ABSTRACT
Foxp3-expressing Treg cells have been well documented to provide immune regulation by promoting immune tolerance and suppressing immune over-reaction. Cimetidine (CIM), used to inhibit stomach acid secretion, has been reported to promote immune responses and suppress Treg cell function in several studies. However, the underlying mechanism is unknown. To investigate CIM effects on the suppressive function of Treg and Foxp3, here we used CIM to stimulate human CD4+CD25+ Treg cells and Jurkat T cells and evaluated changes of Foxp3 expression and stability. Our data showed that CIM leads to a reduction of Foxp3 via E3 ligase Stub1-mediated proteosomal degradation, which is dependent on an activated PI3K-AKT-mTOR pathway. Thus, CIM affects the suppressive function of Treg cells by destabilizing their Foxp3 expression.
KEYWORDS: CD4+CD25+ Treg, cimetidine, Foxp3, Stub1, treg cell
Introduction
Balanced immunity is critical in all host immune system since imbalance leads to host damage. Inflammation is primarily a protective response that involves immune cells, blood vessels, and molecular mediators. The function of inflammation is frequently the elimination of pathogenic microorganisms before they can cause disease or the clearing out of necrotic cells to avoid tissue damage. However, if the inflammatory response is over-reactive, it can become destructive and contribute to disease. Hence, protective immunity requires self-regulation to avoid such over-reactions. We have reported that the anti-inflammatory drug Cimetidine (CIM) modulates immunity, at least in part, by reducing regulatory T cell (Treg) levels1 and here we clarify the mechanism.
CIM, an H2-histamine receptor antagonist, was approved in the USA by the FDA for inhibition of gastric acid secretion in 1979. It has since also been widely used to treat peptic and other gastric ulcers and gastroesophageal reflux,2 herpes zoster 3 and burn injury.4 Additionally, studies have indicated that CIM could also suppress tumor growth.5,6 Early studies had shown that CIM is capable of inhibiting suppressor cell effector function7 and expansion8 and that regulatory T cells possess the H2 receptor.9-11 Experimentally, CIM has been shown to enhance a variety of immunologic functions both in vivo and in vitro, including decrease in the relative abundance or function of Treg cells in several studies.12-14 Indeed, Wang J et al.14 reported that the use of CIM as a vaccine adjuvant in mice could induce expression of pro-inflammatory cytokines such as IL-6 and IL-12 and down-regulate expression of anti-inflammatory cytokines, including IL-10 and TGF-β. The immune adjuvant effects of CIM have been confirmed by several other groups.1,15-18 Furthermore, the adjuvant effects were found to be mediated not only by facilitation of inflammation-related immune activation, but also by impairment of CD4+CD25+ Treg cell-mediated suppression.19,20 Therefore, CIM apparently has an important capacity to down-regulate these Treg cells.
Foxp3 is known to serve as a master regulator for the development and function of CD4+CD25+ Treg cells.21 Fontenot JD et al.22 demonstrated that the expression of Foxp3 is highly restricted to this subset of T cells and correlates with suppressor activity. Constitutive and antigen-induced Foxp3+ Treg cells are specialized cells that control adaptive immunity to avoid autoimmune and allergic diseases.23 When Jeong Kim et al.24 depleted mice of Foxp3+ Treg the mice developed severe autoimmune disorders. In a feedback experiment the autoimmunity could be reversed by adoptive transfer of Tregs into Foxp3+ Treg-depleted hosts. Sakaguchi S et al.25 showed that animals depleted of Foxp3+ Treg developed severe autoimmune inflammatory bowel disease (IBD) and replacing the Foxp3-expressing Treg cells could reverse such disease. Fontenot et al.22 had found that under certain conditions Foxp3 expression and Treg cell function might be unstable. These studies laid a groundwork for subsequent direct demonstration that Foxp3 is a crucial transcription factor for Treg cells. Foxp3 belongs to the Forkhead box (FOX) protein family of master regulators involved in the development and function of regulatory T cells.21
Despite these findings, there has been no direct evidence to explain the relationship between the effects of CIM and the regulation of Foxp3 expression. In this study, we asked how CIM affects Foxp3 expression. We observed that CIM inhibits Treg cells by up-regulating AKT which results in the degradation of Foxp3 by the E3 ligase Stub1 (STIP1 homology and U-Box containing protein 1, which is also known as CHIP, C terminus of Hsc70-interacting protein)-mediated proteosomal degradation pathway.
