Abstract
Purpose
To investigate predictive value of APAF-1 and COX-2 expression in pathologic complete response (pCR) for patients with rectal adenocarcinoma (RAC) who were treated with neoadjuvant chemoradiotherapy (neo-CRT) followed by total mesorectal excision (TME).
Materials and Methods
Immunohistochemistry assay was used to detect expression of APAF-1 and COX-2 in paraffin-wax embedded tissues obtained before neo-CRT for patients with RAC. A 5-point tumor-regression grade (TRG) based on the ratio of residual tumor to fibrosis according to Dworak's scoring system was used to assess neo-CRT response. The relationship between expression of APAF-1 and COX-2 genes and pCR was explored.
Results
pCR (TRG4) was observed in 23 patients (28.0%). pCR were more likely to be achieved for those with APAF-1 over-expression or lower expression of COX-2. pCR rate in patients with combination of high APAF-1 and low COX-2 expression was 56.0%, significantly higher than those with other combination of APAF1 and COX-2 expression. Multivariate analysis showed that over-expression of APAF-1 and suppressed expression of COX-2 were independent predictive factors for pCR.
Conclusion
Immunohistochemical evaluation of APAF-1 and COX-2 expression on pretreatment specimen may be used to predict pCR to neo-CRT in patients with RAC. The potential of the markers in monitoring pCR patient merits further investigation.
Keywords: rectal adenocarcinoma, neoadjuvant chemoradiotherapy, complete pathological response, APAF-1, COX-2
INTRODUCTION
Neoadjuvant chemoradiotherapy (neo-CRT) followed by total mesorectal excision (TME) is the standard of treatment for patients with locally advanced rectal adenocarcinoma. neo-CRT results in varying degrees of tumor regression that range from pathologic complete response (pCR) to modest or no treatment response. Studies have demonstrated that patients with pCR to neo-CRT have shown better prognosis than those with non-pCR [1]. There is a trend that patients who present pCR to neo-CRT might receive local excision [2, 3] or wait-and-see policy [4] to avoid radical surgery related complications. The key to clinical follow-up study is to establish an accurate and accepted model to screen those who may achieve pCR after neo-CRT and before surgery.
Currently, researchers have extensively explored the clinicopathologic factors and molecular markers in predicting pCR to neo-CRT in locally advanced rectal adenocarcinoma. It has been discovered that pre-treatment serum albumin > 3.5 mg/ml, the ratio of neutrophils/lymphocytes < 5 and percent of circulating blood lymphocytes were closely associated with pCR [5] and that pre-treatment hemoglobin (Hb) level could be used to predict not only pCR to neo-CRT but also local tumor recurrence [6]. Other factors such as tumor size, CEA and clinical N stage were also investigated to predict pCR to neo-CRT. Whereas studies have shown that molecular markers such as p53, Ki-67, Bcl-2/Bax cannot be used to predict the tumor response to chemoradiotherapy. Although EGFR, thymidylate synthase, p21 have been reported to be associated with chemoradiotherapy response, further evidence is still warranted [7].
Tumor growth and metastasis depend on angiogenesis; while COX-2 has been reported to be closely associated with cell proliferation and angiogenesis. Apoptosis protease-activating factor 1 (APAF-1) is a key regulator in mitochondrial apoptotic pathway and radiation-induced apoptosis is believed to be the main form of cancer cell death caused by radiotherapy. In this study, we are going to detect expression of COX-2 and APAF-1 genes in tissue samples obtained from pretreatment specimen for patients with locally advanced rectal adenocarcinoma and explore their potential value in predicting treatment response especially pCR to neo-CRT.
RESULTS
Pathologic tumor response to neo-CRT and its association with clinicopathologic features
The tumor response to neo-CRT according to pathological evaluation of tissue samples after TME was reported as TRG 0 in none, TRG 1 in 6 (7.3%), TRG 2 in 33 (40.2%), TRG 3 in 20 (24.4%) and TRG 4 (pCR) in 23 (28.0%), respectively. The relationship between the status of pCR and clinicopathologic factors see Table S1.
