Abstract
A novel phenyl alkene (1) was isolated from a mixture of three Florida sponges, Smenospongia aurea, Smenospongia cerebriformis, and Verongula rigida. Unlike terpenoids or amino acid derivatives, which are commonly known classes of secondary metabolites from these genera, the chemical structure of 1 showed an unprecedented linear phenyl alkene skeleton. Through comprehensive analyses of NMR and MS data, the gross structure of 1 was determined to be (E)-10-benzyl-5,7-dimethylundeca-1,5,10-trien-4-ol. The absolute configuration at C-4 was established as R by a modified Mosher’s method. Based on the relative configuration between C-4 and C-7, the absolute configuration at C-7 was assigned as S. Compound 1 showed in vitro cytotoxic activity against HL-60 human leukemia cancer cells with an IC50 value of 8.1 µM. Molecular docking study suggests that the structure of compound 1 matches the pharmacophore of eribulin required to display cytotoxic activity through the inhibition of microtubule activity.
Keywords: Phenyl alkene; (4R,7S,E)-10-Benzyl-5,7-dimethylundeca-; 1,5,10-trien-4-ol; Cytotoxic activity
Marine sponges are regarded as a rich source of secondary metabolites with chemically diverse structures and potential biological benefits.1 While investigation of secondary metabolites from marine sponges Smenospongia (order Dictyoceratida, family Thorectidae) and Verongula (order Verongida, family Aplysinidae) has received great attention, only a few chemical groups that belong to alkaloids and terpenoids have been isolated from these marine invertebrates.2,3 Of these, sesquiterpene quinones and hydroquinones are the best known classes of secondary metabolites, accounting for more than 170 compounds published from these sponges.4 Our previous study on the isolation of antidepressant compounds from three Florida sponges, Verongula rigida (Esper, 1794), Smenospongia aurea (Hyatt, 1875), and Smenospongia cerebriformis (Duchassaing and Michelotti, 1864),3c showed that the same brominated alkaloids, as well as sesquiterpene quinones and hydroquinones, were found in both V. rigida and S. aurea despite their apparent taxonomic differences. We thus proposed that similar metabolites found in these distinct species of two different genera provide evidence for a microbial origin of the metabolites.3c Due to the similar metabolite profile of these three sponges (as shown by our previous study and LC–MS analysis), we have decided to combine these in order to be able to isolate and characterize compounds present even in minute quantities and to provide larger amounts of brominated metabolites for further animal testing. Comparing to individual isolation of secondary metabolites from each species, we could effectively obtain substantial amounts of brominated indole alkaloids and sesquiterpene quinones from the combined extracts of the three sponges. Recently, our interest in the same sponge materials has focused on the fact that the mixed extracts displayed significant cytotoxicity against HL-60 human leukemia cancer cells. In the course of our continuing research on the isolation of cytotoxic metabolites from the extracts, we found that trace amounts of a novel cytotoxic compound containing an unprecedentedly linear phenyl alkene skeleton existed in each sponge based on LC–MS analyses (Fig. S15). Up to now, to the best of our knowledge, no phenyl alkene type molecule has been identified in these genera. Herein, we describe the isolation and structure elucidation of the new phenyl alkene, and evaluation of cytotoxic activities of the isolate against HL-60 human leukemia cancer cells and MCF-7 human breast cancer cells.
Three sponges5 were obtained during our large-scale collection in October 2008 and mixed together prior to extraction due to a similar profile of secondary metabolites, especially halogenated indole alkaloids with interesting antidepressant activity in behavioral in vivo tests, revealed in our earlier studies.3c The dried ethanol extract (3.6 kg) was subjected to silica gel vacuum liquid chromatography (VLC) and eluted with a stepwise gradient of hexane/acetone/methanol/water to give 13 fractions (Fr. 1–13). Fr. 10 (39.3 g) was further divided into 9 fractions (Fr. 10-1 to 10-9) using silica gel VLC with a gradient order of the same four solvents. Fr. 10-6 (2.7 g) was applied to C18 MPLC (15.5 × 4 cm) with an isocratic solvent system of methanol/water (85:15) to yield 7 subfractions (Fr. 10-6-1 to 10-6-7). Compound 1 (1.9 mg) was obtained from Fr. 10-6-1 (95.1 mg) over C18 HPLC (250 × 21.2 mm, 10 µm) eluted with isocratic methanol/water (78:22). Repeated purification was carried out on Fr. 10-6-2 (457.8 mg) by C18 HPLC (250 × 20.0 mm, 5 µm) with isocratic methanol/water (78:22) to yield 1 (4.4 mg; total yield 6.3 mg, 0.000175% dry weight).