Results
Cimetidine stimulation reduces Foxp3 expression
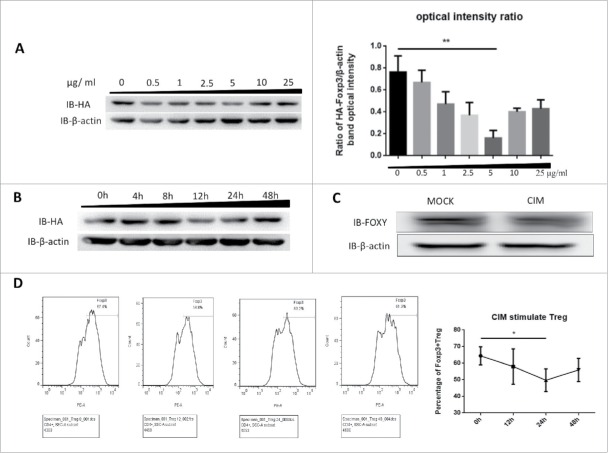
To understand how CIM could enhance immune responses as a vaccine adjuvant and reduce the suppressive function of Treg cells,1,14 we first tested whether Foxp3 protein expression could be affected by CIM treatment. We used HA-Foxp3-expressing Jurkat T cells, which stably express HA-tagged Foxp3 under the control of the constitutive ubiquitin promoter, and stimulated them with 0.5, 1, 2.5, 5, 10 and 25 μg/ml of CIM for 12 h. Foxp3 protein expression was assessed by Western Blot analysis and optical intensity ratio was calculated by Image-pro Plus (Fig. 1A). The optimum concentration of CIM to drive down the level of Foxp3 expression was found to be 5 μg/ml and was used in the rest of study. The optimum duration of treatment to demonstrate the reduction in expression of HA-Foxp3 in Jurkat T cells was found by Western blot analysis to be 12 h (Fig. 1B).
Figure 1.
Foxp3 is destabilized by stimulation with cimetidine. A. Decrease in Foxp3 in Jurkat cells stimulated with different doses of CIM. HA-Foxp3 Jurkat T cells were adjusted to 2×106/ml, and stimulated with 0.5, 1, 2.5, 5, 10, 25 μg/ml cimetidine for 12 h. Four hours before the end of incubation, PMA (50 ng/ml) and ionomycin (1 μg/ml) were added to activate the stimulated cells. The level of Foxp3 protein in cell lysates was detected by immunoblotting (Left). The expression relative to β-actin was analyzed as intensity ratio by Image-Pro Plus, HA/β-actin (Right). B. CIM-induced Foxp3 loss in Jurkat cells treated for different length of time. HA-Foxp3 Jurkat T cells were adjusted to 2×106/ml, and stimulated with cimetidine (5 μg/ml) for 4, 8, 12, 24, or 48 h. PMA (50 ng/ml) and ionomycin (1 μg/ml) were added 4 h before cell harvest. Foxp3 protein in cell lysates was normalized and detected by immunoblotting. C. Decrease in Foxp3 in CIM-stimulated primary human Treg cells. Human primary Treg cells were purified from peripheral blood mononuclear cells of healthy donors at the Shanghai Blood Center and separated by fluorescence cell sorting (FACS) to obtain CD4+CD25hiCD127lo Treg cells. The Treg were then stimulated with cimetidine (5 μg/ml) for 12 h. Foxp3 protein in cell lysates was immunoblotted with FOXY-antibody. D. CIM-induced decrease in the percentage of primary human Treg cells that stained with Foxp3. Human Treg cells stimulated with CIM were stained with CD4, CD25 and CD127 and analyzed by FACS. The sequential changes in percentage of Foxp3+ cells during stimulation are summarized. Data are expressed as means SD from 3 independent experiment. Statistical significance was calculated using t-test (**P < 0.01 and *P < 0.05).
We tested if this was also consistent in human primary Treg cells (CD4+CD25hiCD127lo). The cells were isolated from the peripheral blood mononuclear cells of healthy donors by using FACS ARIA II cell sorter (BD Biosciences, City, USA) and cultured for 2 d at 2 × 106/ml per well then stimulated with 5 μg/ml CIM for 12 h. We found that the expression of Foxp3 was similarly reduced by 12 h stimulation in these cells (Fig. 1C). The effect was confirmed by intracellular staining; the level of intracellular Foxp3 decreased by almost 15% under the CIM stimulation (Fig. 1D). Thus we found that CIM reduced Foxp3 expression in both the isolated human Treg cells and the human T cell line.
