Abstract
Objective:
To report patients with progressive motor impairment resulting from an isolated CNS demyelinating lesion in cerebral, brainstem, or spinal cord white matter that we call progressive solitary sclerosis.
Methods:
Thirty patients were identified with (1) progressive motor impairment for over 1 year with a single radiologically identified CNS demyelinating lesion along corticospinal tracts, (2) absence of other demyelinating CNS lesions, and (3) no history of relapses affecting other CNS pathways. Twenty-five were followed prospectively in our multiple sclerosis (MS) clinic and 5 were identified retrospectively from our progressive MS database. Patients were excluded if an alternative etiology for progressive motor impairment was found. Multiple brain and spinal cord MRI were reviewed by a neuroradiologist blinded to the clinical details.
Results:
The patients' median age was 48.5 years (range 23–71) and 15 (50%) were women. The median follow-up from symptom onset was 100 months (range 15–343 months). All had insidiously progressive upper motor neuron weakness attributable to the solitary demyelinating lesion found on MRI. Clinical presentations were hemiparesis/monoparesis (n = 24), quadriparesis (n = 5), and paraparesis (n = 1). Solitary MRI lesions involved cervical spinal cord (n = 18), cervico-medullary/brainstem region (n = 6), thoracic spinal cord (n = 4), and subcortical white matter (n = 2). CSF abnormalities consistent with MS were found in 13 of 26 (50%). Demyelinating disease was confirmed pathologically in 2 (biopsy, 1; autopsy, 1).
Conclusions:
Progressive solitary sclerosis results from an isolated CNS demyelinating lesion. Future revisions to MS diagnostic criteria could incorporate this presentation of demyelinating disease.
Most patients with multiple sclerosis (MS) either have a relapsing-remitting course associated with subacute, inflammatory clinical attacks that usually resolve or an insidiously progressive course with (secondary progressive MS [SPMS]) or without (primary progressive MS [PPMS]) prior relapses. Disability due to MS principally accrues during the progressive course, the most common clinical presentation being of a progressive myelopathy, although progressive cerebellar and cerebral impairment are less common manifestations.1,2 The pathophysiology of progressive MS is poorly understood and a clinical-radiologic paradox exists with an incomplete association between the number of MS demyelinating lesions and development of a progressive MS course.3 Progressive motor impairment similar to progressive MS disease courses may occur from an isolated demyelinating lesion in the upper cervical cord or brainstem cervico-medullary junction.4–8 Current criteria preclude a diagnosis of MS in such cases because of failure to document dissemination in time and space.9 We describe 30 patients with progressive solitary sclerosis: progressive motor impairment directly attributable to an isolated CNS demyelinating lesion arising in the spinal cord, brainstem, or cerebral white matter.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Mayo Foundation Institutional Review Board (IRB 9007045). All patients consented to the use of their medical records for research purposes at the time of their clinical evaluation at Mayo Clinic.
Patients.
Patients were identified in 2 ways. Twenty-five patients were prospectively identified in our MS clinic from January 1, 1996, to January 2, 2016. Five additional patients were retrospectively identified by reviewing the medical records and MRI of 414 patients in the Mayo Clinic progressive MS database.10 Inclusion criteria were (1) progressive motor impairment for over 1 year duration with a single isolated CNS demyelinating lesion along the corticospinal tracts, (2) absence of other definite CNS demyelinating lesions, and (3) no history of relapses affecting other portions of the CNS. Alternative etiologies of progressive CNS impairment other than demyelinating disease were excluded (e.g., neoplastic or infectious etiologies, nutritional deficiencies, compressive myelopathy).
We attempted to contact patients not seen within the previous year by mail and subsequent telephone interview regarding alternative diagnoses received and disability status. The patients' most recent and interval brain, cervical, and thoracic spinal cord MRI were requested and reviewed. Four patients from this series have been described previously.5 Three additional patients from that series were excluded from this study as they each had 2 demyelinating lesions and did not fulfill our more stringent criteria of having only one demyelinating lesion. In that study, patients with more than 1 demyelinating lesion who did not meet the international panel imaging requirements for dissemination in space (which excludes the symptomatic lesion and requires 2 additional lesions) were included.5
Clinical evaluations.
