Abstract
IMPORTANCE
Comorbidity affects the prognosis of patients with cancer through the direct effects of the comorbid illness and by influencing the patients’ ability to tolerate treatment and mount a host response. However, the prognostic importance of comorbidity in oropharyngeal squamous cell carcinoma is not well characterized in the era of human papillomavirus infection.
OBJECTIVE
To determine the prognostic importance of comorbidity in both p16-positive and p16-negative oropharyngeal squamous cell carcinoma and to explore the relationship between comorbidity and p16.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective cohort study of 305 patients at a single tertiary referral center diagnosed as having oropharyngeal squamous cell carcinoma between June 1996 and June 2010, but without a history of head and neck cancer or distant metastasis at time of diagnosis. The data were analyzed from August 1, 2014, through April 30, 2015.
EXPOSURES
Patients were grouped according to p16 status.
MAIN OUTCOMES AND MEASURES
Overall survival, defined as the time from diagnosis to death from any cause. Disease-free survival, defined as the time from diagnosis to either death from any cause or the first documented local, regional, or distant recurrence.
RESULTS
Of the 305 patients who met eligibility criteria, 230 were p16-positive, 70 were p16-negative, and 5 were not evaluable for p16 status. The final cohort of 300 patients had a mean (SD) age of 56.3 (9.3) years and 262 (87%) were male. In Kaplan-Meier analysis, the 5-year overall survival rates were 71%(95% CI, 65%–76%) for 232 patients with no comorbidity to mild comorbidity and 49%(95% CI, 36%–61%) for 63 patients with moderate to severe comorbidity. In multivariate Cox proportional hazards analysis, moderate to severe comorbidity was associated with an increased risk of death from any cause (adjusted hazards ratio [aHR], 1.52 [95% CI, 0.99–2.32]) and increased risk of death or recurrence (aHR, 1.71 [95% CI, 1.13–2.59]). After stratifying by p16 status and controlling for other variables, moderate to severe comorbidity was significantly associated with increased risk of death from any cause among p16-negative patients (aHR, 1.90 [95% CI, 1.03–3.50]) but not among p16-positive patients (aHR, 1.11 [95% CI, 0.61–2.02]).
CONCLUSIONS AND RELEVANCE
Comorbidity is important to consider when assessing the prognosis of patients with oropharyngeal squamous cell carcinoma and is of greater prognostic value in p16-negative than p16-positive cancer.
The incidence of oropharyngeal squamous cell carcinoma (OPSCC) has risen dramatically in recent years coinciding with the increasing rates of human papillomavirus (HPV) infection.1–4 HPV-related OPSCC is a biologically, epidemiologically, and clinically unique disease entity.5–8 HPV-positive OPSCC tends to arise in young, healthy, white males and is associated with sexual risk factors. These cancers often present as high-grade, small primary tumors with nodal metastasis. In contrast, traditional, HPV-negative OPSCC afflicts older patients, is associated with tobacco and alcohol use, and metastasizes to regional lymph nodes later in the disease process. While HPV-negative OPSCC portends a very poor prognosis, HPV-positive OPSCC is associated with favorable outcomes.7,9–12 The molecular profile of HPV-positive OPSCC is characterized by overexpression of the tumor suppressor protein, p16. Conversely, HPV-negative tumors rarely exhibit p16 overexpression. Thus, p16 is useful as a sensitive and specific marker of HPV in OPSCC.13,14
Compared with HPV-negative OPSCC, HPV-positive cancers are known to arise in patients with less comorbidity.5 Comorbidity is important to consider when assessing the prognosis of patients with cancer because it can have an impact on survival both through the direct effects of the comorbid illness and by influencing the patient’s ability to tolerate treatment and mount a host response.15 In addition, comorbidity often influences treatment selection16 and may affect treatment adherence.5 Comorbidity has been shown to be an important prognostic factor in numerous cancers,17 including cancers of the colon,18 breast,19,20 lung,21,22 cervix,23 and head and neck.24–28 In OPSCC, the prognostic importance of comorbidity is not well defined. Some investigators have shown comorbidity to be an important prognostic factor independent of HPV status,26,29,30 while others have found that among HPV-positive patients, comorbidity is not prognostic.31
In the present study, we evaluated the impact of comorbidity on survival in a large cohort of patients with OPSCC with known p16 status. We hypothesized that the presence of comorbid illness would adversely affect survival, with p16 status modifying this effect. We predicted that comorbidity would be of greater prognostic importance among p16-negative patients compared with p16-positive patients.