Cimetidine leads to Foxp3 protein degradation rather than inhibition of its expression
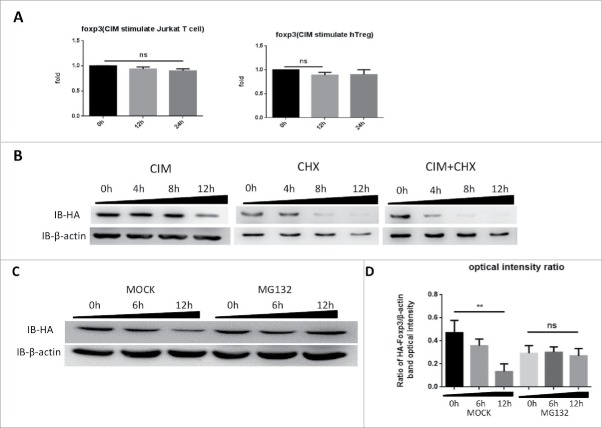
Since we had observed Foxp3 protein reduced under CIM stimulation, we sought to distinguish if the CIM effects were on Foxp3 protein synthesis or degradation. We first assessed the effects of CIM on foxp3 gene expression. We extracted total RNA from CIM-stimulated HA-Foxp3 Jurkat T cells and human Treg cells at intervals and analyzed it by qRT-PCR. As depicted in Figure 2A, the CIM treatment might have slightly affected the foxp3 gene expression but this was not statistically significant.
Figure 2.
Cimetidine stimulation of cells leads to Foxp3 degradation rather than expression inhibition. A. Minimal decrease in foxp3 gene expression in response to CIM stimulation. Total RNA was isolated from HA-Foxp3 Jurkat T cells and Human Treg cells at intervals during stimulation with CIM then reverse-transcribed to cDNA and then qPCR for foxp3 and reference gene GAPDH transcripts was carried out. Normalized-fold changes compared to time zero are shown, and t-test used to analyze the correlation between the groups. The two-tailed P value of Jurkat groups and Treg groups are 0.0614 and 0.1271, respectively. B. Protein synthesis inhibitor CHX enhances the decrease in Foxp3 protein seen during incubation with CIM. HA-Foxp3 Jurkat T cells were stimulated with CIM (5 μg/ml) for 0, 4, 8, or 12 h in the presence or absence of CIM (5 μg/ml) or CHX (5 μg/ml). Cell lysates were normalized for protein content and subjected to Western Blotting. C. Proteasome inhibitor MG132 suppressed the reduction of Foxp3 protein in CIM-stimulated Jurkat cells. HA-Foxp3 Jurkat T cells were stimulated with CIM (5 μg/ml) in the presence or absence of MG132 (5 nM). Cell lysates were normalized for protein content and subjected to Western Blotting. The relative expression of HA bands compared to β-actin bands was analyzed as intensity ratio by Image-Pro Plus (D). Depicted are the representative results of at least 3 independent experiments. Data are expressed as means with SD from 3 independent experiment. Statistical significance was calculated using t-test (**P< 0.01 and *P < 0.05).
To assess the influence of CIM on Foxp3 protein synthesis, HA-Foxp3 Jurkat T cells were stimulated with or without CIM or CHX, an inhibitor of protein biosynthesis in eukaryotic organisms.26 After simultaneous addition of CHX and CIM and incubation of the cells for 4, 8 and 12 h, we observed that CIM had still slightly reduced Foxp3 protein levels after 8 h when compared with the cells treated with CHX alone (Fig. 2B), suggesting that the reduction of protein level caused by CIM is most likely independent of Foxp3 synthesis.
This led us to assess if Foxp3 protein was degraded by proteasomes. When we treated the HA-Foxp3 Jurkat T cells with 5 μM of MG132, a proteasome inhibitor, during the time of the stimulation with 5 μg/ml CIM, we found that the level of Foxp3 protein was sustained (Fig. 2C). Thus it appeared that the reduction of Foxp3 protein in T cells caused by CIM was mainly due to its effect on proteasome-dependent degradation, whereas neither the transcription nor the translation were affected by CIM treatment.
Stub1 mediates the ubiquitination and degradation of Foxp3 under cimetidine stimulation
Having demonstrated the effect of CIM on FOXP3 degradation, we investigated if the ubiquitin ligase Stub1 could be affected in consequence since this protein was reported to be involved in Foxp3 stability.27 The HA-Foxp3 Jurkat T cells were either transfected with a pLV-shStub1 construct to knock down the endogenous Stub1 expression, or with a control construct encoding shGFP. After stable transfection, Stubl expressions was down regulated in the Jurkat T cells with the shStub1-HA-Foxp3, but not in cells with the control construct shCK-HA-Foxp3 (Fig. 3A and B, labeled IB-stub1). These transfected cells were then stimulated with CIM and analyzed by Western Blot. We observed that the level of Foxp3 protein in the cells with the shStub1 knock-down was stable against the CIM treatment (indicated by IB-HA tag detection), but was reduced after 6 hrs by the CIM treatment in the ShCK cells (Fig. 3A and B). We also observed that the level of Stub1 protein was also increased after 6 h CIM treatment (Fig. 3A and C). The data demonstrate that the Stub1 protein is sensitive to the CIM and the increase in Stub1 after CIM treatment might lead to the degradation of Foxp3. Thus, our results suggest that the reduction of Foxp3 protein by CIM is mainly via the regulation of ubiquitin-ligase Stub1 activity.
