Abstract
Early epidemiologic studies have reported that tobacco smoking, which is causally associated with liver cancer, is an independent risk factor for non-alcoholic fatty liver diseases (NAFLD). Lycopene from tomatoes has been shown to be a potential preventive agent against NAFLD and hepatocellular carcinoma (HCC). In the present study, we investigated whether the tobacco carcinogen 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces lesions in both lungs and livers of ferrets with or without lycopene intervention. Male ferrets (6 groups, n = 8-10) were treated either with NNK (50 mg/kg BW, i.p., once a month for four consecutive months) or saline with or without dietary lycopene supplementation (2.2 and 6.6 mg/kg BW/day, respectively) for 26 weeks. Results demonstrate that NNK exposure results in higher incidences of lung tumors, HCC and steatohepatitis (which is characterized by severe inflammatory cell infiltration with concurrent fat accumulation in liver, hepatocellular ballooning degeneration and increased NF-κB expression), as well as elevations in bilirubin and AST levels in ferrets. Lycopene supplementation at two doses prevented NNK-induced expressions of α7 nicotinic acetylcholine receptor in the lung and NF-κB and CYP2E1 in the liver and attenuated the NNK-induced mortality and pathological lesions in both the lungs and livers of ferrets. The present study provided strong experimental evidence that the tobacco carcinogen NNK can induce both HCC and steatohepatitis in the ferrets and can be a useful model for studying tobacco carcinogen-associated NAFLD and liver cancer. Furthermore, lycopene could provide potential benefits against smoke carcinogen-induced pulmonary and hepatic injury.
Keywords: ferret, tobacco carcinogen, liver cancer, steatohepatitis, lycopene
Introduction
Cigarette smoking is recognized to be a strong risk factor for carcinogenesis1 and has been shown to induce lung carcinogenesis in vivo2. Cigarette smoke contains more than 73 carcinogens with the tobacco-specific nitrosamines, such as 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its derivatives, being the most prevalent carcinogens in tobacco products1. NNK is a potent carcinogen that requires activation by the cytochrome p450 (CYP) enzyme system for its tumorigenic activity3 with the production of DNA adducts and single strand breaks of DNA in the lung and liver4. It has been demonstrated that NNK predominantly induces pulmonary carcinogenesis but NNK at high doses (e.g., 200-1800 mg/kg body weight) induces hepatic damage and hepatocellular carcinomas (HCC) in rats5, 6. Tobacco smoking has been shown to be associated with liver diseases7 and is an independent risk factor for non-alcoholic fatty liver disease (NAFLD)8. The risk for liver cancer increases with the duration or the number of cigarettes a person has smoked9. A meta-analysis reported that the pooled relative risk for liver cancer is 1.56 (95% CI = 1.29-1.87) comparing current-smokers to those who have never-smoked, and 1.49 (95% CI = 1.06–2.10) comparing former-smokers to non-smokers, respectively10. In addition, NNK is shown to be a high-affinity ligand for the α7 nicotinic acetylcholine receptor (α7 nAChR)11 . The activation of α7 nAChR modulates the development and progression of lung cancer11, 12, and the pharmacologic or genetic disruption of α7 nAChR significantly reduces angiogenesis and tumor growth13. Indeed, we have demonstrated that ferrets (Mustela putorius furo) exposed to NNK develop pulmonary preneoplastic and plastic lesions, involving high expression of α7 nAChR in the bronchial epithelial cells and pulmonary carcinogenesis14.
Epidemiological studies have suggested an inverse association between the higher intake of lycopene, a major carotenoid from tomatoes and/or tomato products, and the risk of various types of cancer3, 15, 16. Previous in vitro studies suggest that lycopene prevents cell proliferation17, DNA damage18, inhibits neoplastic transformation of normal mouse and human cells and inhibits invasion in hepatoma cell lines19-21. We found that lycopene supplementation inhibited smoke-induced lung squamous metaplasia and induced apoptosis through the up-regulation of insulin-like growth factor-binding protein 3 and the down-regulation of phosphorylation of BAD22. In addition, metabolites of lycopene (apo-10'-lycopenoic acid) induced phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells23, and suppressed NNK-induced lung tumorigenesis24. Lycopene supplementation has been demonstrated to prevent fat accumulation, inflammation, generation of oxidative stress, and regulate hepatic lipid metabolism in animal studies25. Recently, we demonstrated that lycopene and its metabolite apo-10'-lycopenoic acid can inhibit both NAFLD25, 26 and HCC development in mouse models27-29. However, there are very few reports to indicate the efficacy of lycopene on carcinogen-induced lung cancer30 and some researchers have reported no association between lycopene supplementation and lung cancer incidence in vivo31, 32. Unfortunately, these studies are not comparable because of differences in study design regarding species, strains of animals, forms of lycopene (purified lycopene, tomato oleoresin and tomato products), dose ranges, and, importantly, none reported plasma or tissue concentrations of lycopene.
The ferret has been shown to be an excellent non-rodent animal model for studying the effects of micronutrients such as the carotenoids because of the similarities of ferrets and humans in absorption and accumulation33. Further, the ferret is a useful model for pulmonary research studies since the pulmonary structure and airways of ferrets are similar to humans34. Of note, ferrets exposed to NNK alone developed both preneoplastic lesions and pulmonary tumors which are common lesions in humans14. By using this ferret model in the present study, we examined whether the ferrets would develop NAFLD and liver cancer after exposure to NNK, and we investigated the efficacy of different levels of lycopene supplementation against NNK-induced carcinogenesis in both the lungs and livers of the ferrets.
Materials and Methods
Animals, diet, and study groups
Male ferrets (1.1 – 1.7 kg) were obtained from Marshall Farms (North Rose, NY) and maintenance and husbandry of the ferrets was performed as previously described 14, 22. The animals were fed a semi-purified ferret diet (D90001 Research Diets, New Brunswick, NJ: Casein, 34.5%; L-Arginine, 0.5%; L-Methionine, 0.3%; Maltodextrin, 7.5%; Corn starch, 26.5%; Cellulose, 5.0%; Corn oil, 10.5%; Lard, 10.5%; Salt mix (S90002), 3.5%; Vitamin mix (V90002), 1.0%; Choline bitartrate, 0.2%; and lycopene, 0%), with ad libitum access to food and water provided throughout all experiment phases. This diet is suitable for ferret growth 22, and has been used for previous ferret studies14, 22. After a 2 week acclimation, fifty-four ferrets were randomly assigned to one of 6 experimental groups for 26 weeks as follows: (i) control (sham) + placebo (C+P), n = 8; (ii) control + low dose lycopene (C+LL), n = 8; (iii) control + high dose lycopene (C+HL), n = 8; (iv) NNK + placebo (N+P), n = 10; (v) NNK + low dose lycopene (N+LL), n = 10; (vi) NNK + high dose lycopene (N+HL), n = 10. The ferrets were hand-fed lycopene or placebo mixed with peanut butter for 3 weeks (preloading) prior to the NNK or sham injections which continued through the entire study. At the end of the experimental period, ferrets were euthanized by terminal exsanguination under deep isoflurane (Isothesia, Butler Schein, Dublin, OH) anesthesia. The right upper lobe of each lung was inflated and fixed by intratracheal instillation of 10% formalin. Small pieces of liver were collected and fixed in 10% buffered formalin for histological examinations while the remaining lungs and livers were snap frozen in liquid nitrogen and stored at −80°C for later analysis. Plasma was collected and stored at −80°C until analyzed. This study protocol was approved by the Institutional Animal Care and Use Committee at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University.
