Abstract
In 1999, the Global Advisory Committee on Vaccine Safety (GACVS) was established by the World Health Organization (WHO) to provide independent scientific advice on issues relating to the safety of vaccines and immunization. Fifteen years onward, we conducted a multi-faceted review to evaluate the impact, reach and challenges facing GACVS, including the role GACVS plays in informing global, regional and WHO member state vaccine policy. The methods included measures of organizational structure, citation impact, themes approached, and a discussion by previous and current members to evaluate past, present and future challenges. Given the increasing range of data sources and the deployment of many new vaccines, the Committee is facing the complex task of identifying the best available evidence for recommendations on vaccine safety. To help meet the increased demand for public transparency in decision making, GACVS-structured methodology for evidence-based decisions is evolving. GACVS also promotes best practices and capacity building for timely and accurate risk assessment; risk communications; outreach to help countries maintain and, if needed, rebuild public trust in vaccines; and advocacy for bridging the major gaps in vaccine safety capacity globally.
Keywords: Vaccine safety, Global public health, Vaccine adverse events, Global Advisory Committee on Vaccine Safety, Vaccine policy, Immunization program
1. Introduction
Since vaccines are usually administered to healthy individuals to prevent their target vaccine-preventable diseases (VPD), they are typically held to a higher standard of safety than medicinal products used to treat ill patients. To identify common and less common (but not rare) problems at the preapproval stage, vaccines undergo extensive safety and efficacy studies needed to fulfill stringent regulatory licensure requirements [1,2]. Due to the limited size and scope of pre-licensure safety studies, however, post licensure monitoring and evaluation is needed and increasingly performed to identify rare safety concerns. For two centuries, vaccines have demonstrated their public health value in preventing and controlling infectious diseases that previously injured or killed millions of individuals globally each year. However, these successes in controlling and in some cases eliminating their target VPD have paradoxically resulted in increased concerns about the safety of vaccines in recent decades [3]. Occasionally, rare serious adverse vaccine reactions occurring less frequent than one in 10,000 doses may become evident after a new vaccine is in widespread use in the general population [4–6]. But more commonly, as vaccine coverage reach high enough level to decrease the threat of VPDs, there is also a concomitant increase in coincidental adverse event following immunization (AEFI). Due to “post hoc ergo propter hoc”, a common public misunderstandings about logic, these AEFIs may be falsely attributed as being caused by immunization. This misattribution seems particularly common for medical conditions whose etiology and pathophysiology are incompletely understood (e.g. autism, and multiple sclerosis). As information sharing via internet becomes all too easy, however, so is the propagation of such errors [7].
In the absence of adequate capacity to confirm or reject such AEFI's being caused by vaccination in a timely manner, loss of public confidence in a vaccine may occur (manifested as either hesitancy or refusal resulting in reduced coverage), with consequent return of outbreaks of VPDs [8,9]. Enhanced surveillance coupled with sound epidemiologic studies for vaccine safety from the local to the global levels helps provide the best evidence for decision making by parents, patients, providers, policy makers and society. This is a challenging capacity building process, especially in low and middle income countries (LMIC) where AEFI surveillance, investigation and management are often not well established. This process requires long-term commitment and significant resources to create the required safety monitoring infrastructure. The Global Advisory Committee on Vaccine Safety (GACVS) was established by the World Health Organization (WHO) in 1999 due to the growing need for independent review of the (often limited) available evidence and to provide recommendations on emerging vaccine safety issues globally, but especially for LMICs lacking such capacity [10–13]. This paper presents an analysis and review of the work and impact of GACVS over its 15 years of existence with suggestions for further improvements.
2. Methods
The review of GACVS's contributions and challenges was undertaken at the Center for Global Health, University of Colorado Denver, USA along with discussions by a panel of experts who were current or previous members of the GACVS. The WHO GACVS Secretariat provided support and assistance with access to archival documentation. The review encompassed: (a) an organizational evaluation of GACVS to define its composition, expertise, geographical representativeness; (b) an assessment of the GACVS's activities through a quantitative review of its reports, and an estimation of their impact on their global, regional and country target audiences; and finally, (c) a regulator evaluation survey. The GACVS composition, membership history, geography and professional backgrounds were obtained from a previous independent review of WHO immunization advisory committees and the list of current and past members available at the WHO website [14,15].
Given that direct assessment of GACVS's impact on the science of vaccine safety or on the shaping of local, regional and global vaccine policy was difficult, an indirect measure, the citation factor through Google Scholar Impact, was used. To undertake this, all GACVS meeting reports accessible on its website (from 1999 until 2014) as well as other GACVS and WHO publications in the scientific literature and related reports and guidelines were evaluated for thematic content, type of recommendation and impact generated in the published literature. The keywords “gacvs” and “Global Advisory Committee on Vaccine Safety” were used and the relevant publications ranking, number of references linked and their relevance to other scholarly literature was obtained. Whereas most academic databases and search engines allow users to select one factor (e.g. relevance, citation counts, or publication date) to rank results, Google Scholar grades them using a combined algorithm. For all topics reviewed by GACVS, the date of the Committee review was noted and the number of recommendations and conclusions per year recorded. For every GACVS conclusion, we classified the action as: (a) review of the evidence, (b) recommendation of a policy modification, or (c) request for additional research or evidence. The WHO vaccine position papers were also evaluated to determine if GACVS recommendations had been incorporated and cited as part of the review [16].