All patients had symptoms and signs of a progressive CNS disease consistent with progressive forms of MS, most commonly a progressive myelopathic disease course. Disability was assessed using the Expanded Disability Status Scale (EDSS) score and Kurtzke functional assessment.11
Competing diagnoses were excluded, including neuromyelitis optica (NMO) (patients did not conform to international criteria; progressive NMO clinical course is rare12), transverse myelitis (progression to nadir was far greater than 21 days),13 paraneoplastic myelopathy (did not meet the European diagnostic criteria14), neurosarcoidosis (did not fit diagnostic criteria15), primary or metastatic neoplastic myelopathy (patients had long follow-up and often lesion atrophy rather than expansion was found16,17), compressive myelopathy,18 infectious myelopathy, nutritional myelopathy, toxic myelopathy, and inherited spastic paraparesis (solitary focal signal abnormality MRI lesion seen rather than diffuse MRI signal abnormality).
Neuroimaging.
The initial and most recent brain, cervical, and thoracic spine MRI were evaluated by a neuroradiologist (T.J.K.) who was blinded to clinical data. Brain MRI included T1- and T2-weighted imaging, T2-weighted fluid-attenuated inversion recovery imaging, and postgadolinium T1-weighted imaging; spine MRI included T1- and T2-weighted imaging and frequently T2-weighted inversion recovery imaging. Postgadolinium T1-weighted imaging was available in 27 of 30 (90%). Subsequent brain, cervical, and thoracic spine MRI were evaluated for interval evidence of new definitive demyelinating disease lesion burden. Only lesions considered to be unequivocally demyelinating were counted in an approach similar to prior studies.19 MRI characteristics of the solitary CNS demyelinating lesion (location, dimensions, accompanying atrophy, gadolinium enhancement) were recorded. In uncertain cases, a consensus decision about whether the lesion represented demyelination was reached by the investigators (B.M.K., T.J.K., and E.P.F.). In 4 patients where the most recent follow-up MRI was unavailable, the results were reviewed from the official radiology report and interpretation of the consulting neuroradiologist.
