ABSTRACT
Despite the impressive impact of vaccines on public health, the success of vaccines targeting many important pathogens and cancers has to date been limited. The burden of infectious diseases today is mainly caused by antigenically variable pathogens (AVPs), which escape immune responses induced by prior infection or vaccination through changes in molecular structures recognized by antibodies or T cells. Extensive genetic and antigenic variability is the major obstacle for the development of new or improved vaccines against “difficult” targets. Alternative, qualitatively new approaches leading to the generation of disease- and patient-specific vaccine immunogens that incorporate complex permanently changing epitope landscapes of intended targets accompanied by appropriate immunomodulators are urgently needed. In this review, we highlight some of the most critical common issues related to the development of vaccines against many pathogens and cancers that escape protective immune responses owing to antigenic variation, and discuss recent efforts to overcome the obstacles by applying alternative approaches for the rational design of new types of immunogens.
KEYWORDS: antigenically variable pathogens, cancer vaccine, combinatorial peptide library, HIV vaccine, variable epitope library
Introduction
Despite the impressive impact of vaccines on public health, the success of vaccines targeting many important pathogens and cancers has to date been limited.1 The common feature of many important pathogens (such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), dengue virus (DENV), influenza virus, Ebola virus, Plasmodium species, etc.) and cancer cells is their antigenic variability caused by high mutation rate and/or genetic instability which, in turn, represents an obstacle for the development of effective vaccines. However, it is worth mentioning that there are significant differences in the degree of antigenic variability and mutational load between pathogens, ranging from extremely high levels observed in HCV or HIV to moderate/low levels in DENV or Plasmodium species, and the same is true for cancer cells with the highest mutational rates documented in melanoma and lung tumors and less frequent mutations found in pediatric tumors.2-4
Current licensed vaccines, almost exclusively antibody-based in their action, are protective against pathogens with low antigenic variability (examples include vaccines against diphtheria, tetanus, hepatitis A, hepatitis B, measles, mumps, or rubella viruses).1,5,6 Actual strategies for immunogen construction often lack elements of “rational” design specifically targeting genetic/antigenic variability. Moreover, while the induction of neutralizing antibodies, by immunization with whole (modified) pathogen or its' components, may be an important feature explaining the success of the majority of currently available vaccines, humoral immune responses are far less relevant for inducing protection against intracellular pathogens or cancer. Therefore, to develop successful vaccines against antigenically variable pathogens (AVPs) and cancers, novel qualitatively different types of immunogens should be generated, that may replace already existing failed vaccine candidates constructed according to standard approaches accompanied by appropriate immunomodulators.1,5-7
Herein, we highlight some of the most critical common issues related to the development of vaccines against many pathogens and cancers that escape protective immune responses owing to antigenic variation, and discuss recent efforts to overcome the obstacles by applying alternative approaches for the rational design of new types of immunogens.
Antigenically variable pathogens
The burden of infectious diseases today is mainly caused by AVPs, which escape immunity induced by prior infection or vaccination through changes in molecular structures recognized by antibodies (Abs) or T cells.8-11 The development of vaccines against AVPs represents an unprecedented challenge. Researchers should pay close attention to many aspects of the mechanism of action of immune cells as well as to the complex and dynamic nature of the interaction between these cells and rapidly changing epitopes in APVs and cancer cells.
There is compelling evidence that CD8+ T cells are key components of immune response against many intracellular pathogens and tumors and, therefore, effective vaccines against AVPs will likely need to induce broad and potent cellular immune responses.5,6 The observation that whole protein antigens (Ags) are not necessarily essential for inducing protective immunity has led to the emergence of “structural vaccinology.”12-13 Structure-based vaccines are designed on the rationale that suitable epitopes (preferably multiple epitopes) should be sufficient to induce protective immune responses against pathogens, including AVPs.12 Epitope recognition in MHC-restricted T-cell responses involves 2 different binding events: first, small peptides bind to the MHC molecules after Ag processing; then, the resulting peptide-MHC (pMHC) complex is bound by T-cell receptor (TCR) leading to cell activation.5 Current estimates of human αβ TCR diversity suggest that there are <10 8 different Ag receptors in the naïve T cell pool which should recognize >10 15 potential pMHC complexes.14-15 It has been demonstrated that a single TCR could recognize more than one million distinct peptides.14 Unprecedented TCR promiscuity explains how a relatively small number of T cells provide effective immune recognition of all possible peptides or why TCRs are cross-reactive.14-15 To elucidate the nature of TCR promiscuity, combinatorial synthetic peptide libraries were utilized.16 It has been demonstrated that a TCR requires close physico-chemical complementarity with only a few amino acid residues with, on the other hand, considerable structural variability being tolerated at the remaining solvent-exposed residues, thus explaining why TCR/MHC interactions are of low affinity and degenerate.16 This versatility of TCR is particularly important for the development of epitope-based vaccines against AVPs.
It is worth mentioning 2 closely related phenomena that, surprisingly, were not analyzed systematically in current efforts to generate vaccines against AVPs. The first one is “original antigenic sin” (OAS), a phenomenon wherein sequential exposure to closely related pathogen variants reduces Ab and T-cell responses to novel antigenic determinants in the second strain and, consequently, impairs the development of immune memory.17,18 The second phenomenon, referred to as heterologous T-cell immunity, postulates that previous exposure to related or unrelated pathogens can alter immune response of the host and may result in either protective immunity or immunopathology.19,20
Another important emerging challenge faced by scientists is the presence of a large number of potential epitopes in viral, tumor and model systems, that are encoded by short non-primary open reading frames (sORF) and are referred to as defective ribosomal products (DRiPs).21-24 Undoubtedly, the genetic variability of AVPs and genomic instability of cancer cells lead to the generation of these “unexpected” epitopes that are probably the targets of a significant portion of yet unappreciated cellular immune responses.