As there is no formal process to gauge the visibility and consumer use of GACVS recommendations beyond WHO, we designed a nine item qualitative survey. It was administered in a confidential manner to a convenience sample of drug regulatory experts from all six WHO regions who were attending a forum for drug regulation (International Conference of Drug Regulatory Authorities – ICDRA – August 27–29, 2014). Participants were asked about their knowledge of GACVS as well as the WHO's Weekly Epidemiological Record (WER), and the importance of GACVS recommendations in their regulatory work on vaccines.
Finally, to help identify the challenges and opportunities faced by GACVS, we held a discussion amongst previous and current members at the June 2014 GACVS meeting [17]. Presentations on past work of GACVS and results from the organizational and impact evaluation were used to stimulate discussion. These discussions were recorded, transcribed and themes related to contributions to the vision and future work of GACVS were noted.
3. Results
3.1. GACVS organizational assessment
GACVS has been an independent scientific advisory group to WHO, responsible for providing: (a) technical advice on vaccine safety; (b) assessment of risks related to vaccines in order to assist policy-makers in balancing benefits and risks as part of evidence-based policies; and (c) guidance on the development of vaccine safety systems, strategies and mechanisms to strengthen vaccine safety at the national and global levels [18].
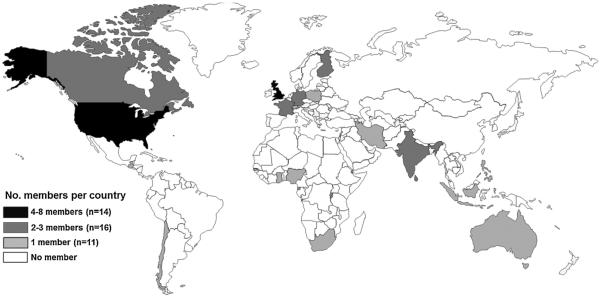
GACVS is composed of experts from around the world in the fields of vaccine safety, vaccinology and allied sciences such as epidemiology, biostatistics, pharmacovigilance, biologic product regulation and clinical medical sciences. The committee's ~15 members are selected by an open application process organized by the WHO secretariat. Members serve on the Committee for three years with possible renewal for a second term. Over the past 15 years, GACVS has had 41 members from 20 different countries, representing all WHO regions, although 26 (63.4%) originate from high-income countries in Europe, North America and Australia as vaccine safety expertise is highly technical (Fig. 1).
Fig. 1.
Total number of GACVS former and current members by region and country 1999–2014.
GACVS members participate in bi-annual in-person meetings; they also work in topic specific sub-groups to develop statements (e.g. vaccines and HIV, vaccines in pregnancy, etc.). The Committee also meets as needed by teleconference in response to new or emerging issues. For example, in early 2010, academic investigators reported finding that one manufacturer's rotavirus vaccine contained DNA from porcine circovirus type 1 [19]. The committee met by teleconference on March 25, 2010, and posted a statement on line the following day [20].
The GACVS agenda is determined by its current and former members, together with the WHO secretariat (who incorporates the needs of the Strategic Advisory Group of Experts (SAGE), WHO's principal advisory group for vaccines and immunizations [21]). National immunization programs and WHO Regional Offices may also bring forward safety issues for consideration. Invited experts and observers may contribute in providing the Committee with up to date specific information but decisions or recommendations are, as a rule, taken by consensus and involve only committee members, not WHO staff or the invited observers or experts.
3.2. Contributions of GACVS to Vaccine Safety Policy
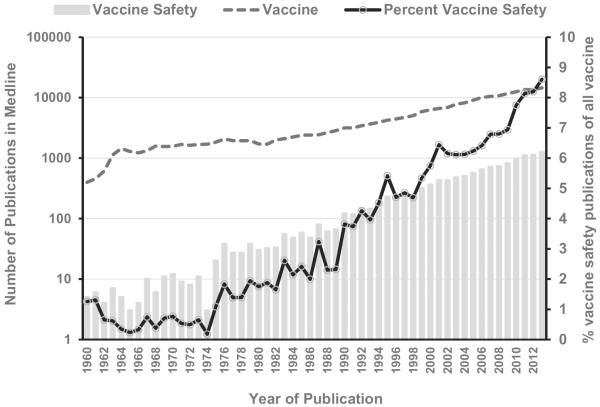
Information on “vaccine safety” issues has increased exponentially in the past 50 years (Fig. 2) – From ≤10 publications per year in the 1960s to >10,000 per year in the past decade. Furthermore, the proportion of publications in Medline related to “vaccine safety” compared to all “vaccine” topics has increased from <1% to 8.3% in 2013. Since its formation, GACVS has been challenged to summarize, analyze and translate the available evidence of vaccine safety. Typically, this evidence can be obtained from (a) clinical trials conducted before regulatory approval for indicated uses; and (b) estimated incidence of AEFI attributable to a vaccine obtained from post-licensure surveillance and other studies. The GACVS uses this evidence to make recommendations that inform public policy and respond transparently to global vaccine safety concerns. At the time of the review, GACVS had produced 106 reports that recap the discussions and determinations of the Committee addressing topics related to vaccine safety and risk assessment of vaccine products (Table 1). Since 2001, GACVS reviews and resolutions have been published in the WER within 2 months following its biannual meetings in June and December. Reports from additional ad hoc meetings by teleconference are also posted on the GACVS website within a few days of the meeting. Table 1 also maps how GACVS followed up on many topics until a policy recommendation could be issued.