Statistical analysis.
The results were reported as median (range, minimum-maximum) for continuous variables and as frequencies and percentages for categorical variables.
RESULTS
Patient characteristics, clinical features, and treatments undertaken.
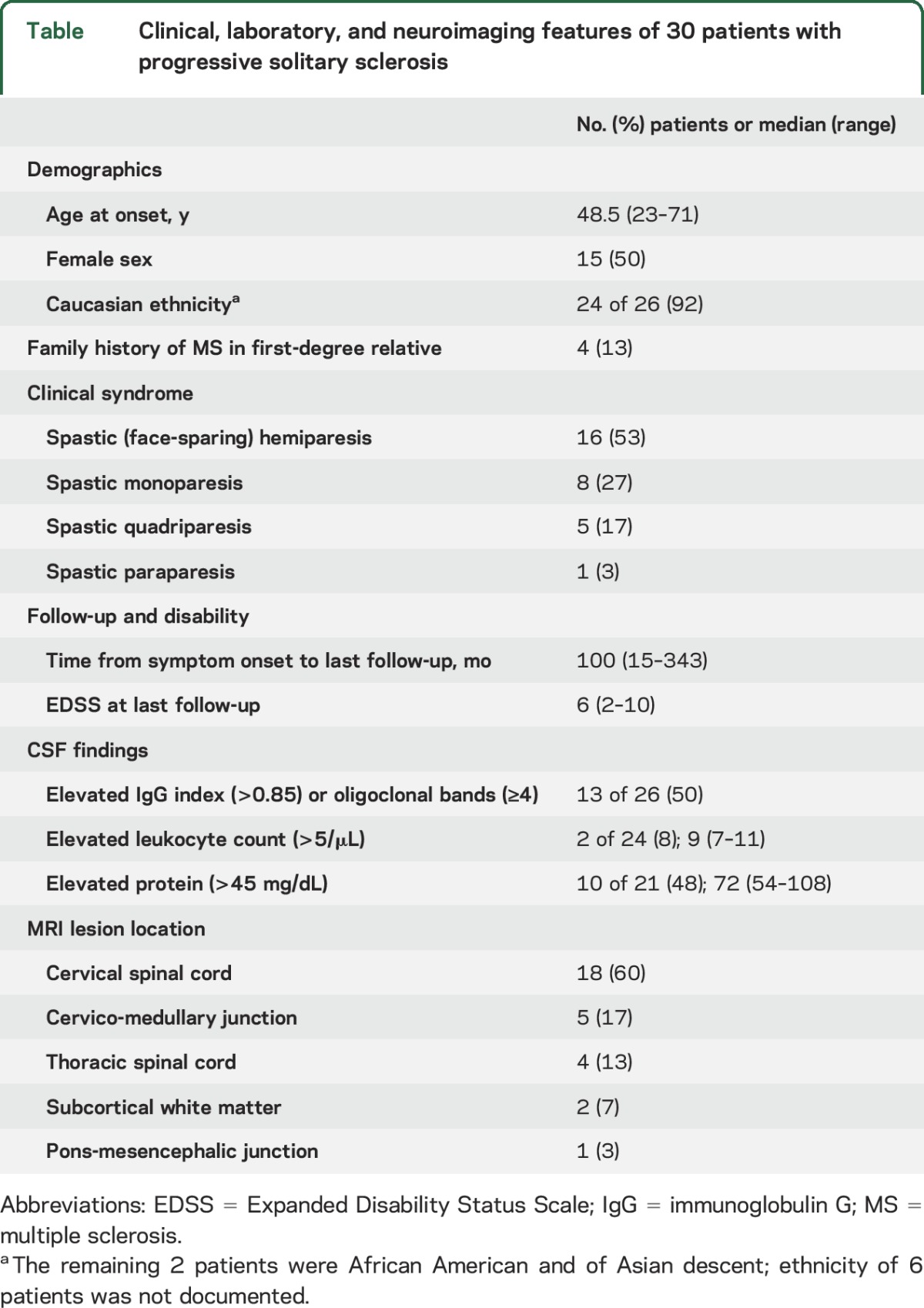
The demographic, clinical, laboratory, and MRI data are outlined in the table. Twenty-five patients presented with a progressive clinical course without a history of any acute inflammatory demyelinating attack. Progressive motor impairment was preceded by an acute inflammatory myelopathy attack with resolution in 5 patients (17%). No patients had intervening clinical attacks of MS following their progressive clinical course in the time of follow-up. Progressive impairment was directly attributable to the solitary CNS demyelinating lesion detected on MRI in all patients. Twenty-four patients (80%) had unilateral progression (monoparesis or hemiparesis) and 6 patients (20%) had bilateral progressive motor impairment (paraparesis or quadriparesis) (table). The median EDSS at last follow-up was 6 (range 2–10). Of the 17 patients who reached EDSS 6 during follow up, the median time from symptom onset to EDSS 6 was 47 months (range 6–331). Associated clinical symptoms included bowel/bladder dysfunction (n = 16), sensory loss (n = 8), Lhermitte symptom (sign) (n = 5), ataxia (n = 3), and tonic spasms (n = 2). Four patients (13%) had a first-degree relative with MS.
Table.
Clinical, laboratory, and neuroimaging features of 30 patients with progressive solitary sclerosis
Five patients underwent variable immunomodulatory or immunosuppressive treatment courses, including IV methylprednisolone (n = 2), cyclophosphamide (n = 2), azathioprine (n = 2), therapeutic plasma exchange (n = 2), mitoxantrone (n = 1), natalizumab (n = 1), glatiramer acetate (n = 1), and methotrexate (n = 1). Subjective improvement in gait was reported in 1 patient treated with cyclophosphamide and 1 of 2 patients treated symptomatically with dalfampridine. Treatments initiated were otherwise deemed to be unsuccessful in arresting progression by the treating clinicians.
Neuroimaging.