Methods
Patients and Study Design
Approval was received from Washington University School of Medicine’s Human Research Protection Office to assemble and analyze a cohort of 305 patients with pathologically confirmed OPSCC, not previously treated, who were identified through a search of separate patient databases maintained by the Departments of Pathology, Otolaryngology, and Radiation Oncology. All data were deidentified. All patients were diagnosed and treated with curative intent at Barnes-Jewish Hospital in St Louis, Missouri, between June 1996 and June 2010. Prospectively gathered demographic, comorbid health, clinicopathological, and outcome data were obtained from the Oncology Data Services tumor registry (Table 1). Vital status was updated through December 2014 using the electronic medical record, and the date of death was confirmed using the Social Security Death Index. Missing values were investigated and resolved when possible using the electronic medical record. Patients with remaining missing values after querying the medical record did not differ from patients with known values in either of the 2 study end points, overall survival (OS) and disease-free survival (DFS). Patients were not compensated and did not provide written informed consent.
Table 1.
Baseline Characteristics of the Cohort Stratified by p16 Statusa
| Characteristic | Total Cohort (n = 300) |
p16 Status | P Valueb | |
|---|---|---|---|---|
| Positive (n = 230) |
Negative (n = 70) |
|||
| Age, mean (SD), y | 56.3 (9.3) | 56.2 (9.0) | 56.4 (10.4) | .87 |
| Sex | .09 | |||
| Male | 262 (87) | 205 (89) | 57 (81) | |
| Female | 38 (13) | 25 (11) | 13 (19) | |
| Race | <.001 | |||
| White | 259 (86) | 218 (95) | 41 (59) | |
| Nonwhite | 41 (14) | 12 (5) | 29 (41) | |
| Smoking | <.001 | |||
| Yes | 215 (72) | 150 (65) | 65 (93) | |
| No | 71 (24) | 67 (29) | 4 (6) | |
| Unknown | 14 (5) | 13 (6) | 1 (1) | |
| Comorbidity | .005 | |||
| None | 119 (40) | 99 (43) | 20 (29) | |
| Mild | 113 (38) | 90 (39) | 23 (33) | |
| Moderate | 42 (14) | 25 (11) | 17 (24) | |
| Severe | 21 (7) | 13 (6) | 8 (11) | |
| Unknown | 5 (2) | 3 (1) | 2 (3) | |
| Clinical T stage | .003 | |||
| TX | 37 (12) | 34 (15) | 3 (4) | |
| T1 | 59 (20) | 52 (23) | 7 (10) | |
| T2 | 85 (28) | 67 (29) | 18 (26) | |
| T3 | 53 (18) | 35 (15) | 18 (26) | |
| T4 | 44 (15) | 27 (12) | 17 (24) | |
| Unknown | 22 (7) | 15 (6) | 7 (10) | |
| Clinical N stage | <.001 | |||
| N0 | 44 (15) | 23 (10) | 21 (30) | |
| N1 | 53 (18) | 41 (18) | 12 (17) | |
| N2A | 38 (13) | 33 (14) | 5 (7) | |
| N2B | 73 (24) | 67 (29) | 6 (9) | |
| N2C | 46 (15) | 33 (14) | 13 (18) | |
| N3 | 22 (7) | 15 (7) | 7 (10) | |
| Unknown | 24 (8) | 18 (8) | 6 (9) | |
| Overall clinical stage | .43 | |||
| I | 6 (2) | 4 (2) | 2 (3) | |
| II | 15 (5) | 10 (4) | 5 (7) | |
| III | 51 (17) | 36 (16) | 15 (21) | |
| IV | 195 (65) | 154 (67) | 41 (59) | |
| Unknown | 33 (11) | 26 (11) | 7 (10) | |
| Treatmentc | <.001 | |||
| Surgery + CRT | 100 (33) | 90 (39) | 10 (14) | |
| Surgery + RT | 108 (36) | 88 (38) | 20 (29) | |
| Surgery alone | 26 (9) | 17 (7) | 9 (13) | |
| RT/CRT | 65 (22) | 35 (15) | 30 (43) | |
Abbreviations: CRT, chemoradiotherapy; RT, radiotherapy.
Unless indicated otherwise, data represent number (%) of patients. Instances of column percentages not adding to 100% are owing to round-off error.
P value comparing p16-positive and p16-negative groups.
One p16-negative patient received chemotherapy alone.