Figure 3.
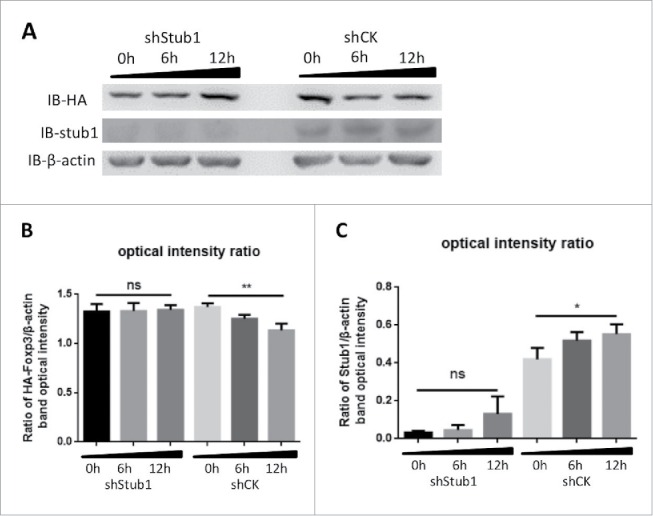
Stub1 mediates the ubiquitination and degradation of Foxp3 during stimulation with cimetidine. A. Stub1 is needed for CIM-induced reduction of Foxp3. ShStub1-HA-Foxp3 Jurkat T cells and ShCK-HA-Foxp3 Jurkat T cells were stimulated with CIM (5 μg/ml) for the indicated time period. Cell lysates were normalized for protein content and subjected to Western Blotting. The expression relative to β-actin was analyzed as intensity ratio by Image-Pro Plus: HA/β-actin (B) and Stub1/β-actin (C). Depicted are the representative results of at least 3 independent experiments. Data are expressed as means with SD from 3 independent experiments. Statistical significance was calculated using t-test (**P< 0.01 and *P < 0.05).
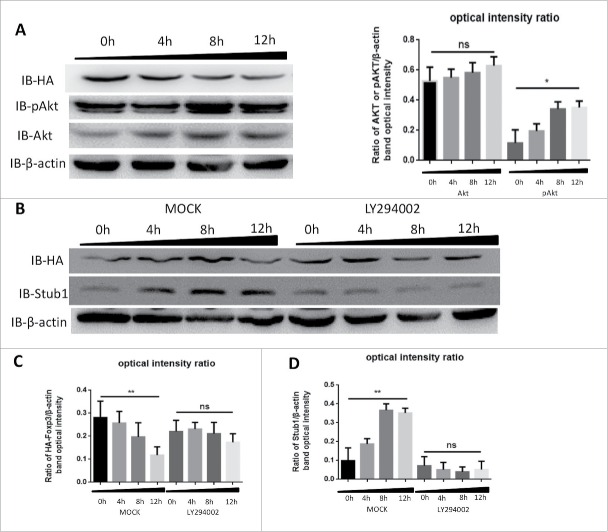
Cimetidine reduces Foxp3 by modulating the PI3K-Akt pathway
Sauer S. et al. had demonstrated that constitutive PI3K-Akt-mTOR activity antagonized Foxp3 induction 28 and our previous study revealed that CIM could activate PI3K-Akt-mTOR pathway,17 therefore we reasoned that CIM might enhance Stub1 activity to destabilize the Foxp3 protein via the same pathway. To assess the relationship of the CIM-induced Foxp3 reduction to Stub1 and PI3K-Akt-mTOR changes, we performed an immunoblotting analysis of the cell lysates of HA-Foxp3 Jurkat T cells after stimulation with CIM. As depicted in Figure 3A, we observed that although the level of Akt was increased, this was not statistically significant (Fig. 4A). In contrast, its phosphorylated form, pAkt, was increased significantly (Fig. 4A). The progressive increase in level of pAkt up to 12 h reciprocally reflected the decreasing level of Foxp3 protein. This result was consistent with our previous observation,17 suggesting that the pAkt activation may affect the Foxp3 protein.
Figure 4.
Cimetidine reduces Foxp3 by modulating the PI3K-Akt pathway. A. Decrease in Foxp3 is accompanied by increase in pAkt during stimulation with CIM. HA-Foxp3 Jurkat T cells were stimulated with CIM (5 μg/ml) for 0, 4, 8 or 12 h then cell lysates were normalized for protein content and subjected to Western Blotting of the Foxp3 (HA), pAkt, Akt and β-actin (left). The expression of Akt and pAkt relative to β-actin was analyzed as intensity ratio by Image-Pro Plus (Right). B. Inhibition of PI3K prevents the decrease of Foxp3 that is induced by stimulation with CIM. HA-Foxp3 Jurkat T cells were stimulated with CIM (5 μg/ml) for the indicated periods in the presence or absence of PI3K blocker LY294002 (50 nM). Cell lysates were normalized for protein content and subjected to Western Blotting. Image-Pro Plus was employed to analyze the relative ratio for HA/β-actin (C) and Stub1/β-actin (D). Data are expressed as means with SD from 3 independent experiments. Statistical significance was calculated using t-test (**P< 0.01 and *P < 0.05).