Brain and spinal cord MRI findings of progressive solitary sclerosis lesions are summarized in the table and representative images are shown in figures 1–3. Twenty-eight patients had brain, cervical, and thoracic spine MRI; 2 patients with a cervical spinal cord lesion explaining their symptoms had a normal brain MRI but did not undergo thoracic spine MRI. The median time from symptom onset to last MRI was 63 months (range 15–343). This MRI included the entire CNS (brain, cervical, and thoracic cord) in 59% confirming no new lesions had developed. The single autopsied case had just one pathologic lesion.
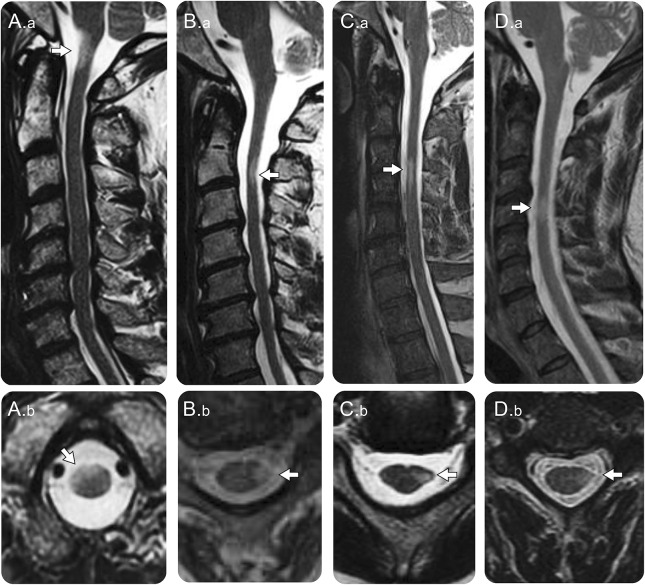
Figure 1. MRI in patients with cervical spinal cord progressive solitary sclerosis.
(A) A 52-year-old man with progressive upper motor neuron quadriparesis with T2-hyperintense lesion at the cervico-medullary junction on sagittal images (A.a, arrow) located in the right more than left anterior cord on axial images (A.b, arrow). (B) A 56-year-old man with progressive (face-sparing) spastic left hemiparesis with a T2-hyperintense lesion in the upper cervical cord on sagittal images (B.a, arrow) located in the left lateral columns on axial images (B.b, arrow) with spinal cord atrophy (B.a and B.b). (C) A 68-year-old man with progressive left (face-sparing) hemiparesis due to a T2-hyperintense lesion in the upper cervical cord seen on sagittal images (C.a, arrow); on axial images, the lesion in the left lateral columns is associated with loss of the normal rounded spinal cord margin indicative of focal atrophy (C.b, arrow). (D) A 57-year-old man presented with a subacute episode of left (face-sparing) hemiparesis with subsequent progressive left hemiparesis with a T2-hyperintense lesion in the mid-cervical cord on sagittal images (D.a, arrow) involving the left lateral columns on axial images with focal atrophy (D.b, arrow).
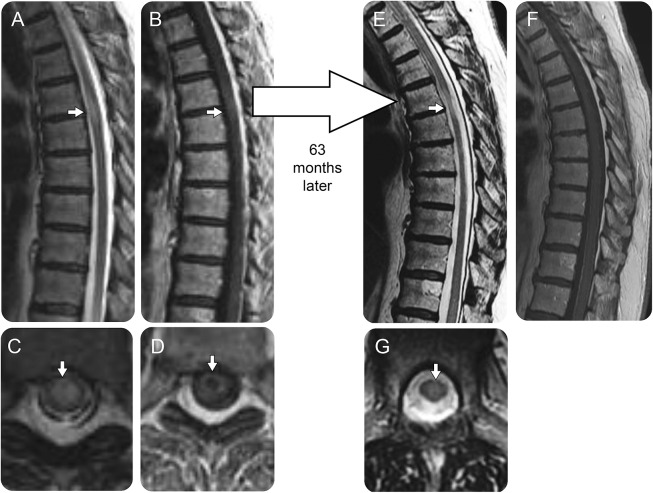
Figure 2. Sequential MRI in a patient with thoracic spinal cord progressive solitary sclerosis.
