Abstract
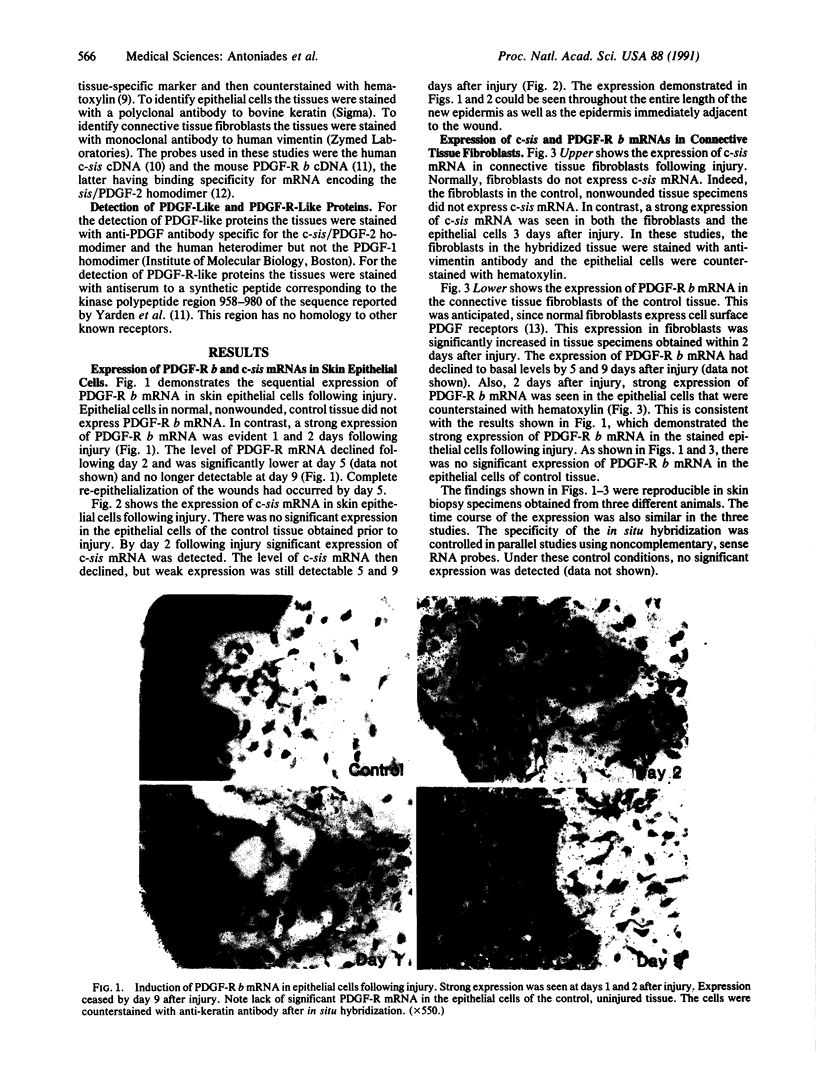
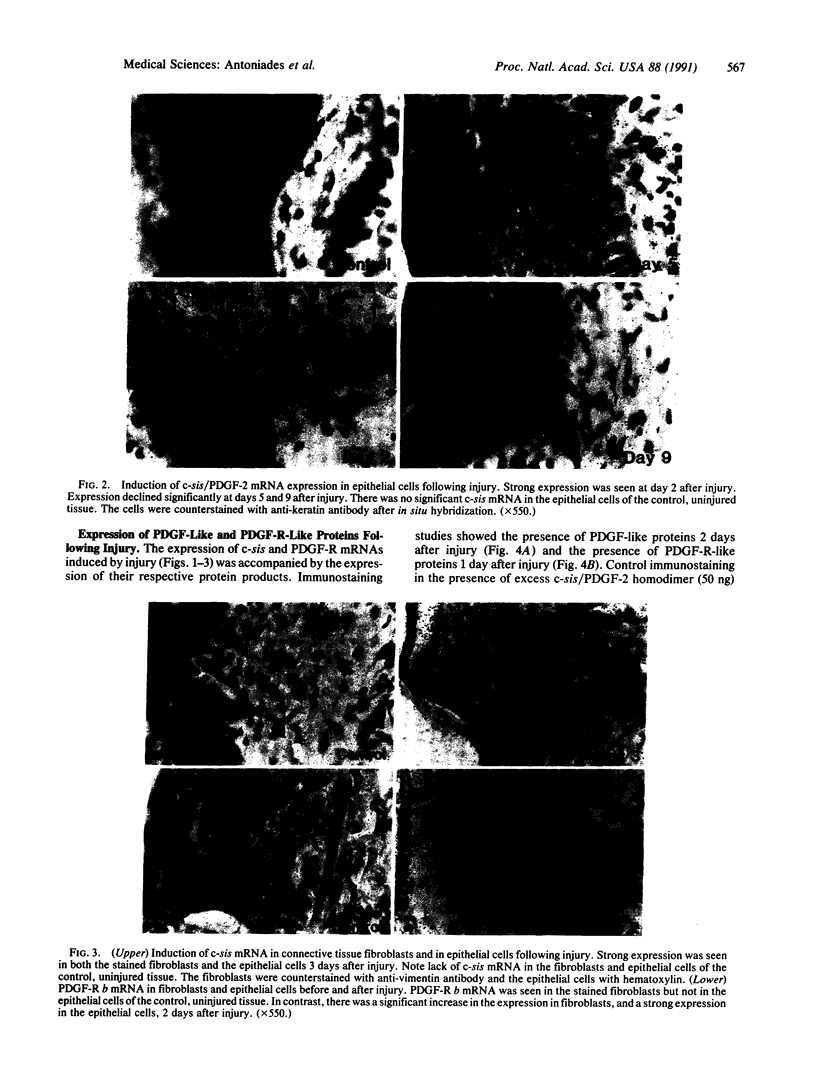
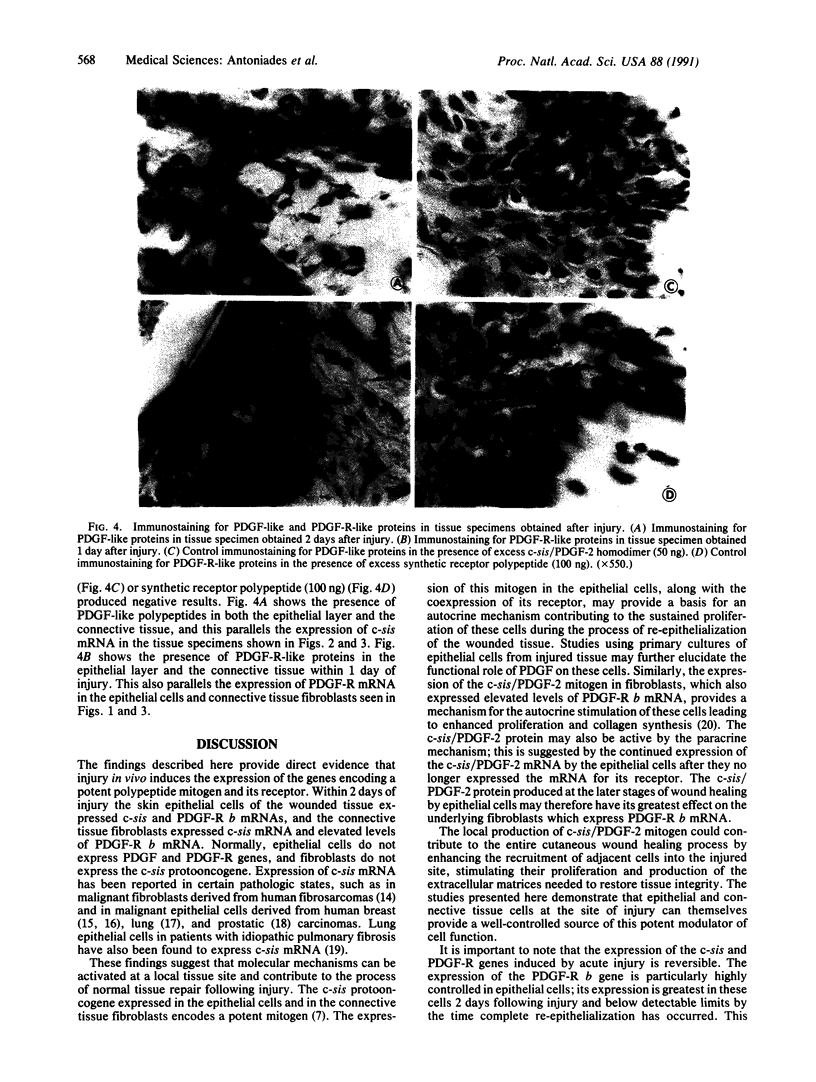
Platelet-derived growth factor (PDGF) stimulates many of the processes important in tissue repair, including proliferation of fibroblasts and synthesis of extracellular matrices. In this study we have demonstrated with in situ hybridization and immunocytochemistry the reversible expression of c-sis/PDGF-2 and PDGF receptor (PDGF-R) b mRNAs and their respective protein products in epithelial cells and fibroblasts following cutaneous injury in pigs. Epithelial cells in control, unwounded skin did not express c-sis and PDGF-R mRNAs, and fibroblasts expressed only PDGF-R mRNA. The expression levels in the injured site were correlated with the stage of tissue repair, being highest during the initial stages of the repair process and declining at the time of complete re-epithelialization and tissue remodeling. It is suggested that the controlled, reversible expression of a potent mitogen and its receptor induced by injury may function in an autocrine/paracrine manner on both epithelial cells and fibroblasts to bring about their sustained proliferation during the normal healing process. These studies provide a molecular basis for understanding the mechanisms contributing to normal tissue repair. We suggest the possibility that a defect in these mechanisms may be associated with defective wound healing. It is also conceivable that "chronic" injury may induce irreversible gene expression leading to pathologic, unregulated cell growth.