NNK treatment
The treatment of NNK in the ferrets was performed as previously described 14. Briefly, each experimental ferret was given an intraperitoneal (i.p.) injection of NNK (Toronto Research Chemicals, Ontario, Canada) at a dose of 50 mg/kg BW, once a month for four consecutive months (total dose of 200 mg/kg BW); control animals were given a sham injection of 0.9% NaCl solution utilizing the same injection schedule.
Lycopene supplementation
Water soluble lycopene beadlets containing 10% w/w lycopene or placebo beadlets (BASF Inc., Basel, Germany) were mixed under red light with 0.5 gram peanut butter (Skippy cream peanut butter, NJ) at two doses to provide lycopene at a dose of 2.2 mg/kg BW /day or 6.6 mg/kg BW/day. Since ferrets spontaneously eat peanut butter, gavaging was not necessary. The amount of lycopene added to the peanut butter was calculated each week and varied with the body weight of ferret so that 0.5 gram peanut butter would provide lycopene at the required dose. Lycopene supplemented peanut butter was mixed once a week and was stored at 4 °C in opaque containers to prevent degradation of lycopene. The lower dose (2.2 mg/kg BW/day) is equivalent to a 30 mg/day intake in a 70 kg man and the higher dose (6.6 mg/kg BW/day) is equivalent to a 90 mg/day intake in a 70 kg man, based on our previous ferret studies22, 33. Although both doses are much higher than the average of intake of lycopene in the United States (9.4 ± 0.3 mg/day)35, these doses are achievable by the use of lycopene supplements and have been verified in prostate cancer trials36.
Histopathological examinations
Preparation of samples for both lung and liver tissues was performed as previously described 14. Four-micrometer sections of formalin-fixed, paraffin-embedded lung and liver tissues were stained with hematoxylin (H) and eosin (E) for histopathologic examination. The sections were examined under light microscopy at 100× and 400× magnification by 2 independent investigators who were blinded to the treatment groups. Lung histopathology of neoplastic lesions was evaluated and classified as preneoplastic lesions (atypical adenomatous hyperplasia and squamous metaplasia) and neoplastic lesions (including dysplasia, adenocarcinoma, and SCC). Liver histopathology of the tumor areas was classified as HCC and SCC. Liver histopathology of non-tumor areas was graded according to the magnitude of steatosis and inflammation as described previously25, 37-39. Briefly, the degree of steatosis was graded 0-4 based on the average percent of H&E stained fat-accumulating hepatocytes per field at 100× magnification in 20 random fields (Grading 0 = <5%, 1 = 5-25%, 2 = 26-50%, 3 = 51-75%, 4 = >75%). The degree of inflammation was graded 0-4 based on the amount of ongoing inflammation (amount/location of inflammatory cells infiltrates) and the degree of hepatic fibrosis and necrosis (Grading 0: No activity, 1: Minimal, 2: Mild, 3: Moderate, 4: Severe).
Immunohistochemical assays
Four-micrometer sections of formalin-fixed, paraffin-embedded lung and liver tissues were immunostained for cytokeratin 19 (A53-B/A2, mouse monoclonal antibody, Santa Cruz Biotechnology, Santa Cruz, CA) using the standard avidin-biotin complex immunoperoxidase method (Vectastain ABC-Elite; Vector Laboratories, Burlingame, CA) as described previously40. Briefly, the sections were incubated with a primary antibody at a dilution of 1:50. Subsequently, the sections were incubated with biotinylated anti-mouse antibody (Vector Laboratories, Inc., Burlingame, CA) diluted 1:250 and further incubated with VECTASTAIN Elite ABC reagent. The sections were subsequently processed with peroxidase substrate solution. The sections were then counterstained with hematoxylin. The sections were examined under light microscopy. The cells were considered to be positive for cytokeratin 19 expression in the cytoplasm if tumor cell cytoplasm stained a brown color above the cytoplasmic background.
Western blots analysis
Western blotting was performed with the lung and liver tissues, as previously described14. Antibodies against α7 nAChR, cyclin D1 and matrix metalloproteinase-2 (MMP-2) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies against nuclear factor-kappa B (NF-κB) were purchased from Cell Signaling (Beverly, MA); antibodies against cytochrome P450 (CYP) 2E1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Millipore (Milford, MA). GAPDH was used as a loading control. Results were quantified using a densitometer and expressed as a fold change in the treatment group as compared to the control group.
HPLC analysis
Plasma and liver concentrations of all-trans and cis isomers (5-cis, 9-cis and13-cis) of lycopene were determined by HPLC analysis according methods described in our previous report21. Briefly, a separation system (Waters Corporate, Milford, MA) fitted with a C30 column was used to separate lycopene. Liver tissue (100 mg) was homogenized in 2 mL of saline and ethanol (1:1 ratio); 1.0 mL of saline and ethanol (1:1 ratio) was added to 1.0 mL of plasma. Lycopene was then extracted separately from both types of samples using 5 mL of hexane and ether (1:1 ratio) by: vortexing for 3 min, centrifuging at 20,000 g for 10 min at 4°C, and collecting the upper layer. Samples were extracted 3 times and were evaporated under nitrogen gas; samples were subsequently reconstituted with 100 μL of ethanol and ether (1:1 ratio); a 50 μL aliquot of the reconstituted extract with ethanol and ether (1:1 ratio) was injected into the system to measure lycopene concentrations. Retinyl acetate and echinenone were used as the internal control to determine the efficiency of extraction. All procedures were conducted under red light.