Because racial disparities exist in survival of head and neck cancer,32 patient-reported race was included in our analysis and was classified as white vs nonwhite. Smoking history was recorded at the time of diagnosis and was dichotomized into nonsmokers and smokers with either a current or former history of smoking. Comorbidity was assessed using the Adult Comorbidity Evaluation-27 (ACE-27) index.17 Clinical stage was assigned according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, Seventh edition, criteria33 and incorporated all information available at the time of diagnosis. Patients who presented with pathologically confirmed squamous cell carcinoma metastatic to regional neck lymph nodes but with a primary tumor of unknown origin were included in the study and designated as having clinical stage TX disease. Head and neck cancers of unknown primary are often treated as occult oropharyngeal cancers and are often subsequently identified as having originated from the oropharynx, especially in p16-positive cancers.34–36 In our cohort, 34 of 37 patients with cancer of unknown primary (92%) were p16-positive. Initial treatment regimens included definitive radiotherapy or chemoradiotherapy, surgery alone, and surgery with adjuvant radiotherapy or chemoradiotherapy. One patient refused surgery and received chemotherapy alone and was excluded from analysis. Patients with a history of head and neck cancer or distant metastases at the time of presentation were excluded.
p16 Immunohistochemical Analysis
p16 Immunohistochemical analysis was conducted by a single pathologist (J.S.L.), blinded to the diagnosis and clinical characteristics of the patients, following standard protocols.37 Representative 4-µm sections cut from formalin-fixed, paraffin-embedded tissue blocks were stained using a monoclonal antibody to p16 (MTM Laboratories CINTEC) on a Ventana Benchmark immunostainer (Ventana Inc) with appropriate positive controls. A specimen was considered p16 positive if at least 50% of tumor cells showed strong and diffuse staining. Of the 230 specimens that were considered positive, 221 (96%) exhibited strong staining in greater than 75% of cells. Of the 70 specimens that were considered negative, 69 (99%) showed staining in less than 25% of tumor cells.
Study End Points
The primary study end point was duration of OS, which was defined as the time from diagnosis to death from any cause. A secondary study end point was duration of DFS, which was defined as the time from diagnosis to either death from any cause or the first documented recurrence. Recurrences were classified as either locoregional failure or distant metastasis and were considered only in patients declared disease-free following initial treatment. Patients who were never disease-free were not classified as having had a locoregional failure or metastasis.
Statistical Analysis
Standard descriptive statistics were used to describe the distribution of characteristics between p16-positive and p16-negative patients. Heterogeneity between these 2 groups was tested using Pearson χ2 or Fisher exact test for categorical data and independent t test for continuous data. The Kaplan-Meier method with the log-rank test and Cox proportional hazards (PH) regression analysis were used for univariate survival analysis. Multivariate Cox PH analysis was conducted to evaluate the independent effect of comorbidity on survival. All variables that were statistically significant predictors of survival at the α = .10 in bivariate analysis were included in the multivariate analysis. Additional Cox PH multivariate analyses were conducted to evaluate the prognostic importance of comorbidity after stratifying by p16 status. The independent prognostic value of comorbidity was expressed as an adjusted hazard ratio (aHR) with a 95% CI. The assumption of proportionality was visually tested for all variables using log − log plots, and the models’ discriminative power was evaluated using Harrell’s c-index.38 All statistical tests were evaluated at the 2-sided α = .05. Statistical analyses were performed using SPSS software (version 22.0; SPSS Inc).
Results
Description of the Cohort
Of the 305 patients initially identified, 300 had tumor specimense valuable for p16 immunohistochemical analysis and were included in the data analysis. Our final cohort of 300 patients consisted of 230 p16-positive patients (77%) and 70 p16-negative patients (23%). Characteristics of the cohort stratified by p16 status are listed in Table 1. Compared with p16-negative patients, p16-positive patients were more likely to be male, white, and a nonsmoker, and have less comorbidity. In addition, p16-positive cancers tended to present as small primary tumors with advanced nodal disease. Among patients who were alive at last follow-up, the median follow-up was 88 months (interquartile range, 68–117 months).
Outcomes
Vital status at last follow-up and the presence of documented recurrence are displayed in Table 2. In Kaplan-Meier analysis, the 5-year OS rates were 77% (95% CI, 72%–82%) for p16-positive patients and 30% (95% CI, 19%–41%) for p16-negative patients for an absolute difference of 47% (95% CI, 35%–59%). The 5-year DFS rates were 74% (95% CI, 68%–79%) for p16-positive patients and 26% (95% CI, 15%–36%) for p16-negative patients resulting in an absolute difference of 48% (95% CI, 36%–60%).
Table 2.