Fig. 2.
Number of publications in Medline related to the search term “vaccine” and “vaccine safety” per year 1960–2013.
Table 1.
Vaccine safety issues addressed by the Global Advisory Committee for Vaccine Safety by year and type of review and decision 1999–2014.
| Safety Issue | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCG | RS | EG | RS | EG | RS | RS | PR | EG | RS | PR | ||||||||||||||||||||
| Dengue candidates | RS | EG | RS | EG | ||||||||||||||||||||||||||
| DPT | RS | RS | EG | |||||||||||||||||||||||||||
| DTwP-HBV-Hib | RS | EG | ||||||||||||||||||||||||||||
| DTaP-HBV-Hib-IPV | RS | EG | RS | PR | ||||||||||||||||||||||||||
| Hepatitis A | ||||||||||||||||||||||||||||||
| Hepatitis B | RS | EG | RS | PR | RS | PR | RS | PR | ||||||||||||||||||||||
| Measles | RS | EG | ||||||||||||||||||||||||||||
| Mumps | RS | EG | RS | EG | RS | EG | EG | RS | PR | |||||||||||||||||||||
| Malaria candidates | RS | EG | ||||||||||||||||||||||||||||
| Meningococcal | RS | PR | RS | RS | EG | RS | EG | RS | PR | RS | EG | |||||||||||||||||||
| Rotavirus | RS | EG | RS | EG | RS | EG | PR | EG | RS | PR | RS | PR | RS | EG | ||||||||||||||||
| Smallpox | RS | EG | RS | |||||||||||||||||||||||||||
| Yellow Fever | PR | EG | RS | PR | RS | EG | RS | PR | RS | PR | RS | EG | ||||||||||||||||||
| Japanese encephalitis | RS | PR | RS | EG | PR | EG | RS | PR | ||||||||||||||||||||||
| HPV | RS | EG | RS | EG | RS | EG | RS | EG | ||||||||||||||||||||||
| Pneumococcal | RS | |||||||||||||||||||||||||||||
| Influenza | RS | EG | RS | PR | RS | EG | EG | EG | RS | EG | RS | EG | RS | EG | RS | PR | RS | PR | RS | EG | ||||||||||
| Varicella zoster | RS | EG | RS | EG | RS | |||||||||||||||||||||||||
| MMR | RS | PR | ||||||||||||||||||||||||||||
| Pregnancy | RS | PR | RS | EG | RS | PR | RS | PR | ||||||||||||||||||||||
| Immune overload | RS | EG | ||||||||||||||||||||||||||||
| Immunocompromissed | EG | RS | PR | RS | ||||||||||||||||||||||||||
| Thiomersal | RS | PR | RS | PR | RS | EG | RS | EG | RS | PR | RS | PR | RS | PR | ||||||||||||||||
| Formulations | RS | EG | RS | EG | ||||||||||||||||||||||||||
| Aluminum | RS | EG | RS | EG | RS | PR | RS | EG | ||||||||||||||||||||||
| Nonspecific effects vaccines | RS | EG | RS | PR | RS | |||||||||||||||||||||||||
| Oculo-respiratory syndrome | RS | EG | ||||||||||||||||||||||||||||
| Adjuvants | RS | EG | RS | EG | RS | EG | ||||||||||||||||||||||||
| TSE | RS | EG | ||||||||||||||||||||||||||||
| VS Systems | VDN | VDN | PAS | PV | VSM | CA | GVSP | CA | ||||||||||||||||||||||
RS, Review Safety (summarized evidence and provided an overview of the data available).
PR, policy recommendation (provides specific instructions based on risk-benefit assessment).
EG, evidence gathering (recommendation to obtain additional evidence on safety of vaccine).
Abbreviations: VS: vaccine safety; VDN: vaccine safety detection networks; PV: pharmacovigilance; VSM: vaccine safety monitoring; CA: causality assessment; GVSP: global vaccine safety plan.
GACVS has also released 21 vaccine safety statements on 10 topics considered to be priorities by member countries or the WHO secretariat [14]. GACVS's conclusions also provide the safety elements of the WHO position papers [16], although not cited as such. GACVS risk assessments and resolutions are regularly presented to SAGE, the Expert Committee on Biological Standards (ECBS), and regional technical advisory groups related to immunization.
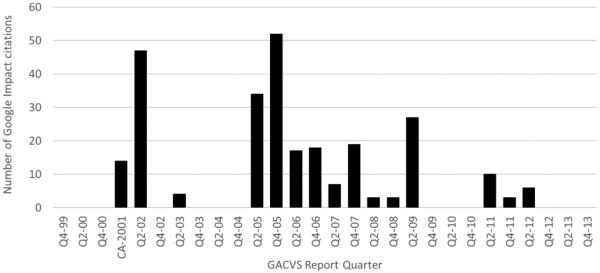
In May 2014, at the time of the review of GACVS reports and articles, Google Scholar contained ~160 million documents. Google Scholar Impact of the 21 vaccine statements and 106 WER GACVS reports found the most cited GACVS meeting report was that from December 2005 with 52 citations (Fig. 3). In that meeting, GACVS had reviewed the safety of Rotashield® vaccine, the potential safety of pandemic influenza vaccination and the safety of adjuvants. The second most cited meeting report, from June 2002, provided an evaluation of the safety of thiomersal-containing vaccines as well as the risk of multiple sclerosis and leukemia after hepatitis B vaccine. The third and fourth most commonly cited meeting reports included discussions on the issues of thiomersal, Japanese encephalitis vaccine safety, hexavalent vaccine safety, safety of novel influenza vaccines and age of administration and safety of rotavirus vaccine.