Liver function analysis
Plasma levels of albumin, bilirubin, alanine aminotransferase (ALT) and aspartic aminotransferase (AST) were analyzed using commercially available assay kits (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Statistical analysis
Results are expressed as means ± SEM or medians (range). Kaplan-Meier survival curves were applied for determination of the survival rate. Differences in survival between experimental groups were analyzed using the log-rank test. One-way ANOVA followed by a Tukey-Kramer post-hoc analysis was performed for mean values of the multiple groups. The incidences of mortality and lesions were evaluated using the Fisher exact test. The grading of steatosis and inflammation was evaluated by a Kruskal-Wallis test for any differences in distribution among groups, followed by a Dunn's multiple comparison test for comparing groups. Results were considered statistically significant at P<0.05.
Results
Mortality, Body weights, liver weights
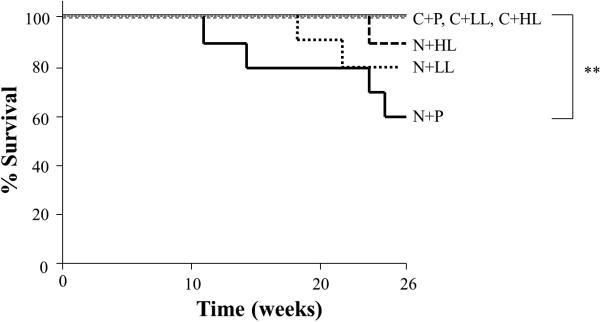
There was no mortality in any of the control groups (C+P, C+LL and C+ HL) prior to the completion of the experiment. Four ferrets died at 11, 14, 24, and 25 weeks, respectively, after the first injection of NNK in the N+P group, which had an overall 40% mortality rate. Although the cause of death was inconclusive for each of these animals, multiple lung tumors and nodules on the hepatic surface of the dead animals were detected at the time of autopsy. We observed statistically significant differences in survival between the control groups and the N+P group by log-rank analysis in the Kaplan-Meier survival curve (P<0.01). Two ferrets died at 18 and 22 weeks in N+LL group (20%), and one ferret died at 24 weeks in N+HL group (11%), respectively (Figure 1). There was no significant difference in mortality when comparing the lycopene supplementation groups (N+LL and N+HL) with the N+P group or controls. There were no significant differences in body weights or liver weights at the end of the experiment between the groups, although NNK treated ferrets (N+P) had non-significant reductions in body liver weights when compared to the control ferrets (Table 1).
Figure 1.
Kaplan - Meier survival analysis in ferrets injected with either sham or NNK with or without lycopene supplementation. **P<0.01 (Long rank test). Abbreviations: C+P: control (sham) + placebo group; C+LL: control + low-dose lycopene group; C+HL: control + high-dose lycopene group; N+P: NNK + placebo group; N+LL: NNK + low-dose lycopene group: N+HL: NNK + high-dose lycopene group.
Table 1.
Body weight, liver weight, hepatic function, and lung and hepatic lesions in the ferrets
| Group | C+P | C+LL | C+HL | N+P | N+LL | N+HL1 |
|---|---|---|---|---|---|---|
| Animal (n) | 8 | 8 | 8 | 10 | 10 | 10 |
| Body weight2 (kg) | 1.58 ± 0.05 | 1.54 ± 0.04 | 1.56 ± 0.07 | 1.42 ± 0.02 | 1.51 ± 0.07 | 1.52 ± 0.05 |
| Liver weight2 (g) | 55.4 ± 2.49 | 52.5 ± 3.72 | 53.6 ± 2.83 | 49.8 ± 2.06 | 45.1 ± 2.64 | 52.5 ± 2.99 |
| Plasma3 | ||||||
| Albumin (g/dL) | 3.86 ± 0.18 | 4.03 ± 0.17 | 4.10 ± 0.19 | 3.20 ± 0.25 | 3.22 ± 0.38 | 3.40 ± 0.19 |
| Bilirubin (mg/dL) | 0.15 ± 0.02a | 0.19 ± 0.02a,b | 0.16 ± 0.02a,b | 0.33 ± 0.06b | 0.32 ± 0.06a,b | 0.28 ± 0.02a,b |
| ALT (IU/L) | 225.3 ± 21.5 | 269.9 ± 65.3 | 182.5 ± 18.9 | 528.4 ± 142.4 | 579.7 ± 152.9 | 513.9±93.4 |
| AST (IU/L) | 70.8 ± 4.4a | 83.3 ± 14.3a | 79.4 ± 4.2a | 231.4 ± 50.7b | 199.8 ± 47.8a,b | 154.0 ± 21.6a,b |
| Lung | ||||||
| Preneoplastic lesions4 Positive/Total animals (%) | 0/8a (0) | 0/8a (0) | 0/8a (0) | 10/10b (100) | 8/10b (80) | 8/9b (89) |
| Neoplastic lesions5 Positive/Total animals (%) | 0/8a (0) | 0/8a (0) | 0/8a (0) | 9/10b (90) | 7/10b (70) | 4/9a,b (44) |
| Liver | ||||||
| Neoplastic lesions,6 Positive/Total animals (%) | 0/8a (0) | 0/8a (0) | 0/8a (0) | 4/10b (40) | 3/10b (30) | 1/9b (11) |
| Steatosis grade7 Median (Range) | 1.5a (0-2) | 1.5a (0-3) | 1.0a (0-3) | 4.0b (2-4) | 3.0a,b (1-4) | 2.0a,b (0-4) |
| Inflammation grade8 Median (Range) | 1.5a (0-2) | 1.0a (0-2) | 1.0a (0-3) | 4.0b (2-4) | 2.0a,b (1-4) | 2.0a,b (0-4) |
C: Control; N: NNK-treated; P: Placebo; LL: Low dose lycopene supplemented; and HL: High dose lycopene supplemented. One ferret in the N+HL group was deleted due to the accidental death (chocked to the hairball) at 6 weeks.
Values are expressed as means ± SEM, n = 6-8, which is the number of animals who were alive at the end of the experimental (26 weeks). Means with different superscripts in a column are significantly different from each other, P<0.05 as determined by ANOVA (Tukey).
Including atypical adenomatous hyperplasia and squamous metaplasia.
Including dysplasia, adenocarcinoma and squamous cell carcinomas.
Including hepatocellular carcinomas and squamous cell carcinomas.
The degree of steatosis is graded 0-4 based on the average percent of fat-accumulated hepatocytes per field (Grading 0 = <5%, 1 = 5-11 25%, 2 = 26-50%, 3 = 51-75%, 4 = >75%, see Method section). Values are expressed as Median (range), n = 6-8/each group. Means with different superscripts in a row are significantly different from each other, P<0.05 as determined by Kruskal-Wallis test.