Vital Status and Presence of Documented Recurrence Stratified by p16 Statusa
| Characteristic | Total Cohort (n = 300) |
p16 Status | Difference, % (95% CI)b | |
|---|---|---|---|---|
| Positive (n = 230) |
Negative (n = 70) |
|||
| Vital status | ||||
| Alive | 181 (60) | 163 (71) | 18 (26) | 45 (32 to 56) |
| Dead | 119 (40) | 67 (29) | 52 (74) | |
| Locoregional failure | ||||
| No | 282 (94) | 222 (97) | 60 (86) | 11 (3 to 20) |
| Yes | 16 (5) | 7 (3) | 9 (13) | |
| Unknown | 2 (1) | 1 (0) | 1 (1) | |
| Distant metastasis | ||||
| No | 271 (90) | 211 (92) | 60 (86) | 6 (−2 to 16) |
| Yes | 27 (9) | 18 (8) | 9 (13) | |
| Unknown | 2 (1) | 1 (0) | 1 (1) | |
Unless indicated otherwise, data represent number (%) of patients. Instances of column percentages not adding to 100% are owing to round-off error.
Percentage of difference (95% CI) between p16-positive and p16-negative groups.
Effect of Baseline Features on Survival
In univariate Cox PH analysis, race, smoking history, comorbidity, p16 status, clinical T stage, and treatment were identified as statistically significant predictors of OS and DFS (Table 3). In addition, age and clinical N stage were statistically significant predictors of OS but were not significant for DFS (Table 3). In multivariate Cox PH analysis, smoking history, p16 status, clinical T stage, clinical N stage, and treatment remained independent prognostic factors for OS, while smoking, comorbidity, p16 status, clinical T stage, and treatment were independently prognostic for DFS (Table 3). p16 status was the most robust prognostic variable in both the OS (aHR, 3.16 [95% CI, 1.97–5.08]) and DFS models (aHR, 2.92 [95% CI, 1.82–4.68]) (Table 3).
Table 3.
Univariate and Multivariate Survival Analysis of the Full Cohort
| Univariate Analysis | Multivariate Analysisa | |
|---|---|---|
| Characteristic | HR (95% CI) | aHR (95% CI) |
| Overall survival | ||
| Age: continuous | 1.02 (1.00–1.04) | 1.00 (0.98–1.02) |
| Sex: male vs female | 1.31 (0.74–2.34) | |
| Race: nonwhite vs white | 2.09 (1.34–3.25) | 0.74 (0.43–1.28) |
| Smoking: yes vs no | 4.15 (2.23–7.72) | 2.27 (1.14–4.52) |
| Comorbidity: moderate/severe vs none/mild | 2.18 (1.48–3.21) | 1.52 (0.99–2.32) |
| p16: Negative vs positive | 4.18 (2.90–6.04) | 3.16 (1.97–5.08) |
| Clinical T stage: T3–T4 vs TX-T2 | 2.70 (1.85–3.93) | 1.98 (1.32–2.98) |
| Clinical N stage: N2B-N3 vs N0-N2A | 1.61 (1.10–2.36) | 1.77 (1.15–2.72) |
| Treatment | ||
| Surgery alone vs RT/CRT | 0.43 (0.22–0.85) | 1.24 (0.57–2.69) |
| Surgery + RT vs RT/CRT | 0.40 (0.26–0.61) | 0.71 (0.44–1.16) |
| Surgery + CRT vs RT/CRT | 0.27 (0.17–0.45) | 0.44 (0.25–0.77) |
| Disease-free survival | ||
| Age: continuous | 1.02 (0.99–1.03) | 0.98 (0.96–1.00) |
| Sex: male vs female | 1.32 (0.76–2.31) | |
| Race: nonwhite vs white | 1.95 (1.27–3.00) | 0.72 (0.42–1.24) |
| Smoking: yes vs no | 3.61 (2.03–6.42) | 2.04 (1.07–3.87) |
| Comorbidity: moderate/severe vs none/mild | 2.12 (1.45–3.09) | 1.71 (1.13–2.59) |
| p16: Negative vs positive | 3.97 (2.78–5.67) | 2.92 (1.82–4.68) |
| Clinical T stage: T3–T4 vs TX-T2 | 2.45 (1.71–3.53) | 2.13 (1.42–3.19) |
| Clinical N stage: N2B-N3 vs N0-N2A | 1.39 (0.96–2.00) | 1.45 (0.96–2.19) |
| Treatment | ||
| Surgery alone vs RT/CRT | 0.72 (0.39–1.31) | 2.31 (1.12–4.74) |
| Surgery + RT vs RT/CRT | 0.45 (0.30–0.69) | 0.78 (0.48–1.25) |
| Surgery + CRT vs RT/CRT | 0.29 (0.17–0.47) | 0.42 (0.24–0.73) |
Abbreviations: aHR, adjusted hazard ratio; CRT, chemoradiotherapy; HR, hazard ratio; RT, radiotherapy.
