl; (NH4)2SO4; 15 mM MgCl2, pH 8,7, Qiagen), 2.5 mM MgCl2, 0,2 mM of each dNTPs (dNTP Mix, Qiagen), 1,75 U HotStarTaq DNA polymerase (Qiagen), the target specific primers and probes, were used at a final concentration of 0.5 µM, finally 5 µl of DNA template was added. The reaction mixture was performed in a final volume of 30 µl. The primers and the probe sequence were selected from a region of the IS6110: Primers IS6110 D-1 (5′- ACCTGAAAGACGTTATCCACCAT-3′) and
IS6110 D-2 (5′-CGGCTAGTGCATTGTCATAGGA-3′) which amplify a 100 bp fragment; the probe: (5′- [6 FAM] TCCGACCGCGCTCCGACCGACG-[TAMRA-Q]-3′), was synthesized and conjugated with the reporter dye FAM and TAMRA quencer dye, which were covalently linked to 5′ and 3′ ends oligonucleotide respectively. The reaction was optimized to obtain the best amplification kinetics and the cycle condition was performed for 1 cycle, 15 min at 95°, 30 s at 95°C and 50 s at 60°C for 50 cycles.39
Disclosure of potential conflict of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Albena Cherneva for excellent technical assistance.
References
- [1].Trunz BB, Fine P, Dye C. BCG vaccination on childhood tuberculosis meningitis and miliary tuberculosis world-wide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006; 367:1173-80; PMID:16616560; http://dx.doi.org/ 10.1016/S0140-6736(06)68507-3 [DOI] [PubMed] [Google Scholar]
- [2].Kaufmann SH. Fact and fiction in tuberculosis vaccine research: 10 years later. Lancet 2011; 11:633-40; PMID:21798463; http://dx.doi.org/ 10.1016/S1473-3099(11)70146-3 [DOI] [PubMed] [Google Scholar]
- [3].Fine PA, Cameiro IAM, Milstein J, Clements CJ. Issues relating to the use of BCG in immunization programmes. A discussion document WHO/V&B/99.23, Geneva: World Health Organization; 1999. [Google Scholar]
- [4].Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995; 346:1339-45; PMID:7475776; http://dx.doi.org/ 10.1016/S0140-6736(95)92348-9 [DOI] [PubMed] [Google Scholar]
- [5].Golditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994; 271:698-702; PMID:8309034; http://dx.doi.org/ 10.1001/jama.1994.03510330076038 [DOI] [PubMed] [Google Scholar]
- [6].Hanekom WA. The immune response to BCG vaccination of newborns. Am J Acad Sci 2005; 1062:69-78; http://dx.doi.org/ 10.1196/annals.1358.010 [DOI] [PubMed] [Google Scholar]
- [7].Orme IM. The Achilles heel of BCG. Tuberculosis (Edinb) 2010; 90:329-32; PMID:20659816; http://dx.doi.org/ 10.1016/j.tube.2010.06.002 [DOI] [PubMed] [Google Scholar]
- [8].Hussey G, Hawkridge T, Hanekom W. Childhood tuberculosis: old and new vaccines. Respir Rev 2007; 8:148-54. [DOI] [PubMed] [Google Scholar]
- [9].McShane H. Tuberculosis vaccines: beyond bacille Calmette–Guérin. Phil Trans R Soc B 2011; 366:2782-9; PMID:21893541; http://dx.doi.org/ 10.1098/rstb.2011.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Finen C, Ota MO, Marchant A, Newport MJ. Natural variation in immune responses to neonatal Mycobacterium bovis bacillus Calmette–Guérin (BCG) vaccination in a cohort of Gambian children. PLoS ONE 2008; 3:e3485; PMID:18941532; http://dx.doi.org/ 10.1371/journal.pone.0003485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Andersen P, Doherty TM. The success and failure of BCG—Implications for a novel tuberculosis vaccine. Nat Rev Microbiol 2005; 3: 656-662; PMID:16012514; http://dx.doi.org/ 10.1038/nrmicro1211 [DOI] [PubMed] [Google Scholar]
- [12].Andersen P, Kaufmann SHE. Novel Vaccination Strategies against Tuberculosis Cold Spring Harb Perspect Med 2014; 4:a018523; PMID:24890836; http://dx.doi.org/ 10.1101/cshperspect.a018523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Markova N, Slavchev G, Michailova L. Presence of mycobacterial L-forms in human blood: Challenge of BCG vaccination. Hum Vaccin Immunother 2015; 11:1192-1200; PMID:25874947; http://dx.doi.org/ 10.1080/21645515.2015.1016682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Markova N, Michailova L, Kussovski V, Jourdanova M. Formation of persisting cell wall deficient forms of Mycobacterium bovis BCG during interaction with peritoneal macrophages in guinea pigs. Electronic J Biol 2008; 4:1-10. [Google Scholar]
- [15].Slavchev G, Michailova L, Markova N. Stress-induced L-forms of Mycobacterium bovis: a challenge to survivability. New Microbiol 2013; 36-:157-66; PMID:23686122 [PubMed] [Google Scholar]
- [16].Prozorovski SV, Kaz LN, Kagan GJ. Bacterial L-forms: Mechanisms of Formation, Structure, Role inPathology. Moscow: Medicine Publishing; 1981. [Google Scholar]
- [17].Domingue GJ. Cell-wall deficient bacteria: basic principles and clinical significance. Reading MA: Addison Wesley Publishing Co.; 1982. [Google Scholar]
- [18].Mattman LH. Cell wall Deficient Forms Stealth Pathogens. Boca Raton, FL USA: CRC Press Inc. 2001. [Google Scholar]
- [19].Domingue GJ. Demystifying pleomorphic forms in persistence and expression of disease: Are they bacteria, and is peptidoglycan the solution? Discov Med 2010; 10:234-46; PMID:20875345 [PubMed] [Google Scholar]