Immunohistochemical staining for expression of APAF-1 gene
The representative data for immunohistochemical staining of APAF-1 gene expression were shown in Figure 1. The expression score for APAF-1 was reported as 1 point in 4 patients, 2 points in 9 patients, 3 points in 11 patients, 4 points in 12 patients, 6 points in 27 patients and 9 points in 19 cases, respectively (Seen in Table 1). Fisher test was used to explore each expression rate with pathological correlation of pCR. 4 points was used as demarcation point of APAF-1 gene expression. Expression score > 4 points was defined as high expression and ≤4 points as low expression (Figure 1a-1d). In the group of APAF-1 high expression, 17 cases were shown to achieve pCR (37.0%) which was significantly higher than those in group of APAF-1 low expression (16.7%)(p=0.042) (Table 2). Detailed analysis did not show significant correlation between the expression level of APAF-1 and clinicopathologic factors except high level of APAF-1 expression in cN0 patients(Table S2).
Figure 1. Detection of APAF-1 expression using immunohistochemical assay.
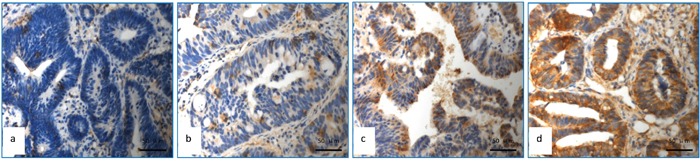
a. APAF-1with no expression; b. APAF-1 low expression (Express degree, 1 score = light yellow granules, 1 score × number of positive tumour cells 25%, 1 score); c. APAF-1 high expression (Express degree, 6 score = brown granular, 3 score×number of positive tumour cells 50%, 2 score); d. APAF-1 high expression (Express degree, 9 score = brown granular, 3 score×number of positive tumour cells 90%, 3 score).
Table 1. The degree of APAF-1 expression in the whole group.
| Expression score | n | pCR (n) | Non-pCR (n) |
|---|---|---|---|
| 1 | 4 | 1 | 3 |
| 2 | 12 | 2 | 10 |
| 3 | 11 | 1 | 10 |
| 4 | 9 | 2 | 7 |
| 6 | 27 | 12 | 15 |
| 9 | 19 | 5 | 14 |
| Total | 82 | 23 | 59 |
Table 2. Relationship between cut-off of APAF-1 and COX-2 and pCR.
| Expression score | pCR (n) | non-pCR (n) | p value |
|---|---|---|---|
| APAF-1 Cut-off of 4 | 0.042 | ||
| 1-4 (low) | 6 | 30 | |
| 5-9 (high) | 17 | 29 | |
| COX-2 Cut-off of 6 | 0.024 | ||
| 1-6 (low) vs | 18 | 30 | |
| 7-9 (high) | 5 | 29 |
Immunohistochemical staining for expression of COX-2 gene
The representative data for immunohistochemical staining of COX2 gene expression were shown in Figure 2. The detailed score for COX-2 expression was shown in Table 3. To explore each expression rate with pathological correlation of pCR using fisher test, 6 points was used as demarcation point, expression score > 6 points is defined as high expression and ≤6 points as low expression. For patients with low expression of COX-2, 18 cases (37.5%) achieved pCR, which was significantly higher than those with COX-2 high expression (14.7%)(p=0.024)(Table 2). No significant correlation between the expression level of COX-2 and clinicopathologic factors was observed. (Table S3).
Figure 2. Detection of COX-2 expression using immunohistochemical staining.