Detailed analysis of the above mentioned features of AVPs may explain the failure to develop a universal safe anti-influenza vaccine. Current influenza vaccines are aimed at inducing Abs specific for hemagglutinin (HA) and neuraminidase (NA). Due to antigenic variability and immune evasion of circulating strains, yearly vaccinations (based on current strain in the population) need to be performed.25-27 A universal influenza vaccine has been an important objective for decades and there have been many failed attempts to design such a vaccine. Hopefully, further advances in the field will lead to the development of a new generation of vaccines. Interestingly, it has been demonstrated that in the absence of preexisting neutralizing Abs indicating a lack of prior exposure, memory cytotoxic T lymphocytes (CTLs) specific for conserved epitopes of novel H7N9 influenza A virus were able to elicit strong CTL responses against any human influenza A virus.28 However, specific HLA alleles expressed by certain ethnic groups (indigenous Australian and Alaskan populations) were associated with poor CTL responses to these conserved H7N9 peptides indicating additional problems for vaccine development.28 Also, recent results highlight the need to design novel high-impact CTL-inducing vaccines with greater capacity to suppress antigenic drift, since prior population immunity may reduce the expected impact of vaccines for pandemic influenza control.29 Some successful preclinical studies offering novel strategies for influenza vaccine development are mentioned below. Thus, vaccination of mice with a mixture of virus-like particles (VLPs) individually displaying 4 different HAs offered significant protection against a variety of influenza A viruses, suggesting a promising strategy for developing a broadly protective or “universal” vaccine.30 Using a rational design and library screening approach, stable HA stem Ags, bearing smaller versions of full-length HA called “mini-HAs” or HA stem mimics and based on an H1 subtype sequence, were engineered and shown to protect mice in lethal heterologous and hetero-subtypic challenge models and to reduce fever after sub-lethal challenge in cynomolgus monkeys.31 Application of a lipopeptide-based Toll-like receptor 2 (TLR2) agonist together with an existing seasonal vaccine provided immediate short-term nonspecific antiviral protection and long-term immunity against vaccine-matched as well as unrelated virus strains, probably by simultaneously stimulating and amplifying both the innate and adaptive immune responses.32
Another AVP is dengue virus (DENV), the etiologic agent of dengue fever, the most significant mosquito-borne viral disease in humans.33,34 The high degree of sequence variation, the existence of 4 viral serotypes and the association of prior DENV infection with an increased risk of more severe disease have presented significant obstacles for vaccine development.34,35 The outcome of DENV infection depends on the balance between favorable (protection against infection or illness) and unfavorable (enhancement of disease) immune responses, and specific Abs and T cells induced by vaccination may have both protective and detrimental effect. The role of OAS in DENV infection has been broadly analyzed, and a live-attenuated tetravalent vaccine (DENGVAXIA, Sanofi Pasteur) containing antigens from all 4 DENV serotypes, thus addressing the issue of OAS to a certain extent, has been licensed recently.36 Importantly, vaccine efficacy was higher in individuals previously infected with DENV compared with participants who had never been exposed to DENV and further research is warranted.37
High genetic/antigenic variability had a negative impact on vaccine development against HCV that causes most of the acute and chronic liver diseases worldwide.38 It is known that T cells are key factors in the outcome of acute HCV infection and in protective immunity upon re-infection, however, viral sequence diversity leads to frequent mutations in T cell epitopes, particularly those responsible for activation of CD8+ T cells, an essential component of immune protection.39 This phenomenon may be implicated in HCV infection persistence and definitely represents a major obstacle for vaccine development.40 Interestingly, structural and biophysical studies along with comprehensive functional analysis of T cells targeted at natural variants of an immunodominant but highly mutable epitope demonstrated that, despite a narrow bias, the TCR accommodated frequent mutations in hypervariable epitope, thereby providing effective viral immunity and indicating the potential for cross-genotype HCV vaccines.40 Importantly, the superior immunogenicity and cross-reactivity of rare HCV epitope variant was reported suggesting that unique epitope variants and potentially altered epitope sequences associated with priming of broadly cross-reactive TCRs should be considered for vaccine design.41
HIV
Perhaps, the most representative example of vaccine failure is the HIV/AIDS vaccine case, despite a huge investment in financial and human resources.42,43 While enormous efforts are focused on development of new adjuvants or stimulatory molecules to enhance efficacy of HIV vaccine, the “correct” immunogens capable of inducing protective and long lasting immune responses have not been generated to date. HIV displays a greater degree of genetic and antigenic variability than any other virus studied44 and this presents a major obstacle in developing an effective vaccine. While there is a general belief that a successful HIV/AIDS vaccine should induce strong and broad cellular and humoral immune responses leading to the generation of CTLs and neutralizing antibodies (nAbs), respectively, current vaccine efforts do not specifically address the antigenic/immunogenic profiles of HIV and its ability to consistently evade anti-viral immune responses.42,43
Current approaches for HIV vaccine design are mainly based on assumption that conserved sequences derived from virus are promising immunologic targets, however, a great number of immunogens bearing conserved or consensus sequences as well as combinations of antigens representing major clades failed to induce protective immunity.42,43 Most of such consensus sequences of HIV and other AVPs have been already naturally selected by the host immune system as antigenic regions, thus, even if epitopes derived from conserved sequences are immunogenic and may be recognized by the host, they likely will not induce protective immunity. There are several mechanisms allowing the virus to escape the immune attack and almost invariably stay one step ahead of the host immune system. These are sequence variation, altered antigen presentation, latency, privileged sites of viral replication, viral adaptation to HLA at a population level, as well as mechanisms causing the loss of effector cells or affecting their functional integrity.45-47 Importantly, the study of HIV-1 adaptation to HLA also represents an opportunity to identify what qualities constitute an effective immune response, how the virus adapts to these pressures, and how we may harness this information to design effective vaccines.9 Thus, in a recent elegant study authors combined deep sequencing technology and comprehensive CD8 T cell epitope mapping and demonstrated that the initial CD8 T cell response in the acute phase of the live attenuated simian immunodeficiency virus infection (SIVΔnef, one of the most effective vaccines in inducing protection against wild-type lentiviral challenge) is mounted predominantly against more variable epitopes, followed by viral escape.48 Interestingly, epitope escape expands the CD8 T cell repertoire that targets highly conserved epitopes (defined as anentropic specificity) generating de novo responses to the escaped epitope variants during the vaccination period.48
The first and the second waves of HIV vaccine development, aimed to induce nAbs and CTL response, respectively, did not address the issue of antigenic variability, and candidate vaccines' capacity to induce immune responses able to recognize variant (mutated) epitopes has not been tested.42,49-51 Clinical trials were halted because of lack of efficacy, and subsequent careful statistical analysis demonstrated that in some cases vaccination in fact enhanced HIV acquisition.50-52 We think that OAS and/or heterologous immunity may explain, in part, the failure of these trials. Interestingly, it has been demonstrated that some immune interference caused by OAS could be avoided by delivering mutant and original CTL epitopes to the immune system simultaneously.53 Importantly, there may be a potential danger in the possible application of current HIV candidate vaccines (which may also be true for other AVPs), since they may reduce the pool of naïve T cells by focusing the immune response on few immunodominant epitopes included in vaccines. The third wave of HIV vaccine studies started in 2009 with the modest efficacy (estimated 31 %) obtained in the RV144 trial evaluating a prime boost combination of an ALVAC vector followed by an envelope glycoprotein.52 An immune correlates analysis showed that, while the RV144 vaccine failed to induce nAbs against HIV primary isolates, the vaccine did induce Abs binding to second variable (V2) loop of gp120 and eliciting Ab dependent cellular cytotoxicity (ADCC).43
Subsequently, a number of elegant studies offered new sophisticated approaches for immunogen construction, however they failed to generate immunogens inducing broadly neutralizing Abs (bnAbs), although helped us to better understand the immune pathways behind the induction and evolution of such antibodies.54-59 We share the opinion that reverse vaccinology, based on the combined use of genomic, structural and immunological information to select relevant protective HIV-1 epitopes with increased antigenicity, may not lead to the discovery of effective immunogens: in other words, vaccine immunogenicity can not be predicted from viral antigenicity.60,61 Clearly, alternative innovative paradigms that could diversify the further search for vaccine candidates are needed.59,60 Recently, an important role of viral antigenic diversity in shaping of bnAb repertoires was analyzed in detailed and systematic manner.61 Thus, the effect of diverse HIV variant epitopes (immunotypes) on driving the neutralization breadth within a family of 33 mAbs, isolated from a superinfected patient was studied demonstrating that early viral escape at key antibody-virus contact sites selects for antibodies that can tolerate these changes.61
Other examples of truly alternative HIV vaccine strategies are hypervariable epitope constructs (HECs),62 multiple epitope immunogens (MEIs)63 and mixotope peptide libraries.64 All of these approaches are aimed at achieving high degree of immunological cross-reactivity between immunogens and viral epitopes, however, they are based on epitope/peptide libraries or peptide cocktails where most frequently found (conserved) amino acids are included in defined amino acid positions, and, consequently, may have serious drawbacks as discussed above. Indeed, the HEC vaccine had no antiviral effect in macaques after challenge with SIV,62 while a vaccine bearing 176 peptide variants induced week nAb responses.62,65 Thus, vaccines based on immunogens carrying a limited number of epitopes, most probably, may hamper or inhibit the activation of naive T or B cells upon encounter with a pathogen bearing slightly different antigen/epitope variants.