To explore this, we added a PI3K-Akt pathway specific inhibitor LY294002 into the system and observed that the level of Foxp3 protein was not affected at 12 h (Fig. 4B and C). However, the treatment with CIM and LY294002 could reduce the Stub1 level after 8 h and there were no changes at Foxp3 protein level at the same time points (Figs. 4B and 3C). The data thus indicated that pAkt inhibition could also suppress the level of Stub1 protein and lead to the stabilization of Foxp3 protein. It further suggested that the CIM-induced Foxp3 destabilization likely could be due to the activation of PI3K-Akt-mTOR pathway which in turn activated Stub1-induced ubiquitination and degradation.
Discussion
Our study demonstrated that the H2-histamine receptor antagonist CIM could reduce Foxp3 expression in Treg cells through E3 ligase Stub1-mediated proteosomal degradation, and this reaction was dependent on the PI3K-Akt pathway. We found that CIM reduced the level of Foxp3 protein in the first 12 h of exposure, without any effect on the level of Foxp3 transcription during that period. These two observations, that degradation of Foxp3 under CIM treatment was dependent on Stub1 and involved the PI3K-Akt pathway in Treg cells, were consistent with a previous report29 that Stub1-induced degradation is accompanied by Akt activation and they clarify the mechanism involved.
The regulation of Foxp3 expression and its protein modifications have not been completely understood until recently. Chen ZJ et al. observed that the level of Foxp3 protein could be down-regulated in response to inflammatory signals 27 and that this regulation was dependent on the ubiquitination of Foxp3 by the E3 ligase Stub1 that is also known as CHIP. They also observed that the suppressive function of Treg could be up regulated by either pharmacologically inhibiting the activity of kinase Pim-2, which phosphorylates Foxp3, leading to decreased suppressive functions of Treg cells, or by genetically knocking out Pim-2 in rodent Treg cells. Additionally, deficiency of Pim-2 activity increased murine host resistance to DSS-induced colitis and a small molecule kinase inhibitor for Pim-2 also modified Treg functions.30 It was reported that the over-expression of Stub1 could activate Akt and inhibit the FoxO transcription factors FoxO1, FoxO3, and FoxO4. Inhibition of PI3K by an antagonist revealed that these events may be critical for Stub1-induced apoptosis resistance.29 Our present results indicated that CIM could activate and elevate Stub1, leading to the degradation of Foxp3, and that Stub1 is an indispensable molecule for the Foxp3 reduction induced by CIM.
Ammann, J et al. reported that inhibition of TCR activation and PI3K/Akt pathway signaling by the use of butyrophilin-related protein Btn2a2, which plays a role in modulation of T lymphocytes, could lead to induction of Foxp3 expression in a dose-dependent manner.31 This indicated that a reduced PI3K/Akt pathway may enhance Foxp3 expression. Therefore, the function of Treg cells could be modulated through changes in the level of Foxp3 protein that are induced by the PI3K/Akt pathway under the influence of local inflammation or various other microenvironmental factors.
The results from our previous study and this one showed that the effect of CIM on Foxp3 could involve the PI3K-Akt-mTOR pathway.17 In this study, we showed that the PI3K inhibitor LY294002 could block the effect of CIM on Foxp3 degradation. This suggested that CIM-induced Foxp3 destabilization likely was due to the activation of PI3K-Akt-mTOR pathway which in turn activated Stub1-induced ubiquitination and degradation. The PI3K-Akt-mTOR pathway is a cell cycle regulating intracellular signaling pathway in which the activation of PI3K phosphorylates and activates Akt.
Besides maintaining Stub1-mediated proteosomal degradation, the activation of Akt has several downstream effects such as activating CREB (cAMP response element-binding protein),32 which could improve cAMP signaling for downstream responses. Zhang W et al. reported that CIM stimulation increased cAMP in DC cells and led to activation of PI3K-Akt.17 Modulation of cAMP may be involved in the PI3K-Akt-mTOR pathway modulated by CIM that leads to Foxp3 degradation with consequent loss of Treg function. However, we have not ruled out the possibility that other pathways may be also involved in the regulation since the PI3K inhibitor LY294002 used may have some other effects.