In a 56-year-old woman with an acute inflammatory myelopathy, thoracic spine MRI demonstrates a T2-hyperintense lesion (A, arrow) in the mid-thoracic cord with mild spinal cord edema on sagittal images, which was located centrally on axial images (C, arrow) and associated with mild ring enhancement after gadolinium administration, more evident on axial (D, arrow) than sagittal images (B, arrow). She recovered from the initial inflammatory myelopathy, but 3.5 years later developed a progressive spastic paraparesis and a repeat MRI 63 months after the initial myelitis episode revealed an apple core atrophic T2-hyperintense lesion (E, arrow) with residual central T2 hyperintensity on axial images (G, arrow) with resolution of the prior gadolinium enhancement on sagittal (F) and axial (images not available) postgadolinium sequences.
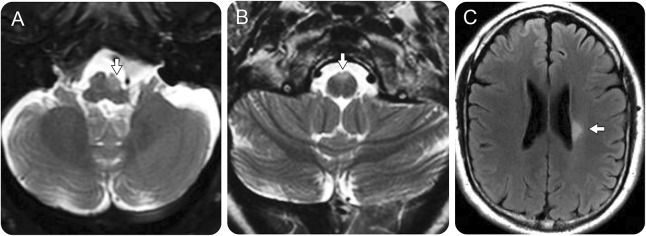
Figure 3. MRI in patients with brainstem and subcortical cerebral white matter progressive solitary sclerosis.
A 50-year-old man with a progressive right hemiparesis had a left medullary T2-hyperintense nonenhancing lesion with accompanying atrophy on axial fluid-attenuated inversion recovery (FLAIR) images (A, arrow). A 46-year-old woman with a progressive spastic quadriparesis was found to have a nonenhancing T2-hyperintense lesion anteriorly involving the right and left pyramids of the medulla (B, arrow). A 45-year-old man had progressive right (face-sparing) spastic hemiparesis associated with a solitary left periventricular nonenhancing T2-hyperintense lesion on axial FLAIR images (C, arrow). The lesion did not change over 5 years of MRI follow-up (not shown); no additional MRI lesions appeared.
Solitary demyelinating CNS lesions were most commonly located within the cervical spinal cord or cervico-medullary junction (table, figure 1). All spinal cord lesions were less than 3 vertebral segments in length. Focal lesion atrophy with loss of the rounded appearance of the spinal cord on axial images (figure 1, B.b, C.b, and D.b) was present on initial imaging or developed over time (figure 2) in 21 of 26 (81%) with adequate axial sequences available; in 2 patients, the atrophy resulted in the appearance of an apple core lesion (figure 2E) associated with paraparesis or quadriparesis. Bilateral lesions involving the medullary pyramids or cervico-medullary junction (figure 3B) were associated with quadriparesis.
The median width of the solitary spinal cord lesion was 5.5 × 4 mm (range 2–28) and the median length was 18 mm (range 7–52). In 2 of 27 (7%), there was gadolinium enhancement of a solitary spinal cord lesion on the initial images that subsequently resolved (figure 2). Nonspecific brain MRI white matter T2 hyperintensities coexisted in 8 patients (27%).
Laboratory investigations.
Half of the patients with evaluable CSF (13 of 26) had abnormalities characteristic of MS (elevations in oligoclonal bands or immunoglobulin G [IgG] index) (table). Visual evoked potentials (18 tested) and brainstem auditory evoked potentials (2 tested) were consistently normal. Somatosensory evoked potentials revealed impaired conduction within central proprioceptive pathways in 1 of 3 tested. Brain biopsy of one patient with a subcortical white matter lesion along the motor tracts showed demyelination, chronic inflammation, and gliosis without evidence of a neoplasm. A second patient had an autopsy that revealed a solitary demyelinating lesion with profound myelin loss and relative axonal preservation in the rostral pontine tegmentum.