The degree of inflammation is graded 0-4 based on the hepatic fibrosis, inflammation and necrosis (Grading 0: No activity, 1: Minimal, 2: Mild, 3: Moderate, 4: Severe). Values are expressed as Median (range), n = 6-8/each group. Means with different superscripts in a row are significantly different from each other, P<0.05 as determined by Kruskal-Wallis test.
Lung lesions
No preneoplastic or neoplastic lesions were observed in the lungs of the control group animals (including C+P, C+LL and C+HL groups, Table 1). Both preneoplastic lesions (atypical adenomatous hyperplasia and squamous metaplasia) and neoplastic lesions (including dysplasia, adenocarcinoma, and SCC) were observed in the lungs of NNK treatment ferrets (Table 1, and Figure 2). The incidence of preneoplastic of lesions in the lungs of all of the NNK treatment group animals was 80 - 100% and there were no significant differences between the NNK treatment groups with or without lycopene supplementation. The incidences of neoplastic lesions in the lungs were 90% in the N+P group, 70% in the N+LL group and 44% in the N+HL group, respectively and there were significant differences in the incidences of neoplastic lesions in the lungs between the control groups and the N+P and the N+LL groups. Differences in the incidence of neoplastic lesions between the control group and the N+HL group were not statistically significant.
Figure 2.
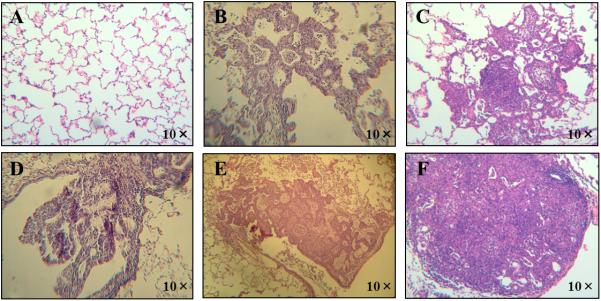
Representative image of histopathologic examination with hematoxylin and eosin staining: normal lung tissue (Panel A), atypical adenomatous hyperplasia (Panel B), squamous metaplasia (Panel C), dysplasia (Panel D), adenocarcinoma (Panel E) and squamous cell carcinoma (Panel F) at 10× magnifications in the ferrets exposed to NNK for 26 weeks.
Hepatic lesions
Only mild steatosis was observed in the livers of ferrets not receiving the NNK treatment (Figure 3-A) but NNK treatment resulted in significant steatohepatitis with typical features of steatosis, inflammatory cell infiltration and ballooning degeneration of the livers (Table 1, Figures 3-B and 3-C). There were no significant differences between the control groups and lycopene supplementation groups (N+LL and N+HL) regarding the grades (severity) of steatosis and inflammation. There were significant differences in the incidence of the most severe steatohepatitis (Score: 4 for steatosis and inflammation) between the N+P group and the N+LL, N+HL groups (P < 0.05). The most severe grading (Score: 4) for both steatosis and inflammation in the N+P groups was observed in five ferrets, however, there was only one ferret with severe steatohepatitis in each of the N+LL and N+HL groups. In the N+HL groups, there was only one ferret without any steatosis and inflammation (both scores: 0).
Figure 3.
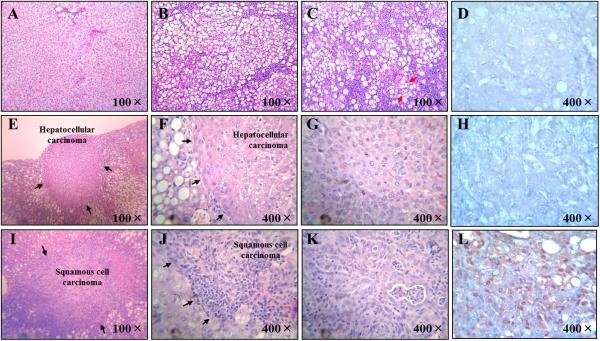
Representative image of normal liver with very mild steatosis (Panel a); hepatic steatosis with inflammatory cell infiltration (Panel b); steatohepatitis with severe fat accumulation, inflammation and hepatocellular ballooning degeneration (Panel c, The red arrow indicated the hepatocellular ballooning degeneration); hepatic non-cancerous tissue immunostained by cytokeratin 19 (Panel d), HCC (Panels e, f and g), HCC immunostained by cytokeratin 19 (Panel h), hepatic SCC (Panels i, j and k) and hepatic SCC immunostained by cytokeratin 19 (Panel l). The black arrows indicated the border between the non-cancerous tissue and cancerous tissue in Panels e, f, i and j.
There were no neoplastic lesions observed in the livers of any of the control groups. We found both HCC and SCC (Figures 3-E, 3-F, 3-G, 3-I, 3-J and 3-K) in the livers of ferrets treated with NNK (40% incidence) which was lower in the lycopene supplemented groups (30% in the N+LL group and 11% in the N+HL group). There was a significant difference in the incidences of hepatic neoplastic lesions between N+P groups and all control groups (P<0.01) but the difference was diminished in the N+HL group.
All of the hepatic tumors induced by NNK injection were HCC except for one tumor with the feature of SCC (similar to that observed in lung) (Figures 2-D and 2-E) in the livers of ferrets in the N+P group (Figures 3-I, 3-J and 3-K). Cytokeratin 19, a member of the Type I acidic subfamily of intermediate filaments, is expressed in various tissues including pulmonary carcinomas (but not hepatic) and has been used to differentiate hepatic metastasis from lung cancer. We found that the SCCs that developed in lungs but observed in liver stained for cytokeratin 19 (Figure 2-F and Figure 3-L) and hepatic non-cancerous tissue and HCCs similarly were not stained for cytokeratin 19 (Figures 3-D and 3-H), suggesting that the hepatic SCCs were metastasized from lung.
We found that the plasma bilirubin and AST levels in ferrets injected with NNK were significantly increased (Table 1). The significant differences in plasma bilirubin and AST level were dramatically reduced in animals treated with lycopene supplementation, as compared to the N+P group (Table 1). There were tendencies of decreased plasma albumin level and increased plasma ALT in ferrets treated with NNK (Table 1).