Additionally, CIM might interfere with the histamine stimulation of immune cells by affecting the H2 receptor. Kumar, A. reported that suppressive T lymphocytes possess histamine2 (H2) receptors and contribute significantly to the function of immune system.11 Jutel M et al. proposed that histamine, through its H2 receptor, positively interferes with the peripheral antigen tolerance that is induced by regulatory T cells via several pathways.33 However, it is uncertain whether histamine is also involved in CIM-stimulated Foxp3 reduction.
In summary, the results from our studies provide direct evidence that effects of CIM on Treg cells mainly occur via destabilizing Foxp3 through activation of E3 ligase Stub1-mediated proteosomal degradation and that activation of Stub1 is a consequence of activation of the PI3K-Akt-mTOR pathway by CIM.
Materials and methods
Quantitative real-time PCR
Total RNA was isolated with the TRIzol reagent (Ambion, Carlsbad, USA) according to the manufacturer's instructions. RNA was quantified, concentration adjusted, then reverse transcribed to complementary DNA with the cDNA archive kit (Applied Biosystem, Foster City, USA) according to the manufacturer's instructions. Quantitative real-time PCR reactions for detecting human genes were accomplished by using SYBR green mix (DRR063A, TAKARA, Shiga, Japan) on ABI Prism 7900 Sequence Detection System. Relative gene expression was quantified by using the formula 2-ΔCT normalized to GAPDH expression. The primers for human gene transcription were as follows: foxp3-forward: 5′-tcccagagttcctccacaac-3′ and foxp3-reverse: 5′-attgagtgtccgctgcttct-3′; gapdh-forward: 5′-ccatgttcgtcatgggtgtgaacca-3′ and gapdh-reverse: 5′-gccagtagaggcagggatgatgttc-3′.
Cell culture and stimulation
Human primary Treg cells were purified from peripheral blood mononuclear cells of healthy donors at the Shanghai Blood Center. By using a fluorescence cell sorting (FACS) ARIA II cell sorter (BD Biosciences, CA, USA), we obtained human CD4+CD25hiCD127lo cells. These purified human Treg were cultured in X-vivo medium with anti-CD3/CD28 beads (Invitrogen, CA, USA) and 500 U/ml IL-2 (R&D, Minn, USA). The Treg cells were expanded by incubation with 100 U/ml IL-2 for 2 d followed by stimulation with the drugs as indicated.
Jurkat T cells were grown in RPMI 1640 supplemented with 10% FCS, penicillin/streptomycin, sodium pyruvate, non-essential amino acids and L-glutamine. HA-Foxp3 Jurkat, shStub1-HA-Foxp3 Jurkat and shHSP70-HA Jurkat cells were constructed in Dr. Bin Li's lab. Cells were adjusted to 2–4 × 106 cell/ml and incubated with 5 μg/ml CIM, which was dissolved in PBS at pH 6 and the pH then adjusted to 7 when the drug was totally dissolved. The cells were treated with PMA (50 ng/ml) and ionomycin (1 μg/ml) for 4 h before culture finished.
Cell lysis and western blot analysis
Cells were lysed in RIPA buffer (50 mM Tris/HCl, pH 7.4, 1% NP-40, 0.5% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, with 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF) and protease inhibitor (Sigma, St. Louis, USA) followed by BCA protein quantification (Beyotime, Shanghai, China). Proteins were denatured by boiling for 10 min in SDS-PAGE loading buffer, subjected to SDS-PAGE electrophoresis, then analyzed by Western blotting. The antibodies used for Western Blot were as follows: anti-HA (F-7, Santa Cruz, Texas, USA), anti-GAPDH (1C4, Sungene Biotech, Tianjin, China), anti-Foxp3 (hFOXY, eBioscience, CA, USA) and anti-Stub1 (H-231, Santa Cruz). MG132 was purchased from Merck and LY294002 was from Beyotime.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We wish to thank Dr. Douglas Lowrie for his critical review and valuable suggestions.
Funding
This work was partly supported by the Nature Science Foundation of China (31430027), National High Technology 863 Projects (2012AA02A407) and National Project against Major Infectious Diseases (2013ZX10002001) to BW.