The following laboratory investigations (number tested) were within normal limits: vitamin B12 (n = 24), human T-lymphotrophic virus serology (n = 16), aquaporin-4 IgG (n = 15), angiotensin-converting enzyme (n = 15), HIV (n = 15), syphilis serology (n = 15), and peroxisomal screen for very-long-chain fatty acids (n = 3). Minimally low serum copper at 0.73 μg/mL (normal 0.75–1.45 μg/mL) was found in 1 of 16 patients tested, deemed to be of no clinical significance. Paraneoplastic autoantibody evaluation revealed one or more positive results in 3 of 15 patients tested, including glutamic acid decarboxylase 65 antibodies, 2 (both 0.04 nmol/L; normal, ≤0.02 nmol/L); P/Q type calcium channel autoantibody, 1 (0.03 nmol/L; normal, ≤0.02 nmol/L); and striated muscle autoantibody, 1 (1:480; normal, <1:120); these results were also considered not to be of clinical significance. No patient had systemic lupus erythematosus; 1 of 16 patients tested serologically had mildly elevated antinuclear antibodies (2.7 units [normal ≤1.0 units]) without antibodies to double-stranded DNA or extractable nuclear antigens. Chest CT was normal in all 8 tested.
DISCUSSION
This study demonstrates that progressive solitary sclerosis results from an isolated CNS demyelinating lesion arising in the spinal cord, brainstem, or cerebral white matter and recapitulates the impairment seen in progressive MS. In all cases, the insidiously progressive upper motor neuron impairment was explained by the location of the solitary CNS lesion location.
In 1990, solitary sclerosis in the cervical spinal cord was described.4 Our prior case series5 showed that a cervico-medullary demyelinating lesion could lead to progressive myelopathy in patients who did not meet criteria for dissemination in time and space for MS. Subsequent to that case series, further patients with solitary sclerosis have been reported,6–8 with comparable clinical and radiologic findings. When compared to prior reports, this study advances our knowledge of progressive solitary sclerosis by expanding the regions involved to the thoracic spinal cord and supratentorial corticospinal tracts, providing more detailed MRI analysis and reporting the outcome in a much larger number of patients with extended follow-up. This study was not able to determine the relative prevalence of this clinical presentation, although it appears to be uncommon, given just 30 patients with progressive solitary sclerosis were identified at our institution over the reviewed duration.
All patients in this study had insidiously progressive CNS disease indistinguishable from patients with PPMS or SPMS who fulfill current diagnostic criteria. Demyelinating disease etiology was supported by consistent CSF findings in 50% and 13% had a first-degree relative with MS. The failure of MS disease-modifying or immunosuppressive therapy is consistent with most studies of progressive MS. Extensive laboratory investigations found no alternative etiologies of the progressive neurologic disorder, although the variable and incomplete ascertainment of laboratory studies in this retrospective study is a limitation that could have reduced diagnostic accuracy. MRI solitary lesions were located in characteristic locations for demyelinating MS plaques along corticospinal tracts (spinal cord lateral columns, anterior medulla, periventricular white matter motor tracts) and the spinal cord lesions were short segment in length (<3 vertebral bodies). Progressive lesional atrophy, rather than expansion, over time was inconsistent with a neoplastic etiology. Long-term follow-up without alternative diagnoses being discovered (median follow-up 100 months from onset) helped exclude other etiologies. In the 27 patients not evaluated for very-long-chain fatty acids serologically, the single focal (often asymmetric) atrophic cord lesions on MRI excluded adrenomyeloneuropathy, in which the MRI may show mild diffuse spinal cord atrophy. The absence of enhancement, hemorrhage, and flow voids and the progressive atrophy helped exclude vascular malformations as the etiology.
The clinical-radiological paradox of incomplete association between the number of MS demyelinating lesions and development of a progressive MS course is challenging.3,20 One theory proposes that the CNS is diffusely affected, as supported by the presence of whole brain atrophy in MS.21 Another plausible explanation is disproportionate contribution of MS lesions located along the motor tracts commonly involving the spinal cord. Recent studies have shown a strong association between MRI cervical spinal cord lesion load and disability accumulation.22 Our cohort of patients with a solitary CNS lesion and progressive disability provides a unique population to demonstrate that disability progression in some patients is anatomically linked to a specific CNS demyelinating lesion. It is not known why patients in this study with a single demyelinating lesion along the motor tracts developed progressive disability while other patients with inflammatory myelopathies with radiologically similar intramedullary lesions do not demonstrate motor progression. Further studies investigating the severity of axonal injury and disparities in the degree of remyelination may resolve this important difference.