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Bravo M. A., Avila R. E., Galanopoulos T., Neville-Golden J., Maxwell M., Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990 Oct;86(4):1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronzert D. A., Pantazis P., Antoniades H. N., Kasid A., Davidson N., Dickson R. B., Lippman M. E. Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5763–5767. doi: 10.1073/pnas.84.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. M., Ross R. Collagen synthesis by monkey arterial smooth muscle cells during proliferation and quiescence in culture. Exp Cell Res. 1977 Jul;107(2):387–395. doi: 10.1016/0014-4827(77)90360-3. [DOI] [PubMed] [Google Scholar]
- Collins T., Ginsburg D., Boss J. M., Orkin S. H., Pober J. S. Cultured human endothelial cells express platelet-derived growth factor B chain: cDNA cloning and structural analysis. Nature. 1985 Aug 22;316(6030):748–750. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Hoefler H., Childers H., Montminy M. R., Lechan R. M., Goodman R. H., Wolfe H. J. In situ hybridization methods for the detection of somatostatin mRNA in tissue sections using antisense RNA probes. Histochem J. 1986 Nov-Dec;18(11-12):597–604. doi: 10.1007/BF01675295. [DOI] [PubMed] [Google Scholar]
- Jennische E., Skottner A., Hansson H. A. Dynamic changes in insulin-like growth factor I immunoreactivity correlate to repair events in rat ear after freeze-thaw injury. Exp Mol Pathol. 1987 Oct;47(2):193–201. doi: 10.1016/0014-4800(87)90074-8. [DOI] [PubMed] [Google Scholar]
- Lawrence W. T., Norton J. A., Sporn M. B., Gorschboth C., Grotendorst G. R. The reversal of an Adriamycin induced healing impairment with chemoattractants and growth factors. Ann Surg. 1986 Feb;203(2):142–147. doi: 10.1097/00000658-198602000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. E., Colvin R. B., Antoniades H. N. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest. 1989 Aug;84(2):640–646. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. E., Nixon J. C., Colvin R. B., Antoniades H. N. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka J., Grotendorst G. R. Two peptides related to platelet-derived growth factor are present in human wound fluid. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4416–4420. doi: 10.1073/pnas.86.12.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Pantazis P., Pelicci P. G., Dalla-Favera R., Antoniades H. N. Synthesis and secretion of proteins resembling platelet-derived growth factor by human glioblastoma and fibrosarcoma cells in culture. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2404–2408. doi: 10.1073/pnas.82.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J., Taylor-Papadimitriou J. Production of PDGF-like growth factor by breast cancer cell lines. Int J Cancer. 1985 Aug 15;36(2):247–252. doi: 10.1002/ijc.2910360218. [DOI] [PubMed] [Google Scholar]
- Sariban E., Sitaras N. M., Antoniades H. N., Kufe D. W., Pantazis P. Expression of platelet-derived growth factor (PDGF)-related transcripts and synthesis of biologically active PDGF-like proteins by human malignant epithelial cell lines. J Clin Invest. 1988 Oct;82(4):1157–1164. doi: 10.1172/JCI113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Sitaras N. M., Sariban E., Bravo M., Pantazis P., Antoniades H. N. Constitutive production of platelet-derived growth factor-like proteins by human prostate carcinoma cell lines. Cancer Res. 1988 Apr 1;48(7):1930–1935. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]