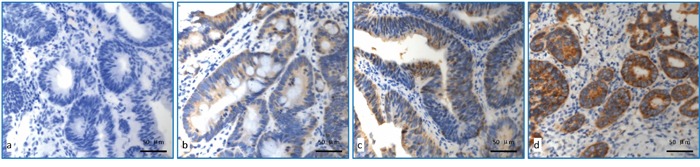
a. COX-2 with no expression; b. COX-2 low expression (Express degree, 1 score = light yellow granules, 1 score×number of positive tumour cells 25%, 1 score); c. COX-2 moderate expression (Express degree, 6 score = brown granular, 3 score × number of positive tumour cells 50%, 2 score); d. COX-2 high expression (Express degree, 9 score = brown granular, 3 score × number of positive tumour cells 95%, 3 score).
Table 3. The degree of COX-2 expression in the whole group.
| Expression score | N | pCR (n) | No-pCR (n) |
|---|---|---|---|
| 1 | 1 | 0 | 1 |
| 3 | 1 | 0 | 1 |
| 4 | 1 | 0 | 1 |
| 6 | 45 | 18 | 27 |
| 9 | 34 | 5 | 29 |
| Total | 82 | 23 | 59 |
Tumor response to neo-CRT and its association with combined expression of APAF-1 and COX-2 genes
Analysis of combined APAF-1 and COX-2 gene expression in predicting pCR showed that patients with high expression of APAF-1/low expression of COX-2 were associated with achieving the highest pCR rate (56.0%), which was significantly higher than those with high expression of APAF-1/high expression of COX-2 (14.3%), low expression of APAF-1/low expression of COX-2 (17.4%), low expression of APAF-1/high expression of COX-2 (15.4%) (p=0.005) (Table 4, Figure 3).
Table 4. Correlation between pCR and combined expression ofAPAF-1and COX-2.
| Group | APAF-1 | COX-2 | No. | pCR (%) | no-pCR (%) |
|---|---|---|---|---|---|
| A | High | Low | 25 | 14 (56.0%) | 11 (44.0%) |
| B | High | High | 21 | 3 (14.3%) | 18 (85.7%) |
| C | Low | Low | 23 | 4 (17.4%) | 19 (82.6%) |
| D | Low | High | 13 | 2 (15.4%) | 11 (84.6%) |
Figure 3. Correlation between the pCR and combined expression of APAF-1 and COX-2 genes.
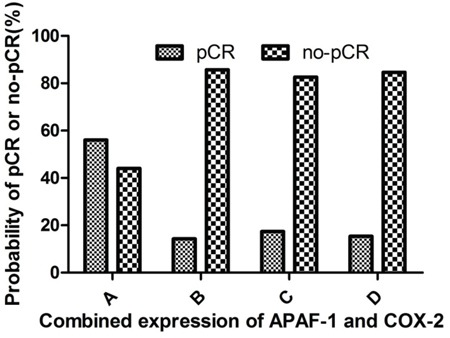
A. high APAF-1/low COX-2 expression group; B. high APAF-1/high COX-2 expression group; C. low APAF-1/low COX-2 expression group; D. low APAF-1/high COX-2 expression group. The probability to achieve pCR rate was 56.0% for patients with high APAF-1/low COX-2 expression, significantly higher than those with high APAF-1/high COX-2 expression group (14.3%), low APAF-1/low COX-2 expression group (17.4%) and low APAF-1/high COX-2 expression group 15.4% (p=0.005).
Univariate logistic analyses of predictors for pCR to neo-CRT
As shown in Table 5, univariate logistic analysis was demonstrated that both APAF-1 and COX-2 gene expression were independent risk factors for pCR after neo-CRT. Other clinical factors including age, gender, histological grade, tumor distance from anal verge, clinical stage and adjuvant chemotherapy were not associated with tumor response to neo-CRT except the serum carcino-embryonic antigen (CEA). The median CEA levels prior to neo-CRT was 2.43 ng/ml (0.69-10.90 ng/ml) in the group of pCR (TRG 4) and 4.38 ng/ml (0.57-206.20 ng/ml) in the group of non-pCR (TRG 0-3), the difference was statistically significant (p=0.001).