Progressive solitary sclerosis does not conform to the current MS diagnostic criteria and accommodation for such patients might be considered in future revisions. Currently recognized MS clinical courses were excluded. Radiologically isolated syndrome was excluded with the progressive clinical symptomatology and the absence of multiple confirmatory MRI lesions meeting criteria.23,24 While a minority had an initial clinical inflammatory myelopathy attack with resolution, clinically isolated syndrome is not appropriate as a diagnosis as all patients were impaired by insidiously progressive motor impairment.25 Relapsing-remitting MS was excluded by the insidious progression occurring without further clinical relapse.9 The patients in this study could not be diagnosed with PPMS or SPMS as only a solitary, symptomatic MRI lesion was found.9 Current MS diagnostic criteria for dissemination in space exclude counting of the symptomatic lesion.9 Modification to the diagnostic criteria for PPMS has been proposed to include symptomatic spinal cord lesions on the grounds that it increases diagnostic sensitivity.26
The study is limited by a number of factors. Follow-up and MRI technique and sequences lacked uniformity between patients. More advanced imaging techniques might detect cortical lesions undetectable with MRI techniques performed.27 It is not possible to be certain that progression is being driven at the level of the solitary demyelinating lesion itself rather than other lesions undetectable by routine MRI; however, based on anatomy, the deficit was consistently explained by the lesion that was seen. Our patients may develop new MRI lesions that satisfy existing MS diagnostic criteria in the future. The long clinical and MRI follow-up in the majority of our patients and a single autopsy patient with progressive symptoms but only a solitary lesion suggest that this type of evolution would be uncommon. Neuropathology was available in only 2 patients as the affected areas in patients with progressive solitary sclerosis are often in neurologically eloquent territory (e.g., spinal cord lateral columns, cervico-medullary junction) but both were diagnostic of CNS demyelination as the cause.
A solitary demyelinating lesion within the spinal cord, brainstem, or cerebral white matter may lead to a progressive syndrome otherwise indistinguishable from progressive forms of MS. Future revisions of MS diagnostic criteria should consider accommodating this subtype of demyelinating disease.
GLOSSARY
- EDSS
Expanded Disability Status Scale
- IgG
immunoglobulin G
- MS
multiple sclerosis
- NMO
neuromyelitis optica
- PPMS
primary progressive multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
AUTHOR CONTRIBUTIONS
Dr. Keegan was involved in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, acquisition of data, and study supervision. Dr. Kaufmann was involved in revising the manuscript for content and analysis and interpretation of data. Dr. Weinshenker was involved in revising the manuscript for content and analysis and interpretation of data. Dr. Kantarci was involved in revising the manuscript for content and analysis and interpretation of data. Dr. Schmalstieg was involved in revising the manuscript for content and analysis and interpretation of data. Dr. Paz Soldan was involved in revising the manuscript for content and analysis and interpretation of data. Dr. Flanagan was involved in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
B. Keegan has served as a consultant to Novartis, Bionest, and Bristol Meyers Squibb and has research funded by Terumo BCT. T. Kaufmann is a consultant for SpineThera. B. Weinshenker: DSMB membership MS trials: Novartis (DSMB member for MS trials); Mitsubishi (DSMB member for MS trial); adjudication committee membership re NMO trials: MedImmune Royalties: RSR Ltd. and Oxford University re patent for NMO-IgG as a diagnostic test for NMO and related disorders. O. Kantarci, W. Schmalsteig, M. Paz Soldan, and E. Flanagan report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol 2007;6:903–912. [DOI] [PubMed] [Google Scholar]