Molecular biomarkers
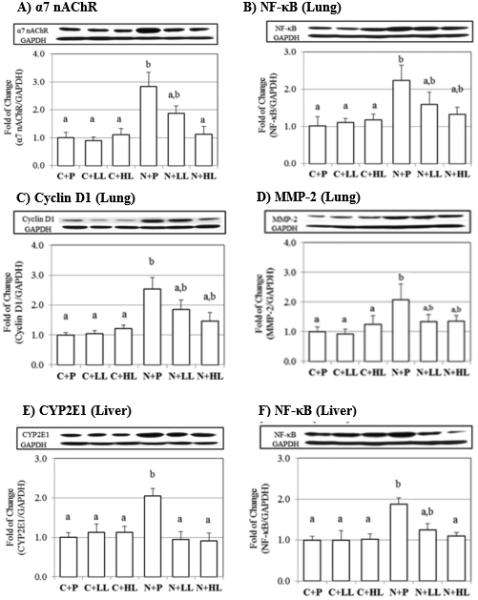
We observed that α7 nAChR protein levels in the lungs of ferrets were significantly increased by NNK treatment, as previously reported10. In addition, we observed that NNK treatment significantly increased the protein levels of cyclin D1, NF-κB and MMP-2 (Figures 4-B, 4-C and 4-D). In contrast, lycopene supplementation decreased NNK-induced α7 nAChR protein levels in a dose-dependent manner (Figure 4-A). Lycopene supplementation tended to decrease NNK-induced NF-κB, cyclin D1 and MMP-2 protein levels (Figures 4-B, 4-C and 4-D).
Figure 4.
The expression of pulmonaryα7 nAChR (a) ,NF-κB (b), Cyclin D1 (c), MMP-2 (d) and hepatic CYP2E1 (e), NF-κB (f) protein levels of ferrets exposed to NNK for 26 weeks with or without lycopene supplementation, measured by western blotting. GAPDH was chosen as internal control for protein equal loading. Values are expressed Mean ± SEM. Different letters for given bars indicate that those values are significantly different from each other (P<0.05, ANOVA (Tukey)).
While there was no significant difference in CYP 2E1 protein levels in the lungs between groups (data not shown), we observed that hepatic CYP 2E1 protein levels were significantly increased in the N+P group when compared to the other experimental groups (Figure 4E); lycopene supplementation at both doses significantly decreased CYP 2E1 protein levels (Figure 4-E). In addition, we observed a significant increase of NF-κB protein levels in the N+P group and lycopene supplementation decreased NNK-induced NF-κB protein levels in a dose-dependent manner (Figure 4-F). We did not detect α7 nAChR protein in the livers of any of the experimental groups (data not shown).
Lycopene isomer concentrations in plasma and liver
All-trans, 5-cis, 9-cis and 13-cis isomers of lycopene were detected in the plasma and livers of lycopene supplemented ferrets (Table 2). Although there were no significant differences, high plasma concentrations of total lycopene were observed in the high lycopene supplemented groups (C+HL) compared to the low dose groups (C+LL) without NNK treatment, however, NNK treatments significantly decreased plasma lycopene concentrations (C+LL, C+HL vs. N+LL, N+HL). Hepatic lycopene concentrations tended to be increased by NNK treatment in the liver. The ratio of trans and cis-isomers in both plasma and liver were not affected by NNK treatment.
Table 2.
Plasma and hepatic lycopene isomer concentrations in the ferret1.
| Group | C+P | C+LL | C+HL | N+P | N+LL | N+HL |
|---|---|---|---|---|---|---|
| Plasma | (nmol/L) | |||||
| Total | ND3 | 142.0 ± 34.6a,b | 230.0 ± 45.7a | ND | 95.3 ± 28.5b | 101.0 ± 12.1b |
| All-trans | ND | 67.7 ± 17.8a,b | 111.2 ± 21.2a | ND | 51.6 ± 10.5b | 43.2 ± 4.1b |
| Sum of cis2 | ND | 74.6 ± 27.8 | 119.6 ± 27.8 | ND | 43.7 ± 21.3 | 58.0 ± 12.7 |
| trans:cis | - | 48:52 | 48:52 | - | 54:46 | 43:57 |
| Liver | (nmol/g tissue) | |||||
| Total | ND3 | 1.4 ± 0.3 | 1.7 ± 0.5 | ND | 1.6 ± 0.3 | 2.6 ± 0.4 |
| All-trans | ND | 0.5 ± 0.1 | 0.5 ± 0.2 | ND | 0.5 ± 0.1 | 0.9 ± 0.2 |
| Sum of cis2 | ND | 0.9 ± 0.1 | 1.2 ± 0.3 | ND | 1.1 ± 0.2 | 1.7 ± 0.3 |
| trans:cis | - | 35:65 | 31:69 | - | 29:71 | 36:64 |
Values are expressed as mean ± SEM, n = 6-8/each group. Different letters for given column in a low indicate that those values are significantly different from each other (P<0.05, ANOVA (Tukey)). C: Control; N: NNK-treated; P: Placebo; LL: Low dose lycopene supplemented; and HL: High dose lycopene supplemented.
Sum of 5-cis, 9-cis and 13-cis of lycopene isomers.
ND: not detected
Discussion
NNK treatment resulted in both lung and liver tumors including HCC and metastasized tumor
Although it is known that NNK is a relatively specific lung carcinogen, in the present study we demonstrated that the ferrets developed both pulmonary tumors (9 out of 10 ferrets) and liver tumors (4 out of 10) with pathologic features of HCC. In addition, the total dose of NNK injected into the ferrets (200 mg/Kg) was much less than that previously (~1800 mg/Kg) used in rats5, 6. Interestingly, we observed one SCC in the liver of a NNK treated ferret, suggesting the possibility that it had metastasized from the lung to the liver. This observation was supported by both pathological analyses (Figures 2-E and 3-K) and positive immunohistochemistry staining using cytokeratin 19 (Figures 2-F and 3-L). Cytokeratin 19 is known to be strongly expressed in bronchial and respiratory epithelium41, not in hepatocytes42, and has been used as a marker for the identification of liver metastasis. As anticipated, the HCCs did not stain for cytokeratin 19 (Figure 2-H) in these ferrets indicating that these HCCs originated from the ferret livers. In addition, pulmonary MMP-2 protein levels were increased by NNK treatment (Figure 4-D), which is consistent with metastasis associated with specific types of carcinomas43. Since lung cancer metastasis is frequent in humans and lung tumors from carcinogen treatment in mice rarely metastasize44, these observations suggest that the ferret may be a relevant model for the study of lung carcinogenesis and tumor metastasis.