Table 5. Uni- and multi-variate logistic analysis of clinicopathologic factors for pCR.
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age (year) | |||||
| ≤56 vs >56 | 1.129 (0.430-2.960) | 0.806 | — | — | |
| Gender | |||||
| Male vs female | 0.743 (0.253-2.186) | 0.590 | — | — | |
| Hg (g/L) | |||||
| ≤110 vs >110 | 4.490 (0.541-37.27) | 0.164 | — | — | |
| Tumor location (cm) | |||||
| ≤5.0 vs >5.0 | 0.762 (0.285-2.033) | 0.587 | — | — | |
| CEA level ( ng/ml) | |||||
| <5.00 vs ≥5.00 | 0.378 (0.124-1.155) | 0.088 | 0.300 (0.089-1.012) | 0.052 | |
| Histologic grade | |||||
| G1-2 vs G3 | 0.983 (0.306-3.156) | 0.977 | — | — | |
| APAF-1expression | |||||
| low vs high | 2.931 (1.014-8.473) | 0.047 | 4.291 (1.342-13.699) | 0.014 | |
| COX-2 expression | |||||
| low vs high | 0.287 (0.094-0.876) | 0.028 | 0.205 (0.059-0.708) | 0.012 | |
| Interval between completion of RT and surgery (weeks)# | |||||
| ≤7 vs >7 | 0.695 (0.263-1.833) | 0.462 | — | — | |
HR: hazard ratio; CI: confidence interval;
interval between neo-CRT and surgery
Multivariate logistic analyses of predictors for pCR to neo-CRT
Multivariate logistic analyses were shown that both APAF-1 and COX-2 gene expression remained to be independent risk factors for pCR. Pre-CRT serum CEA level was not associated with predicting pathologic tumor response to neo-CRT (p=0.052) (Table 5).
DISCUSSION
Our research has shown that evaluation of APAF-1 and COX-2 expression on pretreatment specimen may be used to predict pathologic complete response to neo-CRT in patients with locally advanced rectal adenocarcinoma. We and other researchers have demonstrated that pathologic stage is strongly associated with treatment outcome for thosewho were treated with neoadjuvant chemoradiotherapy followed by radical surgery, especially for those with pCR after neo-CRT can achieve a better prognosis [8, 9]. The ability to monitor pCR patients after neo-CRT before radical surgery would significantly impact subsequent management. Patients who would achieve pCR after neo-CRT may be recommended to have local excision or wait and see treatment strategy to avoid radical surgery-related sequelae and complications [10].
Researchers have mainly focused on the clinical factors, imaging studies and molecular targets to monitor pCR patients after neo-CRT. Our research have shown that clinical factors such as age, gender, hemoglobin (Hb) and histological grade were not associate with pCR [11]. Yet Khan A et al [6] have demonstrated that Hb might be used as a biomarker to predict treatment response of rectal adenocarcinoma to neo-CRT. Garcia-Florez et al have reported that the differentiation level of tumor cells can be used to predict treatment response to neo-CRT in patients with rectal adenocarcinoma [12]. Perez et al [13] have shown that assessment of treatment response with PET/CT imaging at 12 weeks after the completion of CRT may provide additional information for the accurate selection of patients achieving complete clinical response who may avoid unnecessary radical resection. Peng HH et al [14] have reported that transrectal ultrasonography (TRUS) restaging has potential value in screening patients with pCR in 149 patients with locally advanced rectal adenocarcinoma after neo-CRT when compared to postoperative pathological TNM staging.