Fig. 3.
Google Scholar© citation index of GACVS reports by quarter of the year published. Weekly Epidemiological Record 1999–2014. Note: Quarterly data for 2001 is not presented, as GACVS only held one meeting with a specific recommendation.
3.3. Example of a GACVS recommendation on policy globally, then nationally
At the June 2003 meeting, GACVS noted the repeated reports of local and disseminated BCG infection occurring after BCG immunization in persons with HIV and called for closer monitoring of this event [22–25]. In November of 2007, following new evidence from Argentina and South Africa, GACVS concluded that a higher risk of disseminated BCG disease was present in HIV-infected infants and BCG vaccine should not be used in children who are known to be HIV infected; BCG should continue to be provided at birth, however, to infants regardless of HIV exposure in TB highly endemic countries where no early HIV infection confirmation was possible. This recommendation, reviewed again in 2009, allowed many middle-income countries in which HIV early diagnosis is available to prevent such adverse events and stimulated the development of new guidelines for highly endemic countries [26].
3.4. GACVS contributions to vaccine safety methods and systems
During the past 15 years, GACVS has actively contributed to the development of vaccine safety tools, methods and strategies [27–29], frequently bringing together representatives of countries and regions to ensure broad participation. In partnership with the Uppsala Drug Monitoring Center, GACVS promoted a new classification of AEFI, its core data elements and indicators that can be used in country and regional surveillance systems [30]. As result of a WHO and Bill and Melinda Gates Foundation project on the development of a global network for vaccine safety monitoring, GACVS also endorsed the revision of the AEFI causality assessment algorithms [31], vaccine specific safety information documents [32], and facilitated the development of on-line vaccine safety learning modules [33]. The causality assessment algorithm was further revised following input from low and middle income country users [34,35]. These revised tools are available to all countries and are disseminated by WHO through regional workshops and in country meetings. GACVS also reviewed and advised WHO on two key documents: (1) the Global Vaccine Safety Blueprint – WHO's strategy to optimize the safety of vaccines through effective use of pharmacovigilance principles and methods in all countries; and (2) the Global Vaccine Action Plan (GVAP), targeting LMICs to implement practices and tools to allow more systematic and useful vaccine safety information [36,37].
3.5. GACVS outreach and communication
GACVS has continuously expanded its mechanisms for communication of its recommendations to WHO technical audiences using WHO's WER, the GACVS website and through SAGE meetings and its conclusions. The GACVS audiences include national governments and international organizations that deal with policies regarding vaccine safety, professional organizations and immunization technical advisory groups especially those from LMIC. One GACVS initiative to reach out to broader audiences and help counteract misinformation about vaccines was the creation of the Vaccine Safety Net (VSN) launched in 2003 [38]. Through a series of quality indicators developed by GACVS, VSN provides a clearinghouse, a listing of websites that feature scientifically sound and transparent vaccine safety information [39].
The convenience survey of national drug regulatory experts attending ICDRA in 2014 resulted in 75 questionnaire responses from all WHO Regions with 46 (61%) reporting that they knew of GACVS's work, 19 (25%) used its recommendations, but 49 (65%) had never received or read WER. Not receiving or reading WER was associated with not knowing about GACVS (47% vs. 89%), nor using its recommendations (11% vs. 90%). The majority of participants considered GACVS recommendations for their work as regulators as very important (64%) or somewhat important (25%).
3.6. GACVS challenges
The 2-h discussion amongst GACVS members in 2014 involved 6 past and 12 current members. GACVS's work was considered well established and well recognized, with comments made about its value and importance especially to low and middle income countries who do not have significant local vaccine expertise. High income countries also noted the value added, as GACVS gave global interpretation to their local safety findings.
Several challenges illustrating the complexities of global vaccine safety were also identified. First, expert institutions and committees like GACVS are bound to generate their advice based on usually limited albeit up to date evidence (primarily observational studies in the post-licensure phase) along with expert opinion. When the missing information is essential to allow an evidenced-based conclusion, and the risk from delaying a conclusion is low, GACVS has on occasion requested more research on the specific vaccine safety issue. However as GACVS (and sometimes WHO) does not have a discretionary research budget, it is dependent on persuading other stakeholders to do so. For example, when a vaccine against epidemic meningococcal meningitis A in sub-Saharan Africa was made available for mass campaigns in people aged 1–29 years, data on the safety of the vaccine during pregnancy was not available [40,41]. GACVS then made recommendations for monitoring of this safety aspect during the mass vaccination roll-out. As well, vaccine safety issues that have been under consideration at GACVS may undergo periodical revision as new scientific evidence arises. These reviews may be also be in response to requests from other committees or from member states.
Second, while GACVS conclusions are based on the best available evidence and expert opinion, there is increasing pressure to incorporate accepted systems such as Grading of Recommendations, Assessment, Development and Evaluation (GRADE) to qualify that evidence. This presents challenges when it comes to discussing health hazards [42]. Rating the quality of evidence using systematic literature review methods requires resources and adaptation to appraise the quality of observational studies from the post-licensure phase. However, the effort of GACVS to anticipate the need for information and standardization of vaccine safety in post-licensure studies (e.g. malaria and dengue vaccines) can improve its ability to use GRADE in its deliberations.