References
- [1].Xie X, Geng S, Liu H, Li C, Yang Y, Wang B. Cimetidine synergizes with Praziquantel to enhance the immune response of HBV DNA vaccine via activating cytotoxic CD8(+) T cell. Hum Vaccin Immunother 2014; 10:1688-99; PMID:24643207; http://dx.doi.org/ 10.4161/hv.28517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Elder JB, Ganguli PC, Gillespie IE. Gastric cancer in patients who have taken cimetidine. Lancet 1979; 2:245; PMID:89345; http://dx.doi.org/ 10.1016/S0140-6736(79)90251-4 [DOI] [PubMed] [Google Scholar]
- [3].Kapinska-Mrowiecka M, Turowski G. [Efficacy of cimetidine in treatment of Herpes zoster in the first 5 days from the moment of disease manifestation]. Polski Tygodnik Lekarski 1996; 51:338-9; PMID:9273526 [PubMed] [Google Scholar]
- [4].Yoshioka T, Monafo WW, Ayvazian VH, Deitz F, Flynn D. Cimetidine inhibits burn edema formation. Am J Surg 1978; 136:681-5; PMID:717647; http://dx.doi.org/ 10.1016/0002-9610(78)90335-5 [DOI] [PubMed] [Google Scholar]
- [5].Flodgren P. Influence of human leukocyte interferon (HuIFN-alpha/Le/) and cimetidine on the proliferation of cultured melanoma cells in vitro. Anticancer Res 1985; 5:527-31; PMID:4062254 [PubMed] [Google Scholar]
- [6].Tomita K, Izumi K, Okabe S. Roxatidine- and cimetidine-induced angiogenesis inhibition suppresses growth of colon cancer implants in syngeneic mice. J Pharmacol Sci 2003; 93:321-30; PMID:14646250; http://dx.doi.org/ 10.1254/jphs.93.321 [DOI] [PubMed] [Google Scholar]
- [7].Kumar A, Cleveland RP. “Immunoregulatory effects of cimetidine: inhibition of suppressor cell effector function in vivo”. Immunopharmacology and immunotoxicology 1988; 10:327-32; PMID:2974050; http://dx.doi.org/ 10.3109/08923978809041424 [DOI] [PubMed] [Google Scholar]
- [8].Griswold DE, Alessi S, Badger AM, Poste G, Hanna N. Inhibition of T suppressor cell expression by histamine type 2 (H2) receptor antagonists. J Immunol 1984; 132:3054-7; PMID:6202771 [PubMed] [Google Scholar]
- [9].Siegel JN, Schwartz A, Askenase PW, Gershon RK. T-cell suppression and contrasuppression induced by histamine H2 and H1 receptor agonists, respectively. Proc Natl Acad Sci U S A 1982; 79:5052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Babayan RK, Osband ME, Carpinito GA, Ho ZS, Cohen EB, Krane RJ. The relationship of histamine H2 receptor-bearing suppressor cells with the growth and metastasis of FANFT-induced bladder cancer. J Surg Oncol 1983; 24:53-8; PMID:6224980; http://dx.doi.org/ 10.1002/jso.2930240113 [DOI] [PubMed] [Google Scholar]
- [11].Kumar A. Cimetidine: an immunomodulator. DICP 1990; 24:289-95; PMID:2138376 [DOI] [PubMed] [Google Scholar]
- [12].Hahm KB, Kim WH, Lee SI, Kang JK, Park IS. Comparison of immunomodulative effects of the histamine-2 receptor antagonists cimetidine, ranitidine, and famotidine on peripheral blood mononuclear cells in gastric cancer patients. Scandinavian J Gastroenterol 1995; 30:265-71; http://dx.doi.org/ 10.3109/00365529509093275 [DOI] [PubMed] [Google Scholar]
- [13].Adams WJ, Morris DL, Ross WB, Lubowski DZ, King DW, Peters L. Cimetidine preserves non-specific immune function after colonic resection for cancer. Aust N Z J Surg 1994; 64:847-52; PMID:7980260; http://dx.doi.org/ 10.1111/j.1445-2197.1994.tb04562.x [DOI] [PubMed] [Google Scholar]
- [14].Wang J, Su B, Ding Z, Du X, Wang B. Cimetidine enhances immune response of HBV DNA vaccination via impairment of the regulatory function of regulatory T cells. Biochem Biophy Res Commun 2008; 372:491-6; http://dx.doi.org/ 10.1016/j.bbrc.2008.04.191 [DOI] [PubMed] [Google Scholar]
- [15].Chen XZ, Yang XD, Yang W, Chen Y, Liu LL, Shen JL, Sun X. [Study on immune response in BALB/c mice induced by ROP2 protein of Toxoplasma gondii with cimetidine]. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi=Chinese journal of parasitology & parasitic diseases 2013; 31:99-103; PMID:24809187 [PubMed] [Google Scholar]
- [16].Li MJ, Lei JH, Wang T, Lu SJ, Guan F, Liu WQ, Li YL. Cimetidine enhances the protective effect of GST DNA vaccine against Schistosoma japonicum. Exp Parasitol 2011; 128:427-32; PMID:21624364; http://dx.doi.org/ 10.1016/j.exppara.2011.05.012 [DOI] [PubMed] [Google Scholar]