Recently, we have reported a new concept (schematically illustrated in Fig. 1) for generation of vaccine immunogens specifically targeting genetically/antigenically variable pathogens, termed as variable epitope library (VEL).66,67 These new classes of immunogens are combinatorial libraries bearing a mixture of heavily mutated variants of a single immunodominant epitope. In a proof of concept study, we have demonstrated that immunization with VEL expressing a mixture of thousands of variants of an immunodominat HIV-1 gp120 V3 loop-derived CTL epitope68 (RGPGRAFVTI) induced CTLs recognizing more than 50% of heavily mutated variants of wild type epitope.66 Moreover, based on previous reports on neutralizing activity of antibodies induced by the same V3 loop-derived epitope,69,70 we showed that sera from VEL-immunized mice were capable of neutralizing 5 out 10 viral isolates from Tier 2 reference panel, including HIV-1 isolates known to be resistant to neutralization by several potent monoclonal antibodies described previously.67 These data indicate the feasibility of the application of immunogens based on VEL concept as an alternative approach and as a general technological platform for the development of molecular vaccines against AVPs. The hypothesis behind the concept is simple: the activation of diverse pool of B and T lymphocytes requires the immunogens carrying antigenic diversity that mirrors the natural infections with HIV, other AVPs or disease conditions, such as cancer, in respect to interactions between immune system and rapidly evolving epitopes.66,67 Hence, the VELs carrying random mutations may prevent or reduce the likelihood of epitope escape and, therefore, represent a realistic alternative to existing vaccine approaches targeting AVPs.
Figure 1.
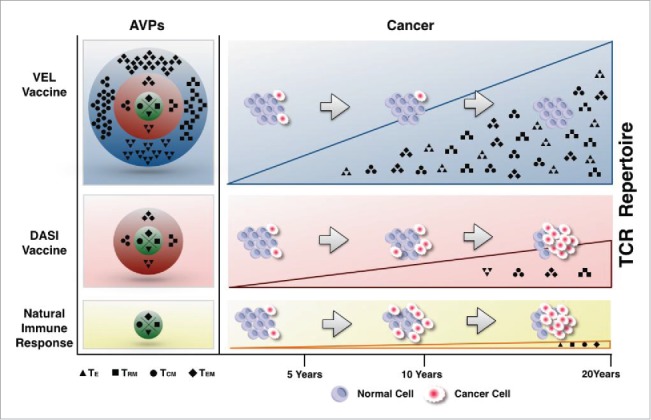
Application of VEL-based vaccine immunogens as an alternative approach for the development of vaccines against AVPs and cancer. An intact immune system responds upon infection with AVPs and against cancer by generating a limited pool of T cells. The vaccination with DASIs induces larger repertoire of lymphocytes, however, these cells were shown to fail to provide protection against APVs and cancer. Vaccines based on VELs generate the largest pool of T cells capable of containing AVPs infection and the development of cancer [60, 61]. TE, TCM, TEM, TRM: effector T cells, central, effector and resident memory T lymphocytes, respectively. DASI: defined antigen sequence immunogen. AVP: antigenically variable pathogen. VEL: variable epitope library.
Cancer
Whereas there are striking similarities between AVPs and cancer regarding the nature of T-cell epitopes due to antigenic variability of tumor cells leading to dynamic changes in epitope landscape, cancers have important additional hindrances, such as: escape from immune surveillance by down-regulating tumor antigen expression/presentation, immune tolerance and immune escape mediated by high epitope mutation rate, immune-suppressive tumor microenvironment and generation of new tumor cells from cancer stem cells.71-73 The cancer “immunoedition” theory, largely accepted in the field, indicates that the interaction between immune system and tumor cells progressively leads to the escape of certain cells from immune attack and to the selection of immune resistant or immunosuppressive cell populations.74 Most recently, a new concept stating that cancer stem cells, key players in cancer initiation and metastasis, arise when transit-amplifying cells with mutant genomes dedifferentiate and enter the stem cell state, has emerged.75
Recent advances in our understanding of molecular biology of different types of cancer have provided opportunities for the development of novel personalized immunotherapies with promising results in pre-clinical models and in clinical trials.76 Today, 3 broad classifications of tumor Ags are accepted: tumor-specific antigens (TSAs) that arise as a consequence of somatic mutations; tumor-associated antigens (TAAs), natural proteins that display aberrant expression in tumor cells; and proteins present only in germ cells and tumor cells, cancer-germline/cancer testis Ags (CTAs).77,78 Recent mAb-based therapies, targeting 2 immunomodulatory receptors on T cells (checkpoint blockade), have yielded promising clinical efficacy and demonstrated that mutant TSAs are not only important targets but they can also be used for personalized cancer therapies.79-81 Advances in DNA and RNA sequencing techniques led to the identification of mutant tumor-specific antigens (TSA, neo-Ags and neo-epitopes) with potential therapeutic capacity.82-85 In an interesting study, a dendritic cell vaccine, tested in patients with advanced melanoma, induced neo-Ag-specific T cells demonstrating that vaccination directed at tumor-encoded amino acid substitutions (mutations) broadens the antigenic breadth and clonal diversity of antitumor immunity.86 In the field of cancer epitope vaccines, the modified, optimized or variant peptides, also known as altered peptide ligands (APLs), mimotopes, heteroclitic peptides or peptide analogs, bearing mutated versions of natural epitopes derived from TAAs are considered as promising candidates and were used to improve vaccine efficacy.87-89 Comprehensive screening strategies, such as combinatorial peptide library screening or testing virtually every single amino acid substitution within an epitope, led to identification of panels of superagonist APLs capable of eliciting potent antitumor patient-specific CTL responses where the native tumor-associated epitope may fail.90,91 Vaccines that incorporate peptide mimics of TAAs, or mimotope vaccines, were shown to elicit increased numbers of T cells cross-reacting with the native tumor Ag, although often these cells had low affinity for the Ag.92 Also, the vaccine immunogens bearing xeno-antigens (proteins that are highly homologous to their autologous counterparts) were used in pre-clinical models and in clinical trials and yielded promising results.93,94 All above mentioned studies may have important implications for optimization of epitope vaccines for cancer immunotherapy. Currently, many clinical trials are underway with the hope to show the therapeutic efficacy of other vaccine candidates, previously tested in pre-clinical studies in animal models. The active immunogenic components of these vaccines are very diverse, ranging from whole cancer cell lysates, protein fractions, cDNA expression libraries, recombinant proteins to oncolytic viruses, recently approved by FDA for the treatment of melanoma patients.11,71,72,76,95-98