Variables that were significant at the α < .10 level in univariate analysis were included in the multivariate model.
Effect of Comorbidity on Survival
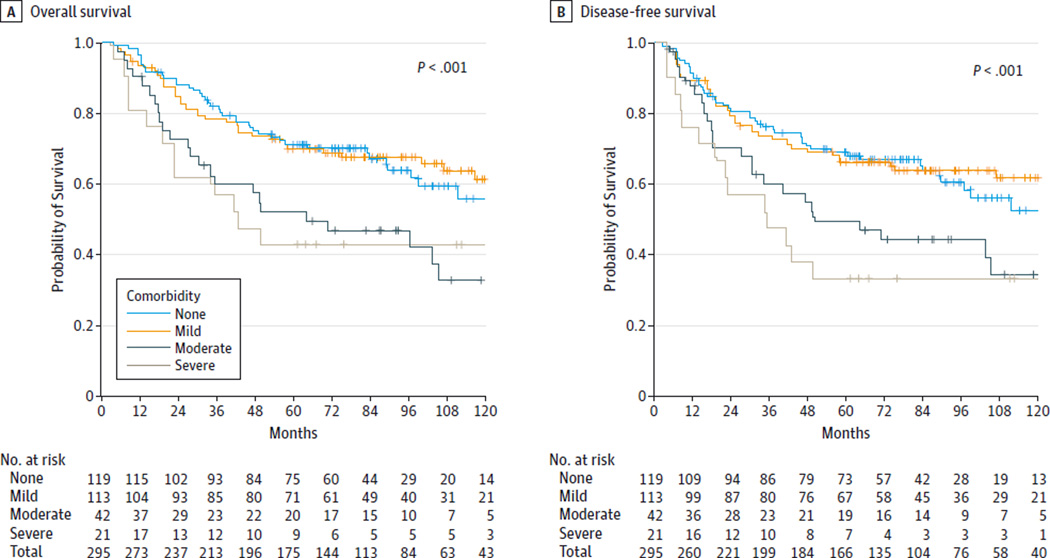
In Kaplan-Meier analysis, comorbidity was a statistically significant predictor of OS and DFS (P < .001 for both end points). As shown in Figure 1, patients with no comorbidity or mild comorbidity had similarly favorable out comes while patients with moderate or severe comorbidity had poor survival. For patients with no comorbidity to mild comorbidity, the 5-year OS rate was 71% (95% CI, 65%–76%) and the 5-year DFS rate was 68% (95% CI, 62%–74%). For patients with moderate to severe comorbidity, the 5-year OS rate was 49% (95% CI, 36%–61%) and the 5-year DFS rate was 44% (95% CI, 31%–57%). In multivariate Cox PH analysis, patients with moderate to severe comorbidity compared with patients with no comorbidity to mild comorbidity had a statistically insignificant yet clinically significant 1.5-fold increased risk of death (aHR, 1.51 [95% CI, 0.99–2.32]) and a statistically significant and clinically meaningful 1.7-fold increased risk of death or recurrence (aHR, 1.71 [95% CI, 1.13–2.59]) (Table 3).
Figure 1. Effect of Comorbidity on Survival.
A, Kaplan-Meier overall survival curves. B, Disease-free survival curves. Patients with no comorbidity and those with mild, moderate, and severe comorbidity are compared. The reported P values indicate the differences among groups by log-rank test.
To investigate the relationship between comorbidity and p16 status, multivariate Cox PH analyses were conducted after stratification by p16 status. Among p16-positive patients, moderate to severe comorbidity was associated with a statistically insignificant worse OS (aHR, 1.11 [95% CI, 0.61–2.02]) and DFS (aHR, 1.34 [95% CI, 0.74–2.43]) after controlling for age, smoking, clinical T stage, clinical N stage, and treatment. Among p16-negative patients, moderate to severe comorbidity was associated with a statistically significant and clinically meaningful 1.9-fold increased risk of death (aHR, 1.90 [95% CI, 1.03–3.50]) and a 2.1-foldincreased risk of death or recurrence (aHR, 2.07 [95% CI, 1.13–3.80]) compared with no comorbidity to mild comorbidity after controlling for age, race, clinical T stage, and treatment. Adjusted survival curves for comorbidity stratified by p16 status are shown in Figure 2. As can be seen, the presence of moderate to severe comorbidity confers a poor prognosis in p16-negative patients (Figure 2, B and D) but has little effect on p16-positive patients (Figure 2, A and C).