NNK treatment resulted in hepatic lesions which closely resembles NAFLD and NASH in humans
It has been reported that cigarette smoking is an independent risk factor for NAFLD8 and cigarette smoking increases lipid accumulation in the hepatocytes of mice45. The interesting aspect of the present study was that NNK treatment resulted not only in steatosis but also NASH (characterized by severe inflammatory cell infiltration with concurrent fat accumulation in liver, hepatocellular ballooning degeneration and increased NF-κB expression and increases to bilirubin and AST levels. Therefore, the development of hepatic lesions (including NASH and HCC) in the ferrets, which closely resembles NAFLD, NASH and HCC in humans, may provide an excellent animal model for dietary intervention studies for hepatic disease. However, the exact mechanism(s) regarding how NNK exposure promoted these hepatic lesions remains unclear. Ferrets are obligate carnivores (meat eaters) and need to obtain a majority of their calories from high fat (~40%), high protein (~38%) and simple carbohydrates diets. While we observed that feeding this high fat diet (40.2% of total calorie from fat) resulted in the development of mild steatosis in the livers of ferrets without NNK exposure, NNK exposure resulted in significant steatosis, NASH and HCC development in the ferrets. Since we did not observe any significant hepatic lesions (e.g., steatosis, NASH and HCC) with the same dose of NNK injection in our previous animal studies fed with chow diet, we believe that the steatosis induced by this high fat diet may prime the liver for the development of more aggressive lesions (such as NASH and HCC) in response to NNK exposure as a “second-hit” in the ferrets. The findings from the current study are consistent with a previous study that reported that nicotine, a precursor of NNK and well known to be a strong agonist of nAChR, can interfere with normal lipid metabolism and has been shown to result in hepatic steatosis in mice fed a high-fat diet47. Our study also confirms the finding of a recent study that found that NNK can alter normal lipid metabolism in the livers of alcohol fed rats46. Unlike lungs, we were not able to detect α7 nAChR protein in the livers of ferrets, possibly due to the low expression levels of hepatic α7 nAChR. Interestingly, NNK exposure resulted in significantly increased hepatic CYP2E1 protein levels which has been shown to play a role in oxidative stress, inflammation, and tumorigenesis53. Since NNK was metabolized by Phase I enzymes (such as CYPs including CYP2E1) which activated its mutagenicity3, 52, it is reasonable to conclude that the induction of hepatic CYP2E1 protein levels promoted a carcinogen-initiated, high fat diet-promoted hepatic oxidative stress, inflammation and carcinogenesis37-39; the induction of CYP2E1 by NNK exposure would contribute to the development of NASH and HCC in the present study.
Lycopene supplementation suppressed NNK-induced pulmonary α7 nAChR and hepatic CYP2E1, which were associated with lower mortality and incidences of both pulmonary and hepatic lesions
We have previously reported that lycopene supplementation prevents cigarette smoke-induced cell proliferation and over-expression of cyclin D1 in both the lungs and gastric mucosa of ferrets40, 48. In the present study, we observed that α7 nAChR and its down-stream protein (cyclin D1, NF-κB and MMP-2) levels were notably decreased by lycopene supplementation, suggesting that lycopene could prevent the activation of proliferation, inflammation and angiogenesis of pulmonary cancerous cells via suppression of pulmonary α7 nAChR. This notion was supported by previous studies that demonstrated that lycopene could inhibit MMPs expression, regulate pathways converging at the NF-κB-binding sites49, and inhibit experimental metastasis involved with the suppression of MMP-2 and other tumor activating markers20. We have recently observed that carotenoid beta-cryptoxanthin supplementation inhibited lung tumorigenesis and was associated with significant reductions of both mRNA and protein levels of α7 nAChR, and mRNA of early growth response-1, an activator to stimulate the promoter activity of α7 nAChR (Anita R. Iskandar AR & Wang XD, Manuscript in preparation). The present study shows that lycopene supplementation decreases both mortality and incidence of pulmonary carcinomas induced by NNK treatment, but statistically significant differences between the N+P, N+LL, and N+HL groups were not reached due to the relatively small sample size for the experimental groups. Regardless, the significant differences on mortality and incidence of pulmonary carcinomas between the N+P group and the control groups were reduced by lycopene supplementation in the NNK-treated ferrets, suggesting potential benefits of lycopene supplementation.
It should be noted that the lycopene concentrations in both plasma (142.0 – 230.0 nmol/L) and liver (1.42 – 1.69 nmol/g) in the ferrets without NNK treatment groups were comparatively lower than the lycopene concentrations reported in humans (260 – 900 nmol/L in plasma and 0.1 – 20.7 nmol/g in liver, respectively)50, suggesting that lycopene's effects in this model took place at low and physiologically relevant concentrations. In addition, the NNK exposure decreased plasma lycopene concentrations in a dose-dependent manner. This is consistent with our previous data using ferrets, in which cigarette smoke exposure decreased plasma lycopene concentrations in both the low-dose and high-dose lycopene groups22. The National Health and Nutrition Examination Survey III reported that smokers have lower serum concentrations of lycopene compared with nonsmokers51. Why cigarette smoking decreases plasma lycopene levels remains unclear. Since the ratio of cis/all-trans lycopene isomers (sum of 5-cis, 9-cis and 13-cis of lycopene isomers vs. all-trans lycopene) in the plasma and livers (46-57% and 64-71%, respectively) of the ferrets was similar to that found in human blood and liver (58-73%)50, one of the possible explanations was that cigarette smoke or its components may increase decomposition of carotenoids by chemical and/or enzymatic reactions, as we previously reported 22. Another possible explanation related to NNK-induced steatohepatitis is that the fat-soluble lycopene might not be transported into circulating blood by the lipoproteins. This possibility was supported by the finding of a slight increase of hepatic lycopene in the NNK-treated ferrets. Further studies are certainly required to investigate the interactive effect of the NNK and lycopene metabolism and dosage and will potentially provide valuable insights into the mechanisms underlying the beneficial effect of lycopene against SCC and HCC development.
An interesting observation from the present study was our demonstration that NNK treatment significantly increased hepatic CYP2E1 protein levels, which was completely inhibited by lycopene supplementation at both doses. This inhibition was associated with decreased levels of hepatic steatosis, inflammation, bilirubin, and AST, as well as the finding of a lower incidence of tumor in NNK-treated ferrets that received lycopene supplementation. Therefore, the inhibition of NNK-induced CYP2E1 contributed significantly to the protective effects of lycopene against hepatic inflammation and carcinogenesis. Our study was consistent with several reports which have reported that lycopene and lycopene metabolites have various physiological functions, such as reduction of hepatic fat accumulation, inhibition of hepatic inflammation, and suppression of both lung and liver tumorigenesis23-27, 29, 39. Therefore, the prevention of NNK-induced CYP2E1 by lycopene supplementation may play a significant role in terms of lycopene protective effects against liver injury.