Third, the current limited capacity of most countries and regions to identify and rigorously assess scientifically plausible and potentially significant vaccine safety signals in a timely manner continues to be a major challenge for the work of GACVS and WHO [43]. Several attempts, to develop multi-country networks for vaccine safety monitoring, supported by WHO, GACVS and other partners but with limited funding have had only modest success due to the variability of capacities and methods used by each participating country [44,45].
Fourth is the issue of transparency and confidentiality. While a technical committee like GACVS should make every effort to make its processes public and clear in order to build trust, the committee acknowledged that to make the most appropriate and evidence-based decisions, GACVS occasionally receives proprietary scientific information that are shared confidentially. Access to this type of information requires balancing safeguards to avoid breaches on proprietary information with the need for the public and partners to be fully informed of the deliberations. The use of innovative tools for decision-making transparency could be of help [46–48].
Finally, the target audiences for GACVS's conclusions needs to be better articulated and defined. In particular, GACVS does not have a communication strategy toward the general public and whether this is best left to the WHO member countries merits further discussion. Engagement of the media has been limited but GACVS has yet to define if media is or is not the best conveyor of its conclusions to the public. Similarly, GACVS has not evaluated if print communication, the primary method used thus far, or new social and scientific media are the best and modern tools for knowledge transfer to its target audiences [49]. The relevance of effective communication is exemplified by some safety concerns that appear to be resolved at the scientific and policy levels (e.g., the non-specific effects of vaccination, adverse effects of thiomersal, whole-cell pertussis vaccines and encephalopathy, HPV vaccine and auto-immune diseases) but continue to surface as public concerns. The use of consistent and explicit language in all statements would also increase the reliability of interpretation of GACVS recommendations and statements by a wider audience.
4. Discussion
GACVS appears to be a valued technical advisory committee for WHO that provides an independent assessment of vaccine safety commonly used by SAGE in their vaccine policy deliberations and position statements. GACVS is also perceived as a valued mechanism by the member states for providing timely independent evidence-based reviews and conclusions on current, emerging or foreseeable vaccine safety issues for policy makers, immunization program managers and technical advisory committees. With many more vaccines becoming available and enhanced AEFI surveillance systems are detecting vaccine safety signals more frequently worldwide [50,51]. Safety concerns are a well-recognized, albeit only one of many, contributing factors to increasing vaccine hesitancy noted in some communities [52–54].
High-income countries have found reassurance and a global perspective when GACVS arrives at similar conclusions to their own expert national technical immunization advisory committees. However, most LMICs lack (and are unlikely to acquire in the near future) the capacity to adequately assess the validity of vaccine safety concerns, therefore they will likely continue to rely on GACVS to provide efficient, timely and clear recommendations to address such safety worries and misinformation. In the medium term, the development of regional safety advisory committees, as well as the enhancement of vaccine safety capacity in countries, as outlined in the Global Vaccine Safety Blueprint [36], could extend the reach and improve participation in vaccine safety surveillance and decision making.
While GACVS has not had a direct role in building vaccine safety capacity globally previously, advocating for its successful deployment in the future could improve detection, investigation and causality evaluation of vaccine safety concerns, and strengthen both a country and GACVS's position to provide more evidence-based conclusions. Unfortunately, despite GACVS recommendations for enhanced post-marketing vaccine safety data to be generated in LMICs when new vaccines are being introduced, the necessary long-term funding remains insufficient. For example it took more than two decades to document the risk of disseminated BCG disease in HIV-infected infants. The deployment of new generation rotavirus vaccines in populations from LMICs also required a significant effort from public health organizations and manufacturers to document the low risk of intussusception related to these vaccines and recommendation for its continued use after a careful benefit-risk analysis. Furthermore, there remains a paucity of information on vaccine safety for special subgroups such as pregnant women, the elderly, those who are immunocompromised or with chronic disabilities, as vaccine pharmacovigilance systems have not focused on these populations and/or have not been well studied during vaccine clinical development [55].
From this review, there is evidence that recommendations from GACVS have been helpful for vaccine policy decision making at global and country levels but there are limitations to these findings. Measuring GACVS impact, as was attempted here, does have several limitations. The use of Google Scholar Citation Index as an indirect measure of scientific use places high weight on the citation counts and words included in a document's title, and does not account for how its reports generated additional inquiries for evidence. Furthermore, all recommendations were treated equally, while at the level of vaccine policy or science, the prioritization of vaccine safety issues is driven by country, public or scholar interest as well as current vaccine context.
Some of the lessons learned from the GACVS 15-year tenure include: (a) its independent expertise to provide vaccine safety assessments to the vaccine decision and policy makers; (b) its active role in the development of tools, methods and best practices useful for vaccine safety assessment; and (c) its increasing inclusion of experts from LMIC to incorporate a diversity perspective to its deliberations. As regulators and immunization programs develop increased capacity in many countries, including LMIC, additional guidelines and innovative methods (eHealth platforms) to perform local post-licensure safety studies will be needed. Systems to share their surveillance information using standardized case definitions and methods could be crucial for policy decision making. In the meantime and until more relevant information becomes available, if the vaccine safety issue of concern requires urgent guidance, GACVS cautionary reports will continue to explain the limitations of the conclusion with: (1) a statement of relevance of the incomplete information; (2) a summary of existing relevant scientific evidence; and (3) the committee's evaluation of possible impacts based upon theoretical approaches or research methods generally accepted in the scientific community.