Figure 2. Effect of Comorbidity on Survival as a Function of p16 Status and Adjustment for Other Prognostic Factors.
A, Adjusted overall survival curves for none to mild comorbidity and moderate to severe comorbidity among p16-positive patients. B, Adjusted overall survival curves for none to mild comorbidity and moderate to severe comorbidity among p16-negative patients. C, Adjusted disease-free survival curves for no comorbidity to mild comorbidity and moderate to severe comorbidity among p16-positive patients. D, Adjusted disease-free survival curves for no comorbidity to mild comorbidity and moderate to severe comorbidity among p16-negative patients. aHR indicates adjusted hazards ratio.
Discussion
In our investigation of comorbidity and survival among patients with OPSCC, comorbidity was found to be an important prognostic variable overall, but not among p16-positive patients. In the full OS and DFS multivariate models, patients with moderate to severe comorbidity had a 50% increased risk of death from any cause and 70% increased risk of death or recurrence compared with patients with no comorbidity to mild comorbidity. Furthermore, given the upper bound of the 95% CI, it is plausible that moderate to severe comorbidity may increase the risk of death from any cause or recurrence by more than 2-fold compared with no comorbidity to mild comorbidity.
These findings indicate the substantial impact that comorbidity has on prognosis and are consistent with results from recent studies. For example, Habbous and colleagues,26 in their study of 525 patients with oropharyngeal cancer, found that after controlling for all other variables including p16 status, a Charlson Comorbidity Index39 (CCI) score of 2 or greater was associated with a 31% increased risk of death from any cause (aHR, 1.31 [95% CI, 1.02–1.70]) compared with CCI scores of 0 or 1. Likewise, in a recent study by Rietbergen and colleagues29 that evaluated 841 patients with oropharyngeal cancer with known HPV status, moderate to severe comorbidity, as assessed by ACE-27 scores, was associated with a 62% increased risk of death from any cause (aHR, 1.62 [95% CI, 1.31–2.01]) compared with no comorbidity to mild comorbidity.
In the present study, multivariate analyses demonstrated a differential effect of comorbidity on survival between p16-negative and p16-positive patients. Comorbidity was the most important prognostic factor among p16-negative patients but was not associated with survival among p16-positive patients. There are at least 2 potential explanations for these findings. First, there are relatively few p16-positive patients with moderate or severe comorbidity. In our cohort, 38 of 230 p16-positive patients (17%) had moderate or severe comorbidity at the time of diagnosis. In contrast, 25 of 70 p16-negative patients (35%) had moderate or severe comorbidity. The small absolute number of p16-positive patients with moderate or severe comorbidity reduces the statistical ability to detect an impact of comorbidity among these patients. Another potential reason that comorbidity is prognostically more important among p16-negative patients is the strong differential effect of smoking on survival in p16-positive but not in p16-negative patients. The prognostic value of comorbidity may be statistically overpowered by smoking among p16-postive patients. However, among p16-negative patients in whom smoking is not prognostic, because very nearly all p16-negative patients are smokers, comorbidity is the most important prognostic variable. In contrast to our results, Rietbergen and colleagues29 found that comorbidity is prognostically important among HPV-positive patients. However, their Dutch cohort consisted almost entirely of smokers, and thus they did not find smoking to be prognostically important. It is possible that among European populations in which smoking rates are high, smoking is less prognostic than comorbidity; but among American populations in which smoking rates are lower, smoking as a prognostic factor outweighs comorbidity. Indeed, in our multivariate analysis smoking was the most prognostic variable after p16 status.