Smoking tobacco is a risk factor for non-alcoholic fatty liver diseases (NAFLD) and hepatocellular carcinoma (HCC). Using the ferret as a model, we have provided strong experimental evidence that a tobacco carcinogen induces not only lung cancer but also NAFLD, steatohepatitis and HCC. Furthermore, dietary lycopene supplementation attenuated pathological lesions in both the lungs and livers of ferrets. The ferret can be a useful non-rodent model for studying tobacco smoking for NAFLD, steatohepatitis and HCC development.
Acknowledgement
This study was supported by the NIH/NCI CA176256 grant, and US Department of Agriculture grants 1950-51000-064S (ARS) and 2015-67017(NIFA). Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of National Institute of Health and the U.S. Department of Agriculture. The authors would also like to thank John Lomartire for his assistance on this manuscript.
Abbreviations
- α7
nAChR α7 nicotinic acetylcholine receptor
- ALT
alanine aminotransferase
- AST
aspartic aminotransferase
- BW
body weight
- CYP
cytochrome P450
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HCC
hepatocellular carcinomas
- MMP-2
matrix metalloproteinase-2
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NF-κB
nuclear factor-kappa B
- NNK
4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone
- SCC
squamous cell carcinoma
Footnotes
Conflict of interest statements
Author Disclosures: Koichi Aizawa, Chun Liu, Sanyuan Tang, Sudipta Veeramachaneni, Kang-Quan Hu, Donald E. Smith, and Xiang-Dong Wang have no conflicts of interest.
References
- 1.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–32. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng HC, Takano Y. NNK-Induced Lung Tumors: A Review of Animal Model. J Oncol. 2011;2011:635379. doi: 10.1155/2011/635379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maser E. Significance of reductases in the detoxification of the tobacco-specific carcinogen NNK. Trends Pharmacol Sci. 2004;25:235–7. doi: 10.1016/j.tips.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Grando SA. Connections of nicotine to cancer. Nat Rev Cancer. 2014;14:419–29. doi: 10.1038/nrc3725. [DOI] [PubMed] [Google Scholar]
- 5.Hecht SS, Chen CB, Ohmori T, Hoffmann D. Comparative carcinogenicity in F344 rats of the tobacco-specific nitrosamines, N′-nitrosonornicotine and 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1980;40:298–302. [PubMed] [Google Scholar]
- 6.Hoffmann D, Rivenson A, Amin S, Hecht SS. Dose-response study of the carcinogenicity of tobacco-specific N-nitrosamines in F344 rats. J Cancer Res Clin Oncol. 1984;108:81–6. doi: 10.1007/BF00390978. [DOI] [PubMed] [Google Scholar]
- 7.El-Zayadi AR. Heavy smoking and liver. World J Gastroenterol. 2006;12:6098–101. doi: 10.3748/wjg.v12.i38.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamabe A, Uto H, Imamura Y, Kusano K, Mawatari S, Kumagai K, Kure T, Tamai T, Moriuchi A, Sakiyama T, Oketani M, Ido A, et al. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol. 2011;46:769–78. doi: 10.1007/s00535-011-0376-z. [DOI] [PubMed] [Google Scholar]
- 9.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 10.Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–64. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 11.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29:151–8. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Improgo MR, Tapper AR, Gardner PD. Nicotinic acetylcholine receptor-mediated mechanisms in lung cancer. Biochem Pharmacol. 2011;82:1015–21. doi: 10.1016/j.bcp.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–36. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aizawa K, Liu C, Veeramachaneni S, Hu K-Q, Smith DE, Wang X-D. Development of ferret as a human lung cancer model by injecting 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Lung Cancer. 2013;82:390–96. doi: 10.1016/j.lungcan.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaud DS, Feskanich D, Rimm EB, Colditz GA, Speizer FE, Willett WC, Giovannucci E. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am J Clin Nutr. 2000;72:990–7. doi: 10.1093/ajcn/72.4.990. [DOI] [PubMed] [Google Scholar]
- 16.Holick CN. Dietary Carotenoids, Serum beta-Carotene, and Retinol and Risk of Lung Cancer in the Alpha-Tocopherol, Beta-Carotene Cohort Study. American Journal of Epidemiology. 2002;156:536–47. doi: 10.1093/aje/kwf072. [DOI] [PubMed] [Google Scholar]
- 17.Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, Sharoni Y. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr Cancer. 1995;24:257–66. doi: 10.1080/01635589509514415. [DOI] [PubMed] [Google Scholar]
- 18.Muzandu K, El Bohi K, Shaban Z, Ishizuka M, Kazusaka A, Fujita S. Lycopene and beta-carotene ameliorate catechol estrogen-mediated DNA damage. Jpn J Vet Res. 2005;52:173–84. [PubMed] [Google Scholar]
- 19.Wang XD. Lycopene metabolism and its biological significance. Am J Clin Nutr. 2012;96:1214S–22S. doi: 10.3945/ajcn.111.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CS, Liao JW, Hu ML. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J Nutr. 2008;138:538–43. doi: 10.1093/jn/138.3.538. [DOI] [PubMed] [Google Scholar]
- 21.Huang CS, Shih MK, Chuang CH, Hu ML. Lycopene inhibits cell migration and invasion and upregulates Nm23-H1 in a highly invasive hepatocarcinoma, SK-Hep-1 cells. J Nutr. 2005;135:2119–23. doi: 10.1093/jn/135.9.2119. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003;63:3138–44. [PubMed] [Google Scholar]
- 23.Lian F, Wang XD. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int J Cancer. 2008;123:1262–8. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28:1567–74. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 25.Ip BC, Liu C, Lichtenstein AH, von Lintig J, Wang XD. Lycopene and apo-10′-lycopenoic acid have differential mechanisms of protection against hepatic steatosis in beta-carotene-9′,10′-oxygenase knockout male mice. J Nutr. 2015;145:268–76. doi: 10.3945/jn.114.200238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung J, Koo K, Lian F, Hu KQ, Ernst H, Wang XD. Apo-10′-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. J Nutr. 2012;142:405–10. doi: 10.3945/jn.111.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int J Cancer. 2010;126:1788–96. doi: 10.1002/ijc.24689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ip BC, Liu C, Ausman LM, von Lintig J, Wang XD. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Cancer Prev Res (Phila) 2014;7:1219–27. doi: 10.1158/1940-6207.CAPR-14-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ip BC, Wang XD. Non-alcoholic steatohepatitis and hepatocellular carcinoma: implications for lycopene intervention. Nutrients. 2014;6:124–62. doi: 10.3390/nu6010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DJ, Takasuka N, Kim JM, Sekine K, Ota T, Asamoto M, Murakoshi M, Nishino H, Nir Z, Tsuda H. Chemoprevention by lycopene of mouse lung neoplasia after combined initiation treatment with DEN, MNU and DMH. Cancer Lett. 1997;120:15–22. doi: 10.1016/s0304-3835(97)00281-4. [DOI] [PubMed] [Google Scholar]