- [20].Zhang Y.Persistent and dormant tubercle bacilli and latent tuberculosis. Front Biosci 2004; 9: 1113-56. [DOI] [PubMed] [Google Scholar]
- [21].Markova N, Slavchev G, Michailova L. Filterable forms and L-forms of Mycobacterium bovis BCG. Hum Vaccin Immunother 2012; 8:759-64; PMID:22495116; http://dx.doi.org/ 10.4161/hv.19698 [DOI] [PubMed] [Google Scholar]
- [22].Alexeev I, Volynetz M, Zamaraeva N, Osipova E, Loginov A, Vladimirskaya E, Rumjanzev A. Umbilical cord blood: Morphological composition and functional characteristics. Gene Technology 1996; 94: 99-106; http://dx.doi.org/ 10.1007/978-3-642-61122-3_6 [DOI] [Google Scholar]
- [23].Hayashida A, Inaba N, Oshima K, Nishikawa M, Shoda A, Hayashida S, Negishi M, Inaba F, Inaba M, Fukasawa I, et al.. Reevaluation of the true rate of hepatitis C virus mother-to-child transmission and its novel risk factors based on our two prospective studies. J Obstet Gynaecol Res 2007; 33: 417-22; PMID:17688606; http://dx.doi.org/ 10.1111/j.1447-0756.2007.00582.x [DOI] [PubMed] [Google Scholar]
- [24].Rodrıguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, et al.. The composition of the gut microbiota throughout life, with an emphasis on early life. Microbial Ecology in Health & Disease 2015; 26: 260-50; http://dx.doi.org/ 10.3402/mehd.v26.26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jime´nez E, Marı´n ML, Martı´n R, Odriozola JM, Olivares M, Xaus J, Ferna´ndez L, Rodrı´guez JM. Is meconium from healthy newborns actually sterile? Research in Microbiology 2008; 159: 187e193; http://dx.doi.org/ 10.1016/j.resmic.2007.12.007 [DOI] [PubMed] [Google Scholar]
- [26].Markova Cell Wall Deficiency in Mycobacteria: Latency and Persistence, in Cardona Pere-Joan. (ed): Understanding tuberculosis – Deciphering the secret life of the bacilli, In Tech, pp 193-216, 2012. [Google Scholar]
- [27].Menéndez MC, Samper S, Otal I, Garcia MJ. Understanding tuberculosis – Deciphering the secret life of the bacilli. Rijeka, Croatia: In Tech; 2012 Chapter 3, IS6110 the double-edged passenger; p. 59–88. [Google Scholar]
- [28].Mathema B, Kurepina NE, Bifani PJ, Kreiswirth BN. Molecular Epidemiology of Tuberculosis: Current Insights. Clin Microbiol Rev 2006; 19: 658-85; PMID:17041139; http://dx.doi.org/ 10.1128/CMR.00061-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mercier R, Kawai Y, Errington J. General principles for the formation and proliferation of a wall-free (L-form) state in bacteria. eLife 2014; 3:e04629; http://dx.doi.org/ 10.7554/eLife.04629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Klieneberger-Nobel E. Filterable forms of bacteria. Bacteriol Rev 1951; 15:77-103; PMID:14847983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kulvietis V, Zalgeviciene V, Didziapetriene J, Rotomskis R. Transport of nanoparticles through the placental barrier. Tohoku J Exp Med 2011; 225:225-35; PMID:22052087; http://dx.doi.org/ 10.1620/tjem.225.225 [DOI] [PubMed] [Google Scholar]
- [32].Capellini I, Nunn CL, Barton RA. Microparasites and Placental Invasiveness in Eutherian Mammals. PLoS ONE 2015. 10(7): e0132563; PMID:26168031; http://dx.doi.org/ 10.1371/journal.pone.0132563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yamashita K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, Nozaki M, Yoshida T, Ogura T, Nabeshi H, Nagano K, et al.. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nanotechnol 2011; 6: 321-8; PMID:21460826; http://dx.doi.org/ 10.1038/nnano.2011.41 [DOI] [PubMed] [Google Scholar]
- [34].Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol 2010; 63: 520-33; PMID:20331583; http://dx.doi.org/ 10.1111/j.1600-0897.2010.00822.x [DOI] [PubMed] [Google Scholar]
- [35].Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T Cells in vitro and in vivo. PLoS ONE 2008. 3(6): e2461; http://dx.doi.org/ 10.1371/journal.pone.0002461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mouillet J-F, Ouyang Y, Bayer A, Coyne CB, Sadovsky Y. The role of trophoblastic microRNAs in placental viral infection. Int J Dev Biol 2014; 58(0): 281-9; PMID:25023694; http://dx.doi.org/ 10.1387/ijdb.130349ys [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Craven CM, Morgan T, Warda K. Decidual Spiral Artery Remodelling Begins Before Cellular Interaction With Cytotrophoblasts. Placenta 1998, 19, 241-52. [DOI] [PubMed] [Google Scholar]
- [38].van Embden JDA, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermas P, Martin C, McAdam R, Shinnick TM. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 1993; 31: 406-9; PMID:8381814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ortu S, Molicotti P, Sechi LA, Pirina P, Saba F, Vertuccio C, Deriu A, Maida I, Mura MS, Zanetti S. Rapid detection and identification of Mycobacterium tuberculosis by Real Time PCR and Bactec 960 MIG. New Microbiol 2006; 29: 75-80; PMID:16608129 [PubMed] [Google Scholar]