To our knowledge, our study is the first to thoroughly investigate the effect of comorbidity on survival and the relationship between comorbidity and p16 status among patients with OPSCC. Our findings are important because they demonstrate the necessity of considering comorbidity when evaluating prognosis and comparative treatment effectiveness of patients with OPSCC, particularly p16-negative OPSCC. Given the superior prognosis of p16-positive OPSCC, current research is focusing on refining the current AJCC staging system to more accurately stratify p16-positive patients into prognostic groups.40 Some investigators have suggested incorporating comorbidity into prognostic models for p16-positive patients29; however, our results indicate that comorbidity may be less important for risk stratification of p16-positive patients. Future research is necessary to confirm the most important prognostic variables for p16-positive OPSCC and to accurately determine which patients have the best prognosis and would benefit the most from treatment de-escalation trials.41
The reliance on observational data is the greatest limitation of this study. Observational data are inherently susceptible to bias from confounding variables. For example, Hess and colleagues5 reported that adherence to radiotherapy may confound the relationship between comorbidity and survival among p16-negative patients. Such confounding variables may lead to an underestimation or overestimation of the effect of comorbidity on survival. In addition, misclassification bias could affect our results because p16 immunohistochemical analysis is not a perfectly sensitive or specific marker of HPV. However, because p16 immunohistochemical analysis was conducted in a blinded fashion, any misclassification bias is likely nondirectional and would bias our results toward the null. Likewise, comorbidity is also susceptible to misclassification bias. However, comorbidity was assessed prospectively without knowledge of outcome status. Thus, any misclassification of comorbidity would also likely be nondirectional and would bias our results toward the null.
Conclusions
Comorbidity is an important prognostic factor to consider when assessing the prognosis of patients with OPSCC. After controlling for other factors, including p16 status, moderate to severe comorbidity is associated with a statistically insignificant yet clinically relevant increased risk of death from any cause. Comorbidity is of greater prognostic importance in p16-negative than p16-positive OPSCC. Our findings should help inform future studies that seek to build predictive models and stratify risk for p16-positive OPSCC.
Key Points.
Question
What is the effect of comorbidity on survival among p16-positive and p16-negative patients with oropharyngeal squamous cell carcinoma?
Findings
In this retrospective cohort study of 300 patients, moderate to severe comorbidity was associated with significantly worse survival than none to mild comorbidity after controlling for other variables including p16 status. In a stratified analysis, comorbidity was significantly associated with survival among p16-negative patients but not among p16-positive patients.
Meaning
Comorbidity is important to consider when assessing the prognosis of patients with oropharyngeal squamous cell carcinoma and is of greater prognostic importance in p16-negative patients than p16-positive patients.
Acknowledgments
Funding/Support: This publication was supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and TL1 TR000449 from the National Center for Advancing Translational Sciences.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Piccirillo had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Skillington, Lewis, Piccirillo.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Skillington, Piccirillo.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Skillington, Kallogjeri.
Obtained funding: Piccirillo.
Study supervision: Piccirillo.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Drs Kallogjeri and Piccirillo own stock in and serve as consultants for Potentia Systems. No other disclosures are reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr Piccirillo was not involved in the editorial evaluation or decision to publish this article.
REFERENCES
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole L, Polfus L, Peters ES. Examining the incidence of human papillomavirus-associated head and neck cancers by race and ethnicity in the U.S., 1995–2005. PLoS One. 2012;7(3):e32657. doi: 10.1371/journal.pone.0032657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein AP, Saha S, Yu M, Kimple RJ, Lambert PF. Prevalence of human papillomavirus in oropharyngeal squamous cell carcinoma in the United States across time. Chem Res Toxicol. 2014;27(4):462–469. doi: 10.1021/tx500034c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess CB, Rash DL, Daly ME, et al. Competing causes of death and medical comorbidities among patients with human papillomavirus-positive vs human papillomavirus-negative oropharyngeal carcinoma and impact on adherence to radiotherapy. JAMA Otolaryngol Head Neck Surg. 2014;140(4):312–316. doi: 10.1001/jamaoto.2013.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 7.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 8.Andl T, Kahn T, Pfuhl A, et al. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res. 1998;58(1):5–13. [PubMed] [Google Scholar]