Importantly, the first cancer vaccine Sipuleucel-T (Provenge) licensed in 2010 for the treatment of asymptomatic or minimally symptomatic metastatic prostate cancer is a therapy designed to target prostatic acid phosphatase (PAP) using patient's own dendritic cells loaded with PAP linked to granulocyte-macrophage colony stimulating factor (GM-CSF).99 However, the vaccine hasn't been shown to stop prostate cancer progression, and the median survival benefit is only 4 months.100 Hopefully, combining such vaccination approaches with adjunctive therapies will enhance clinical benefit.100,101
It is worth noting that the effective cancer vaccine immunogen should not necessarily perfectly match the epitope landscape of the tumor, but should at least closely resemble the complex epitope composition and dynamic changes in epitope evolution of tumor cells at some degree. For example, once adequately activated, T cells may recognize epitopes/antigens not present in the vaccine by interacting with other tumor-derived epitopes by mechanism of intermolecular epitope spreading, Ag cross-presentation or Ag cascade.102 This, in turn, may continuously refine a therapeutic immune response, making it more relevant to a patient's tumor.102
Above mentioned complex phenomena involved in cancer biology and the host immune response may explain why many recent vaccines based on TAAs (for example, vaccines targeting melanoma-associated antigens (MAGE) or epidermal growth factor receptor (EGFR)) have failed in clinical trials although they elicited specific anti-tumor response.71,103,104 Canvaxin, a therapeutic polyvalent vaccine developed from 3 allogeneic melanoma cell lines known to contain more than 20 common immunogenic melanoma-associated antigens, has been discontinuated due to the lack of a survival benefit.105,106
Owing to the clear similarity between AVPs and cancer cells, we recently tested the prophylactic and therapeutic efficacy of vaccine immunogens bearing VELs of survivin-derived CTL epitope/peptide in an aggressive metastatic mouse 4T1 breast tumor model.107 The rationale was that the immunization with VELs carrying a large number of survivin-derived epitope variants will lead to simultaneous presentation of altered/mutated epitopes to immune system and will induce the activation of diverse and broad repertoire of T cells capable of recognizing cancer epitopes present at the time of experimental or spontaneous induction of tumor as well as the variants that would appear later upon disease progression (Fig. 1). Considering that the TCR repertoire and cancer-derived epitopes are of random origin, the VELs carrying random mutations are perfectly suited as immunogens capable of dealing with antigenic variability.107 We showed that VEL-based immunogens induce protective immune responses against mouse adenocarcinoma in both prophylactic and therapeutic settings.107 We think that immunization with VELs may prevent or reduce the likelihood of epitope escape and, therefore, represents a realistic alternative to existing vaccine approaches targeting cancers that have high mutation rates such as melanoma, lung cancer or colon cancer or those with relatively fewer mutations such as glioblastoma or neuroblastoma.77,108 Furthermore, due to the down regulation of MHC molecules in tumor cells, the immunization with VELs may lead to the selection of more “fit” TCRs as a result of competition between peptides for binding to MHC in tumors and the presence of unusually diverse and large pool of newly activated T cells.107 In addition, competitive binding of cognate peptide epitopes to MHC within APCs could be beneficial for the induction and maturation of protective immune responses which are difficult to generate when conventional Ags, such as defined antigenic sequence immunogens (DASIs) regularly leading to activation of both B and T cells with reduced repertoire, are used.
Undoubtedly, the application of modern vaccine delivery systems to VEL-based immunogens, for example, in vivo DNA electroporation, and a combination of active and passive immunotherapy, may induce more effective immune responses.109
Summary
Despite the latest advances in the development of vaccines against AVPs and cancer, the genetic/antigenic variability, unfortunately, was not addressed adequately. Vaccines based on DASIs including those bearing defined mutated sequences can activate only a limited pool of lymphocytes and they likely will not induce protective immunity. An alternative, qualitatively new approaches leading to the generation of disease- and patient-specific immunogens reflecting and/or incorporating complex and rapidly changing epitope/antigen landscapes of vaccine targets, are urgently needed. Definitely, the construction of such complex vaccine immunogens could be accelerated with the application of modern powerful sequencing techniques. Importantly, this type of immunogens bearing personalized epitope profiles will reflect not only the actual immune status of the patient, but will tell us the whole immunological history of the subject, helping to design more potent molecular immune intervention strategies with unprecedented accuracy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Funding provided by CONACyT, MEXICO (166600) to K.M. RSB is recipient of a doctoral scholarship from CONACyT and Programa de Maestría y Doctorado en Ciencias Bioquimicas, UNAM, MEXICO.
References
- [1].Rappuoli R, Pizza M, Del Giudice G, De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci USA 2014; 111(34):12288-12293; PMID:25136130; http://dx.doi.org/ 10.1073/pnas.1402981111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anderson RM, Donnelly CA, Gupta S. Vaccine design, evaluation, and community-based use for antigenically variable infectious agents. Lancet 1997; 350(9089):1466-1470; PMID:9371182; http://dx.doi.org/ 10.1016/S0140-6736(97)03255-8 [DOI] [PubMed] [Google Scholar]
- [3].Trajanoski Z, Maccalli C, Mennonna D, Casorati G, Parmiani G, Dellabona P. Somatically mutated tumor antigens in the quest for a more efficacious patient-oriented immunotherapy of cancer. Cancer Immunol Immunother 2015; 64(1):99-104; PMID:25164877; http://dx.doi.org/ 10.1007/s00262-014-1599-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yu B, O'Toole SA, Trent RJ. Somatic DNA mutation analysis in targeted therapy of solid tumours. Transl Pediatr 2015; 4(2):125-138; PMID:26835368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reinherz EL, Keskin DB, Reinhold B. Forward Vaccinology: CTL Targeting Based upon Physical Detection of HLA-Bound Peptides. Front Immunol 2014; 5:418; PMID:25237310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, Rupert P, Correnti C, Kalyushniy O, Vittal V, et al.. Proof of principle for epitope-focused vaccine design. Nature 2014; 507(7491):201-6; PMID:24499818; http://dx.doi.org/ 10.1038/nature12966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kulp DW, Schief WR. Advances in structure-based vaccine design. Curr Opin Virol 2013; 3(3):322-31; PMID:23806515; http://dx.doi.org/ 10.1016/j.coviro.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fonville JM, Wilks SH, James SL, Fox A, Ventresca M, Aban M, Xue L, Jones TC, Le NM, Pham QT, et al.. Antibody landscapes after influenza virus infection or vaccination. Science 2014; 21;346(6212):996-1000; PMID:25414313; http://dx.doi.org/ 10.1126/science.1256427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carlson JM, Le AQ, Shahid A, Brumme ZL. HIV-1 adaptation to HLA: a window into virus-host immune interactions. Trends Microbiol 2015; 23(4):212-24; PMID:25613992; http://dx.doi.org/ 10.1016/j.tim.2014.12.008 [DOI] [PubMed] [Google Scholar]