- [17].Zhang W, Wang J, Su B, Li R, Ding Z, Kang Y, Wang B. Cimetidine augments Th1/Th2 dual polarized immune responses to recombinant HBV antigens. Vaccine 2011; 29:4862-8; PMID:21481324; http://dx.doi.org/ 10.1016/j.vaccine.2011.03.091 [DOI] [PubMed] [Google Scholar]
- [18].Yamaura K, Yonekawa T, Nakamura T, Yano S, Ueno K. The histamine H2-receptor antagonist, cimetidine, inhibits the articular osteopenia in rats with adjuvant-induced arthritis by suppressing the osteoclast differentiation induced by histamine. J Pharmacolo Sci 2003; 92:43-9; http://dx.doi.org/ 10.1254/jphs.92.43 [DOI] [PubMed] [Google Scholar]
- [19].Jin ZW, Kumar A, Cleveland RP, Murray DL, Kaufman DB. Inhibition of suppressor cell function by cimetidine in a murine model. Clin Immunol Immunopathol 1986; 38:350-6; PMID:2935344; http://dx.doi.org/ 10.1016/0090-1229(86)90245-X [DOI] [PubMed] [Google Scholar]
- [20].Langman MJ, Dunn JA, Whiting JL, Burton A, Hallissey MT, Fielding JW, Kerr DJ. Prospective, double-blind, placebo-controlled randomized trial of cimetidine in gastric cancer. British Stomach Cancer Group. Br J Cancer 1999; 81:1356-62; PMID:10604733; http://dx.doi.org/ 10.1038/sj.bjc.6690457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang L, Zhao Y. The regulation of Foxp3 expression in regulatory CD4(+)CD25(+)T cells: multiple pathways on the road. J Cell Physiol 2007; 211:590-7; PMID:17311282; http://dx.doi.org/ 10.1002/jcp.21001 [DOI] [PubMed] [Google Scholar]
- [22].Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005; 22:329-41; PMID:15780990; http://dx.doi.org/ 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- [23].Ozdemir C, Akdis M, Akdis CA. T regulatory cells and their counterparts: masters of immune regulation. Clin Exp Allergy 2009; 39:626-39; PMID:19422105; http://dx.doi.org/ 10.1111/j.1365-2222.2009.03242.x [DOI] [PubMed] [Google Scholar]
- [24].Kim J, Lahl K, Hori S, Loddenkemper C, Chaudhry A, deRoos P, Rudensky A, Sparwasser T. Cutting edge: depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice. J Immunol 2009; 183:7631-4; PMID:19923467; http://dx.doi.org/ 10.4049/jimmunol.0804308 [DOI] [PubMed] [Google Scholar]
- [25].Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Internat Immunol 2009; 21:1105-11; http://dx.doi.org/ 10.1093/intimm/dxp095 [DOI] [PubMed] [Google Scholar]
- [26].Croons V, Martinet W, Herman AG, Timmermans JP, De Meyer GR. Selective clearance of macrophages in atherosclerotic plaques by the protein synthesis inhibitor cycloheximide. J Pharmacol Exp Therapeut 2007; 320:986-93; http://dx.doi.org/ 10.1124/jpet.106.113944 [DOI] [PubMed] [Google Scholar]
- [27].Chen Z, Barbi J, Bu S, Yang HY, Li Z, Gao Y, Jinasena D, Fu J, Lin F, Chen C, et al.. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 2013; 39:272-85; PMID:23973223; http://dx.doi.org/ 10.1016/j.immuni.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, et al.. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A 2008; 105:7797-802; PMID:18509048; http://dx.doi.org/ 10.1073/pnas.0800928105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lv Y, Song S, Zhang K, Gao H, Ma R. CHIP regulates AKT/FoxO/Bim signaling in MCF7 and MCF10A cells. PloS one 2013; 8:e83312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Deng G, Nagai Y, Xiao Y, Li Z, Dai S, Ohtani T, et al.. Pim-2 kinase influences Tregulatory cell function and stability by mediating Foxp3 N-terminal phosphorylation. J Biol Chem 2015; http://dx.doi.org/ 10.1074/jbc.M115.638221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ammann JU, Cooke A, Trowsdale J. Butyrophilin Btn2a2 inhibits TCR activation and phosphatidylinositol 3-kinase/Akt pathway signaling and induces Foxp3 expression in T lymphocytes. J Immunol 2013; 190:5030-6; PMID:23589618; http://dx.doi.org/ 10.4049/jimmunol.1203325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peltier J, O'Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol 2007; 67:1348-61; PMID:17638387; http://dx.doi.org/ 10.1002/dneu.20506 [DOI] [PubMed] [Google Scholar]
- [33].Jutel M, Blaser K, Akdis CA. Histamine in allergic inflammation and immune modulation. Inter Arch Allergy Immunol 2005; 137:82-92; http://dx.doi.org/ 10.1159/000085108 [DOI] [PubMed] [Google Scholar]