APAF-1 is a key regulating factor of mitochondrial apoptosis pathway. It can combine with cytochrome C, Caspase–9 to form an apoptotic body and therefore activate caspase execution system leading to programmed cell death [15]. Zlobec et al [16] have reported that detection rate of APAF-1 expression for patients who achieved pCR, partial response and no response after neo-CRT was 60%, 51.0% and 27.6%, respectively. Therefore they have concluded that high level of pretreatment APAF-1 expression indicated higher rates of pCR that would be expected for paitents with locally advanced rectal adenocarcinoma when they were treated with neo-CRT. Edden Y et al [17] have also discovered that the level of APAF-1 expression was significantly associated with tumor regression, T downstage and pCR and concluded that APAF-1 was one of the independent factors to predict tumor regression to neo-CRT. Yet Garcia-Florez [12] have made an opposite conclusion that high expression of APAF-1 lead to lower pCR in locally advanced rectal adenocarcinoma after neo-CRT.
COX-2 is the critical enzymes involved in transformation of arachidonic acid into prostaglandins. It works through promoting the production of prostaglandins, which may in turn enhance cell proliferation, promote the formation of blood vessels, inhibit cell apoptosis and exert anti-tumor immune response [18, 19]. Kishi et al [20] have confirmed in nude mouse sarcoma model that COX-2 inhibitors can reduce the expression of prostaglandin E2, suppress tumor angiogenesis, thus inhibit tumor growth. Clinical studies in patients with locally advanced rectal adenocarcinoma have shown that high COX-2 expression conveys poorer sensitivity to radiation and chemotherapy by promoting high expression of angiogenesis factor [21], and using the COX-2 inhibitor celecoxib can improve the sensitivity of tumor cells to radiation therapy [22].
In this study we have focused on the relationship between pCR after neo-CRT and expression level of APAF-1and COX-2 genes in locally advanced rectal adenocarcinoma. Our research data have demonstrated the positive predictive value of a strong APAF-1 pretreatment expression for patients to achieve pCR after neo-CRT and an inverse correlation between increased level of COX-2 and pCR, which were quite similar to the reports [17].
To the best of our knowledge, it is the first research to explore the predicting value of combined expression of APAF-1 and COX-2 genes in pCR to neo-CRT in patients with locally advanced rectal adenocarcinoma. Our data have demonstrated that patients with high expression of APAF-1/low expression of COX-2 would be expected to obtain the highest pCR rate (56.0%), which is significantly higher than those with other combination of their expression, which might be used to monitor pCR patients before neo-CRT.
There may be several factors confounding the results in this study. Firstly it was a retrospective study. We only selected those with preoperative biopsy paraffin blocks available and matched TME surgical specimens. Secondly the sample size was relatively small with only 82 patients included in this study.
In conclusion, the status of APAF-1 and COX-2 expression detected in pretreatment rectal tumor biopsies may be predictive in treatment response to neo-CRT for patients with locally advanced rectal adenocarcinoma. The combination of high expression of APAF1 and low expression of COX-2 might be used in selecting patients with pathologic complete response to neo-CRT. The potential of these two markers used to predict pCR merits further investigation.
MATERIALS AND METHODS
82 consecutive patients with pre-treatment rectal biopsy paraffin tissue blocks and matched TME surgical specimens were treated with neo-CRT and TME from 2005 to 2012. 16 patients (19.5%) presented with stage II and 66 (80.5%) with stage III rectal adenocarcinoma in which pretreatment biopsy paraffin blocks were available for the evaluation of APAF1 and COX2 gene expression and TME surgical specimens for the evaluation of treatment response to neo-CRT. Among them 57 patients were (69.5%) male and 25 (30.5%) female, and the median age was 57 years (range: 15-75). All patients were made clinical and preoperative staging examination including digital rectal exam, chemistry profiling, colonoscopy, endorectal ultrasound, abdominal and pelvic CT and/or MRI, chest radiography.