- [10].Tobin GJ, Trujillo JD, Bushnell RV, Lin G, Chaudhuri AR, Long J, Barrera J, Pena L, Grubman MJ, Nara PL. Deceptive imprinting and immune refocusing in vaccine design. Vaccine 2008; 26:6189-6199; PMID:18852005; http://dx.doi.org/ 10.1016/j.vaccine.2008.09.080 [DOI] [PubMed] [Google Scholar]
- [11].Kissick HT, Sanda MG. The role of active vaccination in cancer immunotherapy: lessons from clinical trials. Curr Opin Immunol 2015; 35:15-22; PMID:26050634; http://dx.doi.org/ 10.1016/j.coi.2015.05.004 [DOI] [PubMed] [Google Scholar]
- [12].Thomas S, Luxon BA. Vaccines based on structure-based design provide protection against infectious diseases. Expert Rev Vaccines 2013; 12(11):1301-11; PMID:24090172; http://dx.doi.org/ 10.1586/14760584.2013.840092 [DOI] [PubMed] [Google Scholar]
- [13].Cozzi R, Scarselli M, Ferlenghi I. Srtuctural vaccinology_ a three-dimensional view for vaccine development. Curr Top Med chem 2013; 13(20):2629-2637; PMID:24066888; http://dx.doi.org/ 10.2174/15680266113136660187 [DOI] [PubMed] [Google Scholar]
- [14].Wooldridge L, Ekeruche-Makinde J, van den Berg HA, Skowera A, Miles JJ, Tan MP, Dolton J, Clement M, Llewellyn-Lacey S, Price DA, et al.. A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem 2012; 287(2):1168-77; PMID:22102287; http://dx.doi.org/ 10.1074/jbc.M111.289488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sewell AK. Why must T cells be cross-reactive? Nat Rev Immunol 2012; 12(9):669-77; PMID:22918468; http://dx.doi.org/ 10.1038/nri3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Boesteanu A, Brehm M, Mylin LM, Christianson GJ, Tevethia SS, Roopenian DC, Joyce S. A molecular basis for how a single TCR interfaces multiple ligands. J Immunol 1998; 61(9):4719-27; PMID:NOT_FOUND [PubMed] [Google Scholar]
- [17].Kim JH, Davis WG, Sambhara S, Jacob J. Strategies to alleviate original antigenic sin responses to influenza viruses. Proc Natl Acad Sci U S A 2012; 109(34):13751-6; PMID:22869731; http://dx.doi.org/ 10.1073/pnas.0912458109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Klenerman P, Zinkernagel RM. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 1998; 394:482-485; PMID:9697771 [DOI] [PubMed] [Google Scholar]
- [19].Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol 2002; 2(6):417-26 [DOI] [PubMed] [Google Scholar]
- [20].Che JW, Selin LK, Welsh RM. Evaluation of non-reciprocal heterologous immunity between unrelated viruses. Virology 2015; 482:89-97; PMID:25838115; http://dx.doi.org/ 10.1016/j.virol.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Simon M, Vanes L, Geahlen RL, Tybulewicz VL. Distinct roles for the linker region tyrosines of Syk in FcepsilonRI signaling in primary mast cells. J Biol Chem 2005; 280(6):4510-7; PMID:15576379; http://dx.doi.org/ 10.1074/jbc.M410326200 [DOI] [PubMed] [Google Scholar]
- [22].Bourdetsky D, Schmelzer CE, Admon A. The nature and extent of contributions by defective ribosome products to the HLA peptidome. Proc Natl Acad Sci U S A 2014; 111(16):E1591-9; PMID:24715725; http://dx.doi.org/ 10.1073/pnas.1321902111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wei J, Gibbs JS, Hickman HD, Cush SS, Bennink JR, Yewdell JW. Ubiquitous Autofragmentation of Fluorescent Proteins Creates Abundant Defective Ribosomal Products (DRiPs) for Immunosurveillance. J Biol Chem 2015; 290(26):16431-9; PMID:25971973; http://dx.doi.org/ 10.1074/jbc.M115.658062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chu Q, Ma J, Saghatelian A. Identification and characterization of sORF-encoded polypeptides. Crit Rev Biochem Mol Biol 2015; 50(2):134-41; PMID:25857697; http://dx.doi.org/ 10.3109/10409238.2015.1016215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yewdell JW. To dream the impossible dream: universal influenza vaccination. Curr Opin Virol 2013; 3(3):316-21; PMID:23835048; http://dx.doi.org/ 10.1016/j.coviro.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Grant EJ, Chen L, Quiñones-Parra S, Pang K, Kedzierska K, Chen W. T-cell immunity to influenza A viruses. Crit Rev Immunol 2014; 34(1):15-39; PMID:24579700; http://dx.doi.org/ 10.1615/CritRevImmunol.2013010019 [DOI] [PubMed] [Google Scholar]
- [27].Nara PL, Tobin GJ, Chaudhuri AR, Trujillo JD, Lin G, Cho MW, Levin SA, Ndifon W, Wingreen NS. How can vaccines against influenza and other viral diseases be made more effective? PLoS Biol 2010; 8(12):e1000571; PMID:21203586; http://dx.doi.org/ 10.1371/journal.pbio.1000571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Quiñones-Parra S, Grant E, Loh L, Nguyen TH, Campbell KA, Tong SY, Miller A, Doherty PC, Vijaykrishna D, Rossjohn J, et al.. Preexisting CD8+ T-cell immunity to the H7N9 influenza A virus varies across ethnicities. Proc Natl Acad Sci U S A 2014; 111(3):1049-54; PMID:Can't; http://dx.doi.org/ 10.1073/pnas.1322229111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bolton KJ, McCaw JM, Brown L, Jackson D, Kedzierska K, McVernon J. Prior population immunity reduces the expected impact of CTL-inducing vaccines for pandemic influenza control. PLoS One 2015; 10(3):e0120138; PMID:25811654; http://dx.doi.org/ 10.1371/journal.pone.0120138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schwartzman LM, Cathcart AL, Pujanauski LM, Qi L, Kash JC, Taubenberger JK. An Intranasal Virus-Like Particle Vaccine Broadly Protects Mice from Multiple Subtypes of Influenza A Virus. MBio 2015; 6(4):e01044; PMID:26199334; http://dx.doi.org/ 10.1128/mBio.01044-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, et al.. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015; 349(6254):1301-6; PMID:26303961; http://dx.doi.org/ 10.1126/science.aac7263 [DOI] [PubMed] [Google Scholar]
- [32].Chua BY, Wong CY, Mifsud EJ, Edenborough KM, Sekiya T, Tan AC, Mercuri F, Rockman S, Chen W, Turner SJ, et al.. Inactivated Influenza Vaccine That Provides Rapid, Innate-Immune-System-Mediated Protection and Subsequent Long-Term Adaptive Immunity. MBio 2015; 6(6):e01024-15; PMID:26507227; http://dx.doi.org/ 10.1128/mBio.01024-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdjyorn S, Duangchinda T, Dong T, Rowland-Jones S, et al.. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 2003; 9(7):921-7; PMID:12808447; http://dx.doi.org/ 10.1038/nm887 [DOI] [PubMed] [Google Scholar]