GACVS and WHO should continue to encourage regional committees and technical advisory groups to address local concerns and to ensure a bidirectional exchange of evidence and recommendations specific to local contexts. The observation by the experts that variability of the language across GACVS recommendations and statements may preclude their interpretation for adoption by users suggests that more standardized wording and GRADE assessments to depict the strength of the evidence and expert opinion guided to action might be helpful. The small survey of national regulators showed their lack of awareness of the conclusions of GACVS. Therefore, to increase GACVS's effectiveness, a knowledge transfer strategy that targets and reaches audiences with GACVS's recommendations needs to be in a format that can be understood and that can be applied to local policies and programs. If its findings and recommendations are to have an impact at country level, communication must also be conveyed to policy-makers. The recently created NITAG resource center [56] or the vaccine safety toolkit [57] illustrates these innovative support channels.
Finally, given the large gaps in capacity to handle vaccine safety signals in a timely manner in many countries worldwide and the resources needed to implement the Global Vaccine Safety Initiative (GVSI), GACVS can play an important advocacy role to help fill these gaps.
5. Conclusions
Technical advisory committees like GACVS may be able to strengthen the policy-making processes of WHO member states by providing in country decision-makers with rigorous scientific evaluation of safety issues. GACVS has had an impact on some vaccine safety topics within WHO member countries and continues to contribute to vaccine recommendations developed by SAGE. Public dissemination of GACVS work deserves additional attention and should, ideally, be further developed in collaboration with WHO member states. GACVS should also serve as a facilitator to bridge the major gaps in vaccine safety capacity globally.
Acknowledgement
This work was funded by the World Health Organization as a commissioned report.
Footnotes
Disclaimer: The findings and views expressed in this white paper are those of the authors alone and do not necessarily reflect those of the World Health Organization or the other authors' institutions.
Author's contributions EJA and PLFZ conceptualized the report. All authors were responsible for analysis and interpretation of data and writing of the manuscript. All authors approved the final version.
Conflicts of interest statement: All the authors with the exception of PLFZ are or have been members of GACVS. PLZ is an employee of WHO, the funding source.
References
- [1].Dellepiane N, Wood DC. Twenty-five years of the WHO vaccines prequalification programme 1987–2012: lessons learned and future perspectives. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.11.066. S0264-S410 (13)01628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Peter G, des Vignes-Kendrick M, Eickhoff TC, Fine A, Galvin V, Levine MM, et al. Lessons learned from a review of the development of selected vaccines. National Vaccine Advisory Committee. Pediatrics. 1999;104(4 Pt 1):942–5. [PubMed] [Google Scholar]
- [3].Chen RT. Vaccine risks: real, perceived and unknown. Vaccine. 1999;17:S41–6. doi: 10.1016/s0264-410x(99)00292-3. [DOI] [PubMed] [Google Scholar]
- [4].Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–72. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- [5].Simonsen L, Viboud C, Elixhauser A, Taylor RJ, Kapikian AZ. More on RotaShield and intussusception: the role of age at the time of vaccination. J Infect Dis. 2005;192:S36–43. doi: 10.1086/431512. [DOI] [PubMed] [Google Scholar]
- [6].Rothman KJ, Young-Xu Y, Arellano F. Age dependence of the relation between reassortant rotavirus vaccine (RotaShield) and intussusception. J Infect Dis. 2006;193(6):898. doi: 10.1086/500217. [DOI] [PubMed] [Google Scholar]
- [7].Dube E, Vivion M, MacDonald NE. The influence of the anti-vaccine movement on parental vaccine decisions: clinical impact and future challenges. Expert Rev Vaccines. 2015;14(1):99–117. doi: 10.1586/14760584.2015.964212. [DOI] [PubMed] [Google Scholar]
- [8].Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K, et al. Measles outbreak – California, December 2014–February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153–4. [PMC free article] [PubMed] [Google Scholar]
- [9].Salmon DA, Haber M, Gangarosa EJ, Phillips L, Smith NJ, Chen RT. Health consequences of religious and philosophical exemptions from immunization laws: individual and societal risk of measles. JAMA. 1999;282(1):47–53. doi: 10.1001/jama.282.1.47. [DOI] [PubMed] [Google Scholar]
- [10].Duclos P, Delo A, Aguado T, Bilous J, Birmingham M, Kieny MP, et al. Immunization safety priority project at the World Health Organization. Semin Pediatr Infect Dis. 2003;14(3):233–9. doi: 10.1016/s1045-1870(03)00038-4. [DOI] [PubMed] [Google Scholar]
- [11].Duclos P. A global perspective on vaccine safety. Vaccine. 2004;22(15–16):2059–63. doi: 10.1016/j.vaccine.2004.01.010. [DOI] [PubMed] [Google Scholar]
- [12].Folb PI, Bernatowska E, Chen R, Clemens J, Dodoo AN, Ellenberg SS, et al. A global perspective on vaccine safety and public health: the Global Advisory Committee on Vaccine Safety. Am J Public Health. 2004;94(11):1926–31. doi: 10.2105/ajph.94.11.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Global Advisory Committee on Vaccine Safety (GACVS) WHO secretariat. Global safety of vaccines: strengthening systems for monitoring, management and the role of GACVS. Expert Rev Vaccines. 2009;8(6):705–16. doi: 10.1586/erv.09.40. [DOI] [PubMed] [Google Scholar]
- [14].WHO [accessed 20.03.15];The Global Advisory Committee on Vaccine Safety. http://www.who.int/vaccine_safety/committee/en/
- [15].WHO IVB Report of the independent review team examining the Advisory Committees of the WHO Department of Immunization. Vaccines Biol. 2007 Jan; [Google Scholar]
- [16].WHO immunization, vaccines and biologicals [accessed 20.03.15]; http://www.who.int/immunization/documents/positionpapers/en/
- [17].Global Advisory Committee on Vaccine Safety 11–12 June 2014 Meeting Report. Wkly Epidemiol Rec. 2014;89(29):332–5. [PubMed] [Google Scholar]
- [18].Global Advisory Committee on Vaccine Safety [accessed 20.03.15]; http://www.who.int/vaccine_safety/committee/GACVS_ToRs.pdf.