- 9.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 10.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 11.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28(27):4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klussmann JP, Gültekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162(3):747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Sun R, Lin H, Hu WH. P16INK4A as a surrogate biomarker for human papillomavirus-associated oropharyngeal carcinoma: consideration of some aspects. Cancer Sci. 2013;104(12):1553–1559. doi: 10.1111/cas.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha P, Kallogjeri D, Piccirillo JF. Assessment of comorbidities in surgical oncology outcomes. J Surg Oncol. 2014;110(5):629–635. doi: 10.1002/jso.23723. [DOI] [PubMed] [Google Scholar]
- 16.Derks W, de Leeuw RJ, Hordijk GJ. Elderly patients with head and neck cancer: the influence of comorbidity on choice of therapy, complication rate, and survival. Curr Opin Otolaryngol Head Neck Surg. 2005;13(2):92–96. doi: 10.1097/01.moo.0000156169.63204.39. [DOI] [PubMed] [Google Scholar]
- 17.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 18.Iversen LH, Nørgaard M, Jacobsen J, Laurberg S, Sørensen HT. The impact of comorbidity on survival of Danish colorectal cancer patients from 1995 to 2006: a population-based cohort study. Dis Colon Rectum. 2009;52(1):71–78. doi: 10.1007/DCR.0b013e3181974384. [DOI] [PubMed] [Google Scholar]
- 19.Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011;103(14):1101–1111. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Land LH, Dalton SO, Jensen M-B, Ewertz M. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990–2008. Breast Cancer Res Treat. 2012;131(3):1013–1020. doi: 10.1007/s10549-011-1819-1. [DOI] [PubMed] [Google Scholar]
- 21.Lüchtenborg M, Jakobsen E, Krasnik M, Linklater KM, Mellemgaard A, Møller H. The effect of comorbidity on stage-specific survival in resected non-small cell lung cancer patients. Eur J Cancer. 2012;48(18):3386–3395. doi: 10.1016/j.ejca.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Wang C-Y, Lin Y-S, Tzao C, et al. Comparison of Charlson comorbidity index and Kaplan-Feinstein index in patients with stage I lung cancer after surgical resection. Eur J Cardiothorac Surg. 2007;32(6):877–881. doi: 10.1016/j.ejcts.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Peipert JF, Wells CK, Schwartz PE, Feinstein AR. Prognostic value of clinical variables in invasive cervical cancer. Obstet Gynecol. 1994;84(5):746–751. [PubMed] [Google Scholar]
- 24.Paleri V, Wight RG, Davies GR. Impact of comorbidity on the outcome of laryngeal squamous cancer. Head Neck. 2003;25(12):1019–1026. doi: 10.1002/hed.10333. [DOI] [PubMed] [Google Scholar]
- 25.Singh B, Bhaya M, Zimbler M, et al. Impact of comorbidity on outcome of young patients with head and neck squamous cell carcinoma. Head Neck. 1998;20(1):1–7. doi: 10.1002/(sici)1097-0347(199801)20:1<1::aid-hed1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Habbous S, Harland LT, La Delfa A, et al. Comorbidity and prognosis in head and neck cancers: differences by subsite, stage, and human papillomavirus status. Head Neck. 2014;36(6):802–810. doi: 10.1002/hed.23360. [DOI] [PubMed] [Google Scholar]
- 27.Yung KC, Piccirillo JF. The incidence and impact of comorbidity diagnosed after the onset of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134(10):1045–1049. doi: 10.1001/archotol.134.10.1045. [DOI] [PubMed] [Google Scholar]
- 28.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110(4):593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Rietbergen MM, Brakenhoff RH, Bloemena E, et al. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment de-escalation trials. Ann Oncol. 2013;24(11):2740–2745. doi: 10.1093/annonc/mdt319. [DOI] [PubMed] [Google Scholar]
- 30.Rios Velazquez E, Hoebers F, Aerts HJ, et al. Externally validated HPV-based prognostic nomogram for oropharyngeal carcinoma patients yields more accurate predictions than TNM staging. Radiother Oncol. 2014;113(3):324–330. doi: 10.1016/j.radonc.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope. 2012;122(S2):S13–S33. doi: 10.1002/lary.23493. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 32.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116(7):1093–1106. doi: 10.1097/01.mlg.0000224939.61503.83. [DOI] [PubMed] [Google Scholar]
- 33.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A., III . AJCC Cancer Staging Manual. 7th. New York, NY: Springer; 2010. [Google Scholar]
- 34.Yasui T, Morii E, Yamamoto Y, et al. Human papillomavirus and cystic node metastasis in oropharyngeal cancer and cancer of unknown primary origin. PLoS One. 2014;9(4):e95364. doi: 10.1371/journal.pone.0095364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zengel P, Assmann G, Mollenhauer M, et al. Cancer of unknown primary originating from oropharyngeal carcinomas are strongly correlated to HPV positivity. Virchows Arch. 2012;461(3):283–290. doi: 10.1007/s00428-012-1290-3. [DOI] [PubMed] [Google Scholar]
- 36.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9(17):6469–6475. [PubMed] [Google Scholar]
- 37.Lewis JS, Jr, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24(11):1413–1420. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3(2):143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 39.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 40.Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol. 2015;33(8):836–845. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 41.Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50(15):2636–2648. doi: 10.1016/j.ejca.2014.07.001. [DOI] [PubMed] [Google Scholar]