- [34].Weiskopf D, Sette A. T-cell immunity to infection with dengue virus in humans. Front Immunol 2014; 5:93; PMID:24639680; http://dx.doi.org/ 10.3389/fimmu.2014.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol 2011; 11(8):532-43; PMID:21760609; http://dx.doi.org/ 10.1038/nri3014 [DOI] [PubMed] [Google Scholar]
- [36].Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, MuhammadIsmail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al.. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373(13):1195-206; PMID:26214039; http://dx.doi.org/ 10.1056/NEJMoa1506223 [DOI] [PubMed] [Google Scholar]
- [37].Guy B, Jackson N. Dengue vaccine: hypothesis to understand CYD-TDV-induced protection. Nat Rev Microbiol 2016; 14(1):45-54; PMID:26639777; http://dx.doi.org/ 10.1038/nrmicro.2015.2 [DOI] [PubMed] [Google Scholar]
- [38].Park SH, Rehermann B. Immune responses to HCV and other hepatitis viruses. Immunity 2014; 40(1):13-24; PMID:24439265; http://dx.doi.org/ 10.1016/j.immuni.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Holz L, Rehermann B. T cell responses in hepatitis C virus infection: historical overview and goals for future research. Antiviral Res 2015; 114:96-105; PMID:25433310; http://dx.doi.org/ 10.1016/j.antiviral.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nivarthi UK, Gras S, Kjer-Nielsen L, Berry R, Lucet IS, Miles JJ, Tracy SL, Purcell AW, Bowden DC, Hellard M, et al.. An extensive antigenic footprint underpins immunodominant TCR adaptability against a hypervariable viral determinant. J Immunol 2014; 193(11):5402-13; PMID:25355921; http://dx.doi.org/ 10.4049/jimmunol.1401357 [DOI] [PubMed] [Google Scholar]
- [41].Ziegler S, Skibbe K, Walker A, Ke X, Heinemann FM, Heinold A, Mok JY, van Esch WY, Yang D, Wolfl M, et al.. Impact of sequence variation in a dominant HLA-A*02-restricted epitope in hepatitis C virus on priming and cross-reactivity of CD8+ T cells. J Virol 2014; 88(19):11080-90; PMID:25008925; http://dx.doi.org/ 10.1128/JVI.01590-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Esparza J. A new scientific paradigm may be needed to finally develop an HIV vaccine. Front Immunol 2015; 6:124; PMID:25852692; http://dx.doi.org/ 10.3389/fimmu.2015.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Haynes BF. New approaches to HIV vaccine development. Curr Opin Immunol 2015; 35:39-47; PMID:26056742; http://dx.doi.org/ 10.1016/j.coi.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ndung'u T, Weiss RA. On HIV diversity. AIDS 2012; 26(10):1255-60; PMID:22706010; http://dx.doi.org/ 10.1097/QAD.0b013e32835461b5 [DOI] [PubMed] [Google Scholar]
- [45].McMichael A, Hanke T. The quest for an AIDS vaccine: is the CD8+ T-cell approach feasible? Nat Rev Immunol 2002; 2:283-291; PMID:12001999; http://dx.doi.org/ 10.1038/nri779 [DOI] [PubMed] [Google Scholar]
- [46].Kawashima Y, Pfafferott K, Frater J, Matthews P, Payne R, Addo M, Gatanaga H, Fujiwara M, Hachiya A, Koizumi H, et al.. Adaptation of HIV-1 to human leukocyte antigen class I. Nature 2009; 458:641-645; PMID:19242411; http://dx.doi.org/ 10.1038/nature07746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med 2012; 63:95-111; PMID:21942424; http://dx.doi.org/ 10.1146/annurev-med-042010-085643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Adnan S, Colantonio AD, Yu Y, Gillis J, Wong FE, Becker EA, Piatak M Jr, Reeves RK, Lifson JD, O'Connor SL, et al.. CD8 T cell response maturation defined by anentropic specificity and repertoire depth correlates with SIVΔnef-induced protection. PLoS Pathog 2015; 11(2):e1004633; PMID:25688559; http://dx.doi.org/ 10.1371/journal.ppat.1004633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Julien JP, Lee PS, Wilson IA. Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev 2012; 250(1):180-98; PMID:23046130; http://dx.doi.org/ 10.1111/imr.12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jones NG, DeCamp A, Gilbert P, Peterson ML, Gurwith M, Cao H. AIDSVAX immunization induces HIV-specific CD8+ T-cell responses in high-risk, HIV-negative volunteers who subsequently acquire HIV infection. Vaccine 2009; 27:1136-1140; PMID:19071176; http://dx.doi.org/ 10.1016/j.vaccine.2008.11.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, et al.. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372:1881-1893; PMID:19012954; http://dx.doi.org/ 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Esparza J, Van Regenmortel MH. More Surprises in the Development of an HIV Vaccine. Front Immunol 2014; 5:329; PMID:25071786; http://dx.doi.org/ 10.3389/fimmu.2014.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Singh RA, Rodgers JR, Barry MA. The role of T cell antagonism and original antigenic sin in genetic immunization. J Immunol 2002; 169:6779-6786; PMID:12471109; http://dx.doi.org/ 10.4049/jimmunol.169.12.6779 [DOI] [PubMed] [Google Scholar]
- [54].Sette A, Rappuoli R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity 2010; 33(4):530-541; PMID:21029963; http://dx.doi.org/ 10.1016/j.immuni.2010.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jardine JG, Ota T, Sok D, Pauthner M, Kulp DW, Kalyuzhniy O, Kod PD, Thinnes TC, Bhullar D, Briney B, et al.. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline targeting immunogen. Science 2015; 349(6244):156-61; PMID:26089355; http://dx.doi.org/ 10.1126/science.aac5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, et al.. HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 2015; 349(6244):aac4223; PMID:26089353; http://dx.doi.org/ 10.1126/science.aac4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dosenovic P, von Boehmer L, Escolano A, Jardine J, Freund NT, Gitlin AD, McGuire AT, Kulp DW, Oliveira T, Scharf L, et al.. Immunization for HIV-1 broadly neutralizing antibodies in human Ig Knockin Mice. Cell 2015; 161(7):1505-15; PMID:26091035; http://dx.doi.org/ 10.1016/j.cell.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mascola JR. HIV. The modern era of HIV-1 vaccine development. Science 2015; 349(6244):139-40; PMID:26160931; http://dx.doi.org/ 10.1126/science.aac7800 [DOI] [PubMed] [Google Scholar]