- 31.Hecht SS, Kenney PM, Wang M, Trushin N, Agarwal S, Rao AV, Upadhyaya P. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137:123–30. doi: 10.1016/s0304-3835(98)00326-7. [DOI] [PubMed] [Google Scholar]
- 32.Guttenplan JB, Chen M, Kosinska W, Thompson S, Zhao Z, Cohen LA. Effects of a lycopene-rich diet on spontaneous and benzo[a]pyrene-induced mutagenesis in prostate, colon and lungs of the lacZ mouse. Cancer Lett. 2001;164:1–6. doi: 10.1016/s0304-3835(00)00705-9. [DOI] [PubMed] [Google Scholar]
- 33.Wang XD, Krinsky NI, Marini RP, Tang G, Yu J, Hurley R, Fox JG, Russell RM. Intestinal uptake and lymphatic absorption of beta-carotene in ferrets: a model for human beta-carotene metabolism. Am J Physiol. 1992;263:G480–6. doi: 10.1152/ajpgi.1992.263.4.G480. [DOI] [PubMed] [Google Scholar]
- 34.Vinegar A, Sinnett EE, Kosch PC, Miller ML. Pulmonary physiology of the ferret and its potential as a model for inhalation toxicology. Lab Anim Sci. 1985;35:246–50. [PubMed] [Google Scholar]
- 35.Institute of Medicine (US) Panel on Micronutrients . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US); Washington (DC): 2001. [PubMed] [Google Scholar]
- 36.Clark PE, Hall MC, Borden LS, Jr., Miller AA, Hu JJ, Lee WR, Stindt D, D'Agostino R, Jr., Lovato J, Harmon M, Torti FM. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67:1257–61. doi: 10.1016/j.urology.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Nonalcoholic steatohepatitis induced by a high-fat diet promotes diethylnitrosamine-initiated early hepatocarcinogenesis in rats. Int J Cancer. 2009;124:540–6. doi: 10.1002/ijc.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–17. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Ip BC, Hu KQ, Liu C, Smith DE, Obin MS, Ausman LM, Wang XD. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev Res (Phila) 2013;6:1304–16. doi: 10.1158/1940-6207.CAPR-13-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu C, Bronson RT, Russell RM, Wang XD. beta-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev Res (Phila) 2011;4:1255–66. doi: 10.1158/1940-6207.CAPR-10-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chyczewski L, Niklinski J, Chyczewska E, Laudanski J, Furman M. Immunohistochemical analysis of tissue localization of cytokeratin 19 in lung cancer. Rocz Akad Med Bialymst. 1997;42(Suppl 1):162–72. [PubMed] [Google Scholar]
- 42.Lai YS, Thung SN, Gerber MA, Chen ML, Schaffner F. Expression of cytokeratins in normal and diseased livers and in primary liver carcinomas. Arch Pathol Lab Med. 1989;113:134–8. [PubMed] [Google Scholar]
- 43.Rojiani MV, Alidina J, Esposito N, Rojiani AM. Expression of MMP-2 correlates with increased angiogenesis in CNS metastasis of lung carcinoma. Int J Clin Exp Pathol. 2010;3:775–81. [PMC free article] [PubMed] [Google Scholar]
- 44.Nikitin AY, Alcaraz A, Anver MR, Bronson RT, Cardiff RD, Dixon D, Fraire AE, Gabrielson EW, Gunning WT, Haines DC, Kaufman MH, Linnoila RI, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64:2307–16. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]
- 45.Yuan H, Shyy JY, Martins-Green M. Second-hand smoke stimulates lipid accumulation in the liver by modulating AMPK and SREBP-1. J Hepatol. 2009;51:535–47. doi: 10.1016/j.jhep.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zabala V, Tong M, Yu R, Ramirez T, Yalcin EB, Balbo S, Silbermann E, Deochand C, Nunez K, Hecht S, de la Monte SM. Potential contributions of the tobacco nicotine-derived nitrosamine ketone (NNK) in the pathogenesis of steatohepatitis in a chronic plus binge rat model of alcoholic liver disease. Alcohol Alcohol. 2015;50:118–31. doi: 10.1093/alcalc/agu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman TC, Sinha-Hikim I, Parveen M, Najjar SM, Liu Y, Mangubat M, Shin CS, Lyzlov A, Ivey R, Shaheen M, French SW, Sinha-Hikim AP. Additive effects of nicotine and high-fat diet on hepatic steatosis in male mice. Endocrinology. 2012;153:5809–20. doi: 10.1210/en.2012-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu C, Russell RM, Wang XD. Lycopene supplementation prevents smoke-induced changes in p53, p53 phosphorylation, cell proliferation, and apoptosis in the gastric mucosa of ferrets. J Nutr. 2006;136:106–11. doi: 10.1093/jn/136.1.106. [DOI] [PubMed] [Google Scholar]
- 49.Huang CS, Fan YE, Lin CY, Hu ML. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J Nutr Biochem. 2007;18:449–56. doi: 10.1016/j.jnutbio.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, Clinton SK. Prostate Carcinogenesis in N-methyl-N-nitrosourea (NMU)-Testosterone-Treated Rats Fed Tomato Powder, Lycopene, or Energy-Restricted Diets. CancerSpectrum Knowledge Environment. 2003;95:1578–86. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 51.Wei W, Kim Y, Boudreau N. Association of smoking with serum and dietary levels of antioxidants in adults: NHANES III, 1988-1994. Am J Public Health. 2001;91:258–64. doi: 10.2105/ajph.91.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karamanakos PN, Trafalis DT, Geromichalos GD, Pappas P, Harkitis P, Konstandi M, Marselos M. Inhibition of rat hepatic CYP2E1 by quinacrine: molecular modeling investigation and effects on 4-(methyl nitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced mutagenicity. Arch Toxicol. 2009;83:571–80. doi: 10.1007/s00204-008-0350-6. [DOI] [PubMed] [Google Scholar]
- 53.Seitz HK, Wang XD. The Role of Cytochrome P450 2E1 in Ethanol-Mediated Carcinogenesis. In:Cytochrome P450 2E1: Its Role in Disease and Drug Metabolism. Subcellular Biochemistry. 2013;67:131–143. doi: 10.1007/978-94-007-5881-0_3. [DOI] [PubMed] [Google Scholar]