Concurrent chemoradiotherapy was administered to all patients. Target volume was defined according to the recommendations of the ICRU reports No. 50 and 62 and Myerson RJ et al [23]. The gross tumour volume (GTV) was delineated further according to the information obtained from the diagnostic CT and MRI, including the rectal primary tumor and invaded lymph nodes. Two clinical target volumes (CTVs) were defined: CTV1 was the GTV plus the corresponding mesorectum and presacral region plus a margin of 2-5 cm in the cranio-caudal direction. CTV2 included the whole rectum and loco-regional lymph nodes at risk of involvement, the posterior part of prostate and seminal vesicles in male patients and the posterior of vaginal wall and cervix in female patients. The uppermost border for CTV2 was at the bifurcation of abdominal aorta approximated the sacral promontory and its lowermost border was at the anal verge covered the rectosigmoid junction and the whole rectum with its mesentery. PTV1, PTV2 were obtained by adding non-uniform margins to CTV1, CTV2 as below: the margins of the cranio-caudal, the anterior and posterior, and the lateral were 0.9cm, 0.7cm and 0.8cm, respectively. The organs at risk (OAR) volumes were outlined in the small bowel, the bladder, and the femoral heads. Radiotherapy with 50 Gy to the rectum as clinical tumor volume, CTV1) and 46 Gy was administered to the region of pelvic lymph node as clinical tumor volume, CTV2) in 1.8-2.0 Gy/fraction over a period of 5 weeks. Once the treatment planning was completed, the plan was normalized to cover 100% of the PTVs with ≥ 95% of the prescribed dose.
One of two chemotherapeutic regimens was delivered concurrent with RT: (1) FOLFOX: fluorouracil 3.0 g/m2, CIV lasting for 48 h; calcium folinate 200.0 mg/m2, day 1; oxaliplatin 100.0 mg/m2, day 1; repeated for three weeks (n=6 patients, 7.3%); and (2) XELOX: capecitabine 1000.0 mg/m2 bid, days 1–14; oxaliplatin 100.0 mg/m2, day 1; repeated for three weeks (n = 76 patients, 92.7%).
Surgery was performed approximately 7 weeks (range: 4-20 weeks) after the completion of neo-CRT. The surgical procedure was either low anterior resection/double stapling method (n=54 patients, 65.9%) or abdominoperineal resection (n = 28 patients, 34.1%).
52 patients received post-operative adjuvant chemotherapy with either XELOX (63%) or FOLFOX (34%). The regimen of adjuvant chemotherapy was consistent with the pre-operative chemotherapy with median cycle of 3 (range: 2–6) cycles.
Immunohistochemical assay
Paraffin block from the pretreatment biopsy sample were sectioned at 3 μm in thickness, placed on positively charged slides. The immunohistochemical staining was made according to the recommendation by the company. The tissue sections were stained with the following antibodies: COX-2 (1:100, ABGENT, USA) and APAF-1 (1:100, ABGENT, USA). Negative control was made by substituting PBS for the primary antibodies.
Analysis of immunohistochemical assay
The results of immunohistochemical staining for COX-2 and APAF-1 gene expression was evaluated by two independent pathologists blinded to the tumor response grade (TRG) to neo-CRT and final pathological staging. As recommended by Edden Y and Smith FM [17, 24], the intensity of staining and the number of positive tumor cells were used to classify cytoplasmic markers based on the following criteria: 0, none; 1, light yellow granules; 2, yellow granules; 3, brown granular. Number of positive tumor cells: 0, no staining; 1, up to 25% of positive cells; 2, 26–50% of positive cells; and 3, more than 50% of positive cells. The express degree score = Intensity of staining score × Number of positive tumor cells score.
The histological sections were originally examined by two experiencedpathologists independently. TRG was assessed by utilizing Dworak's scoring system [25] which defined the amount of residual carcinoma in relation to fibrosis on a five-point scale as follows: TRG 0, no regression; TRG 1, tumor regression less than 25%; TRG 2, tumor regression between 25%-50%; TRG 3, tumor regression more than 50% with fibrosis outgrowing the tumor mass; TRG4, complete pathologic response, only fibrosis (pCR).
Statistical analysis
SPSS v17.0 software was used for statistical analysis. Classification variables were analyzed using chi-square test or fish precise inspection, quantitative variables using student t test or rank and inspection. Kaplan Meier method was adopted for survival analysis, and the log rank test was used between variables, and a p value < 0.05 was considered to be significant.