- [59].Bricault CA, Kovacs JM, Nkolola JP, Yusim K, Giorgi EE, Shields JL, Perry J, lavine CL, Cheung A, Ellingson-Strouss K, et al.. A multivalent clade C HIV-1 Env trimer cocktail elicits a higher magnitude of neutralizing antibodies than any individual component. J Virol 2015; 89(5):2507-19; PMID:NOT_FOUND; http://dx.doi.org/ 10.1128/JVI.03331-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Van Regenmortel MH. An Outdated Notion of Antibody Specificity is One of the Major Detrimental Assumptions of the Structure-Based Reverse Vaccinology Paradigm, Which Prevented It from Helping to Develop an Effective HIV-1 Vaccine. Front Immunol 2014; 5:593; PMID:25477882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bhiman JN, Anthony C, Doria-Rose NA, Karimanzira O, Schramm CA, Khoza T, Kitchin D, Botha G, Gorman J, Garrett NJ, et al.. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat Med 2015; 21(11):1332-6; PMID:26457756; http://dx.doi.org/ 10.1038/nm.3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Anderson DE, Singapuri A, Kang KH, Montefiori DC, Torres JV. Timing of retroviral infection influences anamnestic immune response in vaccinated primates. Viral Immunol 2005; 18:689-694; PMID:16359235; http://dx.doi.org/ 10.1089/vim.2005.18.689 [DOI] [PubMed] [Google Scholar]
- [63].Hewer R, Meyer D. Peptide immunogens based on the envelope region of HIV-1 are recognized by HIV/AIDS patient polyclonal antibodies and induce strong humoral immune responses in mice and rabbits. Mol Immunol 2003; 40:327-335; PMID:14522014; http://dx.doi.org/ 10.1016/S0161-5890(03)00163-9 [DOI] [PubMed] [Google Scholar]
- [64].Oliveira E, Jimenez-Clavero MA, Nunez JI, Sobrino F, Andreu D. Analysis of the immune response against mixotope peptide libraries from a main antigenic site of foot-and-mouth disease virus. Vaccine 2005; 23:2647-2657; PMID:15780448; http://dx.doi.org/ 10.1016/j.vaccine.2004.10.041 [DOI] [PubMed] [Google Scholar]
- [65].Azizi A, Anderson DE, Torres JV, Ogrel A, Ghorbani M, Soare C, Sandstrom P, Fournier J, Diaz-Mitoma F. Induction of broad cross-subtypespecific HIV-1 immune responses by a novel multivalent HIV-1 peptide vaccine in cynomolgus macaques. J Immunol 2008; 180:2174-2186; PMID:18250424; http://dx.doi.org/ 10.4049/jimmunol.180.4.2174 [DOI] [PubMed] [Google Scholar]
- [66].Pedroza-Roldan C, Charles-Niño C, Saavedra R, Govezensky T, Vaca L, Avaniss-Aghajani E, Gevorkian G, Manoutcharian K. Variable epitope library-based vaccines: shooting moving targets. Mol Immunol 2009; 47(2–3):270-82; PMID:19853920; http://dx.doi.org/ 10.1016/j.molimm.2009.09.024 [DOI] [PubMed] [Google Scholar]
- [67].Charles-Niño C, Pedroza-Roldan C, Viveros M, Gevorkian G, Manoutcharian K. Variable epitope libraries: new vaccine immunogens capable of inducing broad human immunodeficiency virus type 1-neutralizing antibody response. Vaccine 2011; 29(32):5313-21; PMID:Can't; http://dx.doi.org/ 10.1016/j.vaccine.2011.05.007 [DOI] [PubMed] [Google Scholar]
- [68].Fomsgaard A, Nielsen HV, Bryder K, Machuca R, Bruun L, Hansen J, Buus S. Improved humoral and cellular immune responses against the 120 V3 loop of HIV-1 following genetic immunization with a chimeric DNA vaccine encoding the V3 inserted into the hepatitis B surface antigen. Scand J Immunol 1998; 47:289-295; PMID:9600309; http://dx.doi.org/ 10.1046/j.1365-3083.1998.00323.x [DOI] [PubMed] [Google Scholar]
- [69].Pantophlet R, Wrin T, Cavacini LA, Robinson JE, Burton DR. Neutralizing activity of antibodies to the V3 loop region of HIV-1 gp120 relative to their epitope fine specificity. Virology 2008; 381(2):251-260; PMID:18822440; http://dx.doi.org/ 10.1016/j.virol.2008.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zolla-Pazner S, Sharpe Cohen S, Krachmarov C, Wang S, Pinter A, Lu S. Focusing the immune response on the V3 loop, a neutralizing epitope of the HIV-1 gp envelope. Virology 2008; 372:233-246.70; PMID:18061228; http://dx.doi.org/ 10.1016/j.virol.2007.09.024 [DOI] [PubMed] [Google Scholar]
- [71].Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, et al.. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 2014; 11(9):509-24; PMID:25001465; http://dx.doi.org/ 10.1038/nrclinonc.2014.111 [DOI] [PubMed] [Google Scholar]
- [72].Madorsky Rowdo FP, Baron A, Urrutia M, Mordoh J. Immunotherapy in cancer: A combat between tumors and the immune system; you win some, you lose some. Front Immunol 2015; 6:127; PMID:25859247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Natrajan R, Sailem H, Mardakheh FK, Arias Garcia M, Tape CJ, Dowsett M, Bakal C, Yuan Y. Microenvironmental heterogeneity parallels breast cancer progression: A histology-genomic integration analysis. PLoS Med 2016; 13(2):e1001961; PMID:26881778; http://dx.doi.org/ 10.1371/journal.pmed.1001961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol 2014; 27:16-25; PMID:24531241; http://dx.doi.org/ 10.1016/j.coi.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chaffer CL, Weinberg RA. How does multistep tumorigenesis really proceed? Cancer Discov 2015; 5(1):22-4; PMID:25583800; http://dx.doi.org/ 10.1158/2159-8290.CD-14-0788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Melero I, Berman DM, Aznar MA, Korman AJ, Pérez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer 2015; 15(8):457-72; PMID:26205340; http://dx.doi.org/ 10.1038/nrc3973 [DOI] [PubMed] [Google Scholar]
- [77].Heemskerk B, Kvistborg P, Schumacher TN. The cancer antigenome. EMBO J 2013; 32(2):194-203; PMID:23258224; http://dx.doi.org/ 10.1038/emboj.2012.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest 2015; 125(9):3413-21; PMID:26258412; http://dx.doi.org/ 10.1172/JCI80008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al.. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014; 515(7528):577-81; PMID:25428507; http://dx.doi.org/ 10.1038/nature13988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Eggermont AM, Maio M, Robert C. Immune checkpoint inhibitors in melanoma provide the cornerstones for curative therapies. Semin Oncol 2015; 42(3):429-35; PMID:25965361; http://dx.doi.org/ 10.1053/j.seminoncol.2015.02.010 [DOI] [PubMed] [Google Scholar]
- [81].Desrichard A, Snyder A, Chan TA. Cancer Neoantigens and Applications for Immunotherapy. Clin Cancer Res 2016; 22(4):807-12. DOI: 10.1158/1078-0432.CCR-14-3175.82 [DOI] [PubMed] [Google Scholar]
- [82].Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al.. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 2013; 19(6):747-52; PMID:23644516; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].van Buuren MM, Calis JJ, Schumacher TN. High sensitivity of cancer exome-based CD8 T cell neo-antigen identification. Oncoimmunology 2014; 3:e28836; PMID:25083320; http://dx.doi.org/ 10.4161/onci.28836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lu YC, Yao X, Crystal JS, Li YF, El-Gamil M, Gross C, Davis L, Dudley ME, Yang JC, Samuels Y, et al.. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin Cancer Res 2014; 20(13):3401-10; PMID:24987109; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Neiller C, Shinde J, Soysouvanh F, et al.. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 2015; 47(5):505-11; PMID:25822088; http://dx.doi.org/ 10.1038/ng.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, et al.. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015; 348(6236):803-8; PMID:25837513; http://dx.doi.org/ 10.1126/science.aaa3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Platsoucas CD, Fincke JE, Pappas J, Jung WJ, Heckel M, Schwarting R, Magira E, Monos D, Freedman RS. Immune responses to human tumors: development of tumor vaccines. Anticancer Res 2003; 23(3A):1969-96; PMID:12894571 [PubMed] [Google Scholar]