- 2.Staff NP, Lucchinetti CF, Keegan BM. Multiple sclerosis with predominant, severe cognitive impairment. Arch Neurol 2009;66:1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 2002;15:239–245. [DOI] [PubMed] [Google Scholar]
- 4.Weinshenker BG, Gilbert JJ, Ebers GC. Some clinical and pathologic observations on chronic myelopathy: a variant of multiple sclerosis. J Neurol Neurosurg Psychiatry 1990;53:146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmalstieg WF, Keegan BM, Weinshenker BG. Solitary sclerosis: progressive myelopathy from solitary demyelinating lesion. Neurology 2012;78:540–544. [DOI] [PubMed] [Google Scholar]
- 6.Cohen M, Lebrun C, Ayrignac X, Labauge P, Assouad R. Solitary sclerosis: experience from three French tertiary care centres. Mult Scler 2015;21:1216. [DOI] [PubMed] [Google Scholar]
- 7.Lattanzi S, Logullo F, Di Bella P, Silvestrini M, Provinciali L. Multiple sclerosis, solitary sclerosis or something else? Mult Scler 2014;20:1819–1824. [DOI] [PubMed] [Google Scholar]
- 8.Rathnasabapathi D, Elsone L, Krishnan A, Young C, Larner A, Jacob A. Solitary sclerosis: progressive neurological deficit from a spatially isolated demyelinating lesion: a further report. J Spinal Cord Med 2015;38:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paz Soldan MM, Novotna M, Abou Zeid N, et al. . Relapses and disability accumulation in progressive multiple sclerosis. Neurology 2015;84:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 12.Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002;59:499–505. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan EP, McKeon A, Lennon VA, et al. . Paraneoplastic isolated myelopathy: clinical course and neuroimaging clues. Neurology 2011;76:2089–2095. [DOI] [PubMed] [Google Scholar]
- 15.Zajicek JP, Scolding NJ, Foster O, et al. . Central nervous system sarcoidosis–diagnosis and management. QJM 1999;92:103–117. [DOI] [PubMed] [Google Scholar]
- 16.Flanagan EP, O'Neill BP, Habermann TM, Porter AB, Keegan BM. Secondary intramedullary spinal cord non-Hodgkin's lymphoma. J Neurooncol 2012;107:575–580. [DOI] [PubMed] [Google Scholar]
- 17.Flanagan EP, O'Neill BP, Porter AB, Lanzino G, Haberman TM, Keegan BM. Primary intramedullary spinal cord lymphoma. Neurology 2011;77:784–791. [DOI] [PubMed] [Google Scholar]
- 18.Flanagan EP, Krecke KN, Marsh RW, Giannini C, Keegan BM, Weinshenker BG. Specific pattern of gadolinium enhancement in spondylotic myelopathy. Ann Neurol 2014;76:54–65. [DOI] [PubMed] [Google Scholar]
- 19.Brex PA, Ciccarelli O, O'Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 2002;346:158–164. [DOI] [PubMed] [Google Scholar]
- 20.Hackmack K, Weygandt M, Wuerfel J, et al. . Can we overcome the “clinico-radiological paradox” in multiple sclerosis? J Neurol 2012;259:2151–2160. [DOI] [PubMed] [Google Scholar]
- 21.De Stefano N, Giorgio A, Battaglini M, et al. . Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology 2010;74:1868–1876. [DOI] [PubMed] [Google Scholar]
- 22.Kearney H, Altmann DR, Samson RS, et al. . Cervical cord lesion load is associated with disability independently from atrophy in MS. Neurology 2015;84:367–373. [DOI] [PubMed] [Google Scholar]
- 23.Okuda DT, Siva A, Kantarci O, et al. . Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One 2014;9:e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantarci OH, Lebrun C, Siva A, et al. . Primary progressive multiple sclerosis evolving from radiologically isolated syndrome. Ann Neurol 2016;79:288–294. [DOI] [PubMed] [Google Scholar]
- 25.Swanton JK, Rovira A, Tintore M, et al. . MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: a multicentre retrospective study. Lancet Neurol 2007;6:677–686. [DOI] [PubMed] [Google Scholar]
- 26.Kelly SB, Kinsella K, Duggan M, Tubridy N, McGuigan C, Hutchinson M. A proposed modification to the McDonald 2010 criteria for the diagnosis of primary progressive multiple sclerosis. Mult Scler 2013;19:1095–1100. [DOI] [PubMed] [Google Scholar]
- 27.Calabrese M, Battaglini M, Giorgio A, et al. . Imaging distribution and frequency of cortical lesions in patients with multiple sclerosis. Neurology 2010;75:1234–1240. [DOI] [PubMed] [Google Scholar]