SUPPLEMENTARY TABLES
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
GRANT SUPPORT
This work was supported by National Natural Science Foundation of China (No. 81172209); Guangdong Natural Science Foundation (No. S2011020003612).
REFERENCES
- 1.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. The British journal of surgery. 2012;99:918–928. doi: 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- 2.Kundel Y, Brenner R, Purim O, Peled N, Idelevich E, Fenig E, Sulkes A, Brenner B. Is local excision after complete pathological response to neoadjuvant chemoradiation for rectal cancer an acceptable treatment option? Diseases of the colon and rectum. 2010;53:1624–1631. doi: 10.1007/DCR.0b013e3181f5b64d. [DOI] [PubMed] [Google Scholar]
- 3.Belluco C, De Paoli A, Canzonieri V, Sigon R, Fornasarig M, Buonadonna A, Boz G, Innocente R, Perin T, Cossaro M, Polesel J, De Marchi F. Long-term outcome of patients with complete pathologic response after neoadjuvant chemoradiation for cT3 rectal cancer: implications for local excision surgical strategies. Annals of surgical oncology. 2011;18:3686–3693. doi: 10.1245/s10434-011-1822-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolthuis AM, Penninckx F, Haustermans K, Ectors N, Van Cutsem E, D'Hoore A. Outcome standards for an organ preservation strategy in stage II and III rectal adenocarcinoma after neoadjuvant chemoradiation. Annals of surgical oncology. 2011;18:684–690. doi: 10.1245/s10434-010-1324-5. [DOI] [PubMed] [Google Scholar]
- 5.Krauthamer M, Rouvinov K, Ariad S, Man S, Walfish S, Pinsk I, Sztarker I, Charkovsky T, Lavrenkov K. A study of inflammation-based predictors of tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Oncology. 2013;85:27–32. doi: 10.1159/000348385. [DOI] [PubMed] [Google Scholar]
- 6.Khan AA, Klonizakis M, Shabaan A, Glynne-Jones R. Association between pretreatment haemoglobin levels and morphometric characteristics of the tumour, response to neoadjuvant treatment and long-term outcomes in patients with locally advanced rectal cancers. Colorectal disease. 2013;15:1232–1237. doi: 10.1111/codi.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. International journal of radiation oncology, biology, physics. 2009;74:673–688. doi: 10.1016/j.ijrobp.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Negri FV, Campanini N, Camisa R, Pucci F, Bui S, Ceccon G, Martinelli R, Fumagalli M, Losardo PL, Crafa P, Bordi C, Cascinu S, Ardizzoni A. Biological predictive factors in rectal cancer treated with preoperative radiotherapy or radiochemotherapy. British journal of cancer. 2008;98:143–147. doi: 10.1038/sj.bjc.6604131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng H, You K, Wang C, Huang R, Chang H, Zhou G, Zeng Z, Liu M, Wen B, Gao Y. Prognosis of clinical and pathological stage in locally advanced rectum cancer after neo-chemoradiotherapy. Chinese Journal of Radiation Oncology. 2013;22:439–442. [Google Scholar]
- 10.Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, Hulsewe KW, Buijsen J, Beets GL. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. Journal of clinical oncology. 2011;29:4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 11.Wen B, Zhang L, Wang C, Huang R, Peng H, Zhang T, Dong J, Xiao W, Zeng Z, Liu M, Gao Y. Prognostic significance of clinical and pathological stages on locally advanced rectal carcinoma after neoadjuvant chemoradiotherapy. Radiat Oncol. 2015;10:124. doi: 10.1186/s13014-015-0425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Florez LJ, Gomez-Alvarez G, Frunza AM, Barneo-Serra L, Martinez-Alonso C, Fresno-Forcelledo MF. Predictive markers of response to neoadjuvant therapy in rectal cancer. The Journal of surgical research. 