- [88].Jordan KR, McMahan RH, Kemmler CB, Kappler JW, Slansky JE. Peptide vaccines prevent tumor growth by activating T cells that respond to native tumor antigens. Proc Natl Acad Sci U S A 2010; 107(10):4652-7; PMID:20133772; http://dx.doi.org/ 10.1073/pnas.0914879107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hoppes R, Oostvogels R, Luimstra JJ, Wals K, Toebes M, Bies L, Ekkebus R, Rijal P, Celie PH, Huang JH, et al.. Altered peptide ligands revisited: vaccine design through chemically modified HLA- A2-restricted T cell epitopes. J Immunol 2014; 193(10):4803-13; PMID:25311806; http://dx.doi.org/ 10.4049/jimmunol.1400800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Abdul-Alim CS, Li Y, Yee C. Conditional superagonist CTL ligands for the promotion of tumor- specific CTL responses. J Immunol 2010; 184(11):6514-21; PMID:20483791; http://dx.doi.org/ 10.4049/jimmunol.0900448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ekeruche-Makinde J, Clement M, Cole DK, Edwards ES, Ladell K, Miles JJ, Mattews KK, Fuller A, Lloyd KA, Madura F, et al.. T-cell receptor-optimized peptide skewing of the T-cell repertoire can enhance antigen targeting. J Biol Chem 2012; 287(44):37269-81; PMID:22952231; http://dx.doi.org/ 10.1074/jbc.M112.386409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Buhrman JD, Jordan KR, Munson DJ, Moore BL, Kappler JW, Slansky JE. Improving antigenic peptide vaccines for cancer immunotherapy using a dominant tumor- specific T cell receptor. J Biol Chem 2013; 288(46):33213-25; PMID:24106273; http://dx.doi.org/ 10.1074/jbc.M113.509554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Suresh K, Scheid E, Klotz L, Venkateswaran V, Gauldie J, Foley R. Induction of specific human cytotoxic T cells using dendritic cells transduced with an adenovector encoding rat epidermal growth factor receptor 2. Int J Oncol 2011; 39(4):907-13; PMID:21769423 [DOI] [PubMed] [Google Scholar]
- [94].Strioga MM, Darinskas A, Pasukoniene V, Mlynska A, Ostapenko V, Schijns V. Xenogeneic therapeutic cancer vaccines as breakers of immune tolerance for clinical application: to use or not to use?. Vaccine 2014; 32(32):4015-24; PMID:24837511; http://dx.doi.org/ 10.1016/j.vaccine.2014.05.006 [DOI] [PubMed] [Google Scholar]
- [95].Milani A, Sangiolo D, Aglietta M, Valabrega G. Recent advances in the development of breast cancer vaccines. Breast Cancer (Dove Med Press) 2014; 6:159-68; PMID:25339848; http://dx.doi.org/ 10.2147/BCTT.S38428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pol J, Bloy N, Buqué A, Eggermont A, Cremer I, Sautès-Fridman C, Galon J, Tartour E, Zitgovel L, Kroemer G, et al.. Trial Watch: Peptide-based anticancer vaccines. Oncoimmunology 2015; 4(4):e974411; PMID:26137405; http://dx.doi.org/ 10.4161/2162402X.2014.974411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cody JJ, Hurst DR. Promising oncolytic agents for metastasis breast cancer treatment. Oncolytic Virotherapy 2015; 4:63-73; PMID:NOT_FOUND [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sokolowski NAS, Rizos H, Diefenbach RJ. Oncolytic virotherapy using herpes simplexvirus: how far have we come?. Oncolytic Virotherapy 2015; 4:207-219; PMID:NOT_FOUND [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pieczonka CM, Telonis D, Mouraviev V, Albala D. Sipuleucel-T for the treatment of patients with metastatic castrate-resistant prostate cancer: considerations for clinical practice. Rev Urol 2015; 17(4):203-10; PMID:26839517 [PMC free article] [PubMed] [Google Scholar]
- [100].Bourzac K. An immune one-two punch. Nature 2015; 528(7582):S134-6; PMID:26672788; http://dx.doi.org/ 10.1038/528S134a [DOI] [PubMed] [Google Scholar]
- [101].Datta J, Berk E, Cintolo JA, Xu S, Roses RE, Czerniecki BJ. Rationale for a multimodality strategy to enhance the efficacy of dendritic cell-based cáncer immunotherapy. Front Immunol 2015; 6:271; PMID:26082780; http://dx.doi.org/ 10.3389/fimmu.2015.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gulley JL. Therapeutic vaccines: the ultimate personalized therapy?. Hum Vaccin Immunother 2013; 9(1):219-21; PMID:22995839; http://dx.doi.org/ 10.4161/hv.22106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pol J, Bloy N, Buque A, Eggermont A, Cremer I, Sautes-Fridman C, Galon J, Tartour E, Zitgovel L, Kroemer G, et al.. Trial watch: peptide-based anticancer vaccines. Oncoimmunology 2015; 4(4):e974411; PMID:26137405; http://dx.doi.org/ 10.4161/2162402X.2014.974411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Vansteenkiste JF, Cho BC, Vanakesa T, De Pas T, Zielinski M, Kim MS, Jassem J, Yoshimura M, Dahabreh J, Nakayama H, et al.. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive nonsmall-cell lung cáncer (MAGRIT): a randomized-double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016; 17(6): 822–835; http://dx.doi.org/ 10.1016/S1470-2045(16)00099-1 [DOI] [PubMed] [Google Scholar]
- [105].Hsueh EC, Morton DL. Antigen-based immunotherapy of melanoma: Canvaxin therapeutic polyvalent cáncer vaccine. Semin Cancer Biol 2003; 13(6):401-7; PMID:15001158; http://dx.doi.org/ 10.1016/j.semcancer.2003.09.003 [DOI] [PubMed] [Google Scholar]
- [106].Kelland L. Discontinued drugs in 2005: oncology drugs. Expert Opin Investig Drugs 2006; 15(11):1309-18; PMID:17040193; http://dx.doi.org/ 10.1517/13543784.15.11.1309 [DOI] [PubMed] [Google Scholar]
- [107].Noe Dominguez-Romero A, Zamora-Alvarado R, Servín-Blanco R, Pérez-Hernández EG, Castrillon-Rivera LE, Munguia ME, Acero G, Govezensky T, Gevorkian G, Manoutcharian K. Variable epitope library carrying heavily mutated survivin-derived CTL epitope variants as a new class of efficient vaccine immunogen tested in a mouse model of breast cancer. Hum Vaccin Immunother 2014; 10(11):3201-13; PMID:25483665; http://dx.doi.org/ 10.4161/hv.29679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, Bignel GR, Bolli N, Borg A, Borresen-Dale AL, et al.. Signatures of mutational processes in human cancer. Nature 2013; 500:415-421; PMID:23945592; http://dx.doi.org/ 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Muthumani K, Block P, Flingai S, Muruganantham N, Chaaithanya IK, Tingey C, Wise M, Reuschel EL, Chung C, Muthumani A, et al.. Ra´pid and long-term immunity elicited by DNA encoded antibodu prophylaxis and DNA vaccination against Chikungunya virus. J Infect Dis 2016; PMID:27001960; http://dx.doi.org/ 10.1093/infdis/jiw111 [DOI] [PMC free article] [PubMed] [Google Scholar]