- [19].Victoria JG, Wang C, Jones MS, Jaing C, McLoughlin K, Gardner S, et al. Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus. J Virol. 2010;84:6033–40. doi: 10.1128/JVI.02690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Global Advisory Committee on Vaccine Safety [accessed 20.03.15]; http://www.who.int/vaccine_safety/committee/topics/rotavirus/rotarix_statement_march_2010/en/
- [21].Strategic Advisory Group of Experts (SAGE) [accessed 20.03.15];Terms of reference. http://www.who.int/immunization/sage/Full_SAGE_TORs.pdf.
- [22].O'Brien KL, Ruff AJ, Louis MA, Desormeaux J, Joseph DJ, McBrien M, et al. Bacillus Calmette-Guérin complications in children born to HIV-1-infected women with a review of the literature. Pediatrics. 1995;95(3):414–8. [PubMed] [Google Scholar]
- [23].Waddell RD, Lishimpi K, von Reyn CF, Chintu C, Baboo KS, Kreiswirth B, et al. Bacteremia due to Mycobacterium tuberculosis or M. bovis, Bacille Calmette-Guérin (BCG) among HIV-positive children and adults in Zambia. AIDS. 2001;15(1):55–60. doi: 10.1097/00002030-200101050-00009. [DOI] [PubMed] [Google Scholar]
- [24].Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, Schaaf HS, et al. Bacille Calmette-Guérin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis. 2006;42(4):548–58. doi: 10.1086/499953. [DOI] [PubMed] [Google Scholar]
- [25].Azzopardi P, Bennett CM, Graham SM, Duke T. Bacille Calmette-Guérin vaccine-related disease in HIV-infected children: a systematic review. Int J Tuberc Lung Dis. 2009;13(11):1331–44. [PubMed] [Google Scholar]
- [26].WHO Revised BCG vaccination guidelines for infants at risk for HIV infection. Wkly Epidemiol Rec. 2007;82(21):193–6. [PubMed] [Google Scholar]
- [27].Letourneau M, Wells G, Walop W, Duclos P. Improving global monitoring of vaccine safety: a survey of national centres participating in the WHO Programme for International Drug Monitoring. Drug Saf. 2008;31(5):389–98. doi: 10.2165/00002018-200831050-00003. [DOI] [PubMed] [Google Scholar]
- [28].Letourneau M, Wells G, Walop W, Duclos P. Improving global monitoring of vaccine safety: a quantitative analysis of adverse event reports in the WHO Adverse Reactions Database. Vaccine. 2008;26(9):1185–94. doi: 10.1016/j.vaccine.2007.12.033. [DOI] [PubMed] [Google Scholar]
- [29].Mehta U, Milstien JB, Duclos P, Folb PI. Developing a national system for dealing with adverse events following immunization. Bull World Health Organ. 2000;78(2):170–7. [PMC free article] [PubMed] [Google Scholar]
- [30].Labadie J. Vaccine safety. Int J Risk Saf Med. 2011;23(2):113–5. doi: 10.3233/JRS-2011-0524. [DOI] [PubMed] [Google Scholar]
- [31].Tozzi AE, Asturias EJ, Balakrishnan MR, Halsey NA, Law B, Zuber PL. Assessment of causality of individual adverse events following immunization (AEFI): a WHO tool for global use. Vaccine. 2013;31(44):5041–6. doi: 10.1016/j.vaccine.2013.08.087. [DOI] [PubMed] [Google Scholar]
- [32].WHO Global Vaccine Safety [accessed 20.03.15]; http://www.who.int/vaccine_safety/publications/en/
- [33].WHO E-learning course on Vaccine Safety Basics [accessed 20.03.15]; http://www.who.int/vaccine_safety/initiative/tech_support/ebasic/en/
- [34].MacDonald NE, Guichard S, Amarasinghe A, Balakrishnan MR. Strengthening of causality assessment of adverse events following immunization in the WHO South East Asia and Western Pacific Regions: lessons from the 2014 SEAR Inter-country Workshop. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.01.033. http://dx.doi.org/10.1016/j.vaccine.2015.01.033. S0264-410X(15)00052-3. [DOI] [PubMed]
- [35].WHO Adverse Events Following Immunizaton Aide-memoire on Causality Assessment. http://www.who.int/vaccine_safety/initiative/investigation/New_aide_mem_causal_assmt.pdf.