2015;194:120–126. doi: 10.1016/j.jss.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Perez RO, Habr-Gama A, Gama-Rodrigues J, Proscurshim I, Juliao GP, Lynn P, Ono CR, Campos FG, Silva e Sousa AH, Jr, Imperiale AR, Nahas SC, Buchpiguel CA. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation: long-term results of a prospective trial (National Clinical Trial 00254683) Cancer. 2012;118:3501–3511. doi: 10.1002/cncr.26644. [DOI] [PubMed] [Google Scholar]
- 14.Peng HH, You KY, Wang CT, Huang R, Shan HB, Zhou JH, Pei XQ, Gao YH, Wen BX, Liu MZ. Value of transrectal ultrasonography for tumor node metastasis restaging in patients with locally advanced rectal cancer after neoadjuvant chemoradiotherapy. Gastroenterology report. 2013;1:186–192. doi: 10.1093/gastro/got028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams JM, Cory S. Apoptosomes: engines for caspase activation. Current opinion in cell biology. 2002;14:715–720. doi: 10.1016/s0955-0674(02)00381-2. [DOI] [PubMed] [Google Scholar]
- 16.Zlobec I, Vuong T, Compton CC. The predictive value of apoptosis protease-activating factor 1 in rectal tumors treated with preoperative, high-dose-rate brachytherapy. Cancer. 2006;106:284–286. doi: 10.1002/cncr.21600. [DOI] [PubMed] [Google Scholar]
- 17.Edden Y, Wexner SD, Berho M. The use of molecular markers as a method to predict the response to neoadjuvant therapy for advanced stage rectal adenocarcinoma. Colorectal disease. 2012;14:555–561. doi: 10.1111/j.1463-1318.2011.02697.x. [DOI] [PubMed] [Google Scholar]
- 18.Sheehan KM, Steele C, Sheahan K, O'Grady A, Leader MB, Murray FE, Kay EW. Association between cyclooxygenase- 2-expressing macrophages, ulceration and microvessel density in colorectal cancer. Histopathology. 2005;46:287–295. doi: 10.1111/j.1365-2559.2005.02083.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishi K, Petersen S, Petersen C, Hunter N, Mason K, Masferrer JL, Tofilon PJ, Milas L. Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer research. 2000;60:1326–1331. [PubMed] [Google Scholar]
- 21.Min BS, Choi YJ, Pyo HR, Kim H, Seong J, Chung HC, Rha SY, Kim NK. Cyclooxygenase-2 expression in pretreatment biopsy as a predictor of tumor responses after preoperative chemoradiation in rectal cancer. Arch Surg. 2008;143:1091–1097. doi: 10.1001/archsurg.143.11.1091. discussion 1097. [DOI] [PubMed] [Google Scholar]
- 22.Davis TW, O'Neal JM, Pagel MD, Zweifel BS, Mehta PP, Heuvelman DM, Masferrer JL. Synergy between celecoxib and radiotherapy results from inhibition of cyclooxygenase- 2-derived prostaglandin E2, a survival factor for tumor and associated vasculature. Cancer research. 2004;64:279–285. doi: 10.1158/0008-5472.can-03-1168. [DOI] [PubMed] [Google Scholar]
- 23.Myerson RJ, Garofalo MC, El Naqa I, Abrams RA, Apte A, Bosch WR, Das P, Gunderson LL, Hong TS, Kim JJ, Willett CG, Kachnic LA. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. International journal of radiation oncology, biology, physics. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith FM, Reynolds JV, Kay EW, Crotty P, Murphy JO, Hollywood D, Gaffney EF, Stephens RB, Kennedy MJ. COX-2 overexpression in pretreatment biopsies predicts response of rectal cancers to neoadjuvant radiochemotherapy. International journal of radiation oncology, biology, physics. 2006;64:466–472. doi: 10.1016/j.ijrobp.2005.07.961. [DOI] [PubMed] [Google Scholar]
- 25.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. International journal of colorectal disease. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