- [36].Department of Immunization, Vaccines and Biologicals . Global Vaccine Safety Blueprint. World Health Organization; 2012. WHO/IVB/12.07. [Google Scholar]
- [37].World Health Assembly . Global vaccine action plan 2011–2020. WHO; 2013. ISBN 9789241504980. [Google Scholar]
- [38].World Health Organization Global Advisory Committee on Vaccine Safety World Health Organization develops guidance for vaccine safety information on the web. Euro Surveill. 2005 Jan;10(1):E050113.2. [PubMed] [Google Scholar]
- [39].Martínez-Mora M, Alvarez-Pasquín MJ, Rodríguez-Salvanés F. Vaccines and Internet: characteristics of the vaccine safety net web sites and suggested improvements. Vaccine. 2008;26(52):6950–5. doi: 10.1016/j.vaccine.2008.09.093. [DOI] [PubMed] [Google Scholar]
- [40].Ouandaogo CR, Yaméogo TM, Diomandé FV, Sawadogo C, Ouédraogo B, Ouédraogo-Traoré R, et al. Adverse events following immunization during mass vaccination campaigns at first introduction of a meningococcal A conjugate vaccine in Burkina Faso, 2010. Vaccine. 2012 May;30(Suppl. 2):B46–51. doi: 10.1016/j.vaccine.2011.12.112. [DOI] [PubMed] [Google Scholar]
- [41].Chaibou MS, Bako H, Salisou L, Yaméogo TM, Sambo M, Kim SH, et al. Monitoring adverse events following immunization with a new conjugate vaccine against group A meningococcus in Niger, September 2010. Vaccine. 2012 Jul;30(35):5229–34. doi: 10.1016/j.vaccine.2012.06.006. [DOI] [PubMed] [Google Scholar]
- [42].Duclos P, Durrheim DN, Reingold AL, Bhutta ZA, Vannice K, Rees H. Developing evidence-based immunization recommendations and GRADE. Vaccine. 2012;31(1):12–9. doi: 10.1016/j.vaccine.2012.02.041. [DOI] [PubMed] [Google Scholar]
- [43].Amarasinghe A, Black S, Bonhoeffer J, Carvalho SM, Dodoo A, Eskola J, et al. Effective vaccine safety systems in all countries: a challenge for more equitable access to immunization. Vaccine. 2013;31(Suppl. 2):B108–14. doi: 10.1016/j.vaccine.2012.10.119. [DOI] [PubMed] [Google Scholar]
- [44].Labadie J. Network for vaccines. Uppsala Reports. 2009:46, 11. [Google Scholar]
- [45].WHO GACVS meeting of 7–8 December 2011: Global network for post-marketing surveillance and AEFI monitoring. Wkly Epidemiol Rec. 2012 [Google Scholar]
- [46].Fischer KE, Rogowski WH, Leidl R, Stollenwerk B. Transparency vs. closed-door policy: do process characteristics have an impact on the outcomes of coverage decisions? A statistical analysis. Health Policy. 2013;112(3):187–96. doi: 10.1016/j.healthpol.2013.04.011. [DOI] [PubMed] [Google Scholar]
- [47].Byington CL. Vaccines: can transparency increase confidence and reduce hesitancy? Pediatrics. 2014;134(2):377–9. doi: 10.1542/peds.2014-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bujkiewicz S, Jones HE, Lai MC, Cooper NJ, Hawkins N, Squires H, et al. Development of a transparent interactive decision interrogator to facilitate the decision-making process in health care. Value Health. 2011;14(5):768–76. doi: 10.1016/j.jval.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dittmann S. Vaccine safety: risk communication – a global perspective. Vaccine. 2001;19(17–19):2446–56. doi: 10.1016/s0264-410x(00)00470-9. [DOI] [PubMed] [Google Scholar]
- [50].Wijnans L, de Bie S, Dieleman J, Bonhoeffer J, Sturkenboom M. Safety of pandemic H1N1 vaccines in children and adolescents. Vaccine. 2011;29(43):7559–71. doi: 10.1016/j.vaccine.2011.08.016. [DOI] [PubMed] [Google Scholar]
- [51].Ropero-Álvarez AM, Whittembury A, Bravo-Alcántara P, Kurtis HJ, Danovaro-Holliday MC, Velandia-González M. Events supposedly attributable to vaccination or immunization during pandemic influenza A (H1N1) vaccination campaigns in Latin America and the Caribbean. Vaccine. 2015;33(1):187–92. doi: 10.1016/j.vaccine.2014.10.070. [DOI] [PubMed] [Google Scholar]
- [52].Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–9. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- [53].Dubé E, Gagnon D, Nickels E, Jeram S, Schuster M. Mapping vaccine hesitancy – country-specific characteristics of a global phenomenon. Vaccine. 2014;32(49):6649–54. doi: 10.1016/j.vaccine.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].WHO Report of the SAGE working group on vaccine hesitancy. 01.10.14. http://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf. [Google Scholar]
- [55].Klein N. Vaccine safety in special populations. Hum Vaccines. 2011;7(2):269–71. doi: 10.4161/hv.7.2.13860. [DOI] [PubMed] [Google Scholar]
- [56].Agence de Médecine Préventive [accessed 15.09.15]; http://www.nitag-resource.org/
- [57].WHO [accessed 15.09.15];Global vaccine safety initiative technical supports and training. http://www.who.int/vaccine_safety/initiative/tech_support/en/