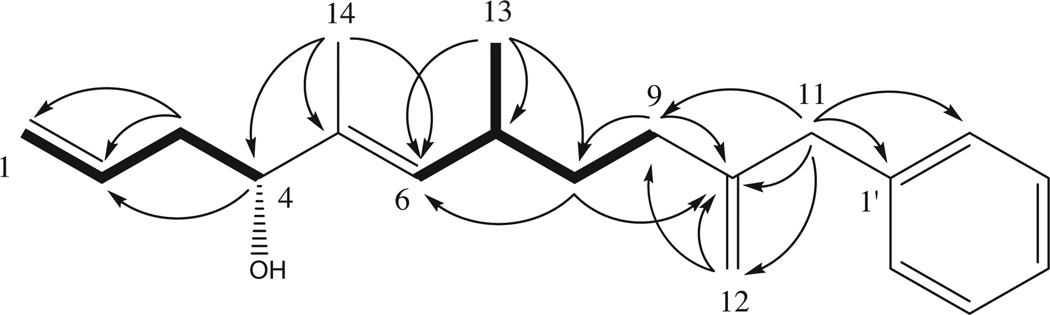
Compound 16 was isolated as colorless oil. The HRFABMS showed a molecular ion peak at m/z 285.2215 in positive mode with the molecular formula C20H29O [M+H]+ (calcd 285.2218) with seven degrees of unsaturation. The 1H and 13C NMR data in CDCl3 indicated that 1 has a mono-substituted benzene moiety [δH 7.27 (2H, dd, J = 7.5, 7.5 Hz), 7.20 (1H, dd, J = 7.5, 7.5 Hz), 7.13 (2H, d, J = 7.5 Hz) and δC 138.39 (C-1′), 129.09 (C-2′), 128.60 (C-3′), 126.68 (C-4′)] and six olefinic protons and carbons of three double bonds [δH 5.79 (2H, s), 5.72 (1H, m), 5.12 (1H, d, J = 9.6 Hz), 5.08 (1H, br d, J = 17.4 Hz), 5.04 (1H, d, J = 10.2 Hz) and δC 142.46 (C-10), 135.86 (C-5), 135.00 (C-2), 132.78 (C-6), 117.67 (C-1), 113.96 (C-12)], which accounted for four and three degrees of unsaturation, respectively (Table 1). An oxygenated methine [δH 3.99 (1H, t, J = 6.6 Hz) and δC 76.84 (C-4)], a benzylic methylene [δH 3.33 (2H, s) and δC 41.33 (C-11)], an allylic methylene [δH 2.05 (2H, t, J = 8.4 Hz) and δC 28.36 (C-9)], an olefinic methyl [δH 1.57 (3H, d, J = 1.2 Hz) and δC 11.87 (C-14)], and a secondary methyl [δH 0.92 (3H, d, J = 6.6 Hz) and δC 21.02 (C-13)] were distinctively observed in the 1H and 13C NMR spectra as well (Table 1). As shown in Figure 1, the detailed analysis of the 1H–1H COSY, HSQC, HMBC, NOESY, ROESY, and HRFABMS data suggested that the structure of 1 should be (E)-10-benzyl-5,7-dimethylundeca-1,5,10-trien-4-ol. The HMBC correlations from the benzylic protons at δH 3.33 (H2-11) to two olefinic carbons at δC 142.46 (C-10) and 113.96 (C-12), as well as the allylic methylene carbon at δC 28.36 (C-9), indicated the (2-methylenealkyl)-benzene framework (Fig. 1). The consecutive analyses of the 1H–1H COSY correlations from the allylic protons at δH 2.05 (H-9) to the olefinic proton at δH 5.12 (H-6) and the secondary methyl protons at δH 0.92 (H-13) showed that an isopentane unit comprised the alkyl chain, in agreement with the analysis of HMBC data where the cross-peaks from H-13 to the olefinic carbon at δC 132.78 (C-6), a methylene carbon at δC 34.76 (C-8), and a methine carbon at δC 32.31 (C-7) were detected (Fig. 1). The next partial structure was deduced by the HMBC correlations from the olefinic methyl protons at δH 1.57 (H-14) to the olefinic carbons at δC 135.86 (C-5) and 132.78 (C-6), and oxygenated methine carbon at δC 76.84 (C-4), presenting a connection to the isopentane group at C-6 (Fig. 1). The analyses of successive 1H–1H COSY correlations from the oxygenated methine proton at δH 3.99 (H-4) to the olefinic protons at δH 5.08 and 5.04 (H2-1) established the last partial structure, which turned out to be a secondary alcohol, joining the existing part of the structure at C-4. Corresponding analysis of the HMBC data verified the arrangement in which the cross-peaks from the H-4 to the olefinic carbon at δC 135.00 (C-2), as well as from methylene protons at δH 2.26 (H-3) to the olefinic carbons at δC 117.67 (C-1) and C-2 were observed (Fig. 1). The E-geometry of the C-5/C-6 olefin was supported by 13C NMR chemical shift of C-14 at δC 11.877 (vs δC 20.5 for Z-geometry)7 and confirmed by the NOE correlation between H-7 and H-14 (and not between H-6 and H-14 which would be seen in the case of Z-geometry).
Table 1.
NMR spectroscopic data of compound 1 in CDCl3
| Position | δC, mult.a |
δH, mult. (J in Hz)b | COSY | HMBC | NOE |
|---|---|---|---|---|---|
| 1 | 117.7, CH2 |
5.04, d (10.2); 5.08, br d (17.4) |
H-2 | ||
| 2 | 135.0, CH |
5.72, m | H-1, 3 | ||
| 3 | 40.1, CH2 |
2.26, m | H-2, 4 | C-1, 2 | H- 13 |
| 4 | 76.8, CH |
3.99, t (6.6) | H-3 | C-2 | H-6 |
| 5 | 135.9, C | ||||
| 6 | 132.8, CH |
5.12, d (9.6) | H-7 | H-4 | |
| 7 | 32.3, CH |
2.31, m | H-6, 8, 13 |
H- 14 |
|
| 8 | 34.8, CH2 |
1.24, m; 1.39, m | H-7, 9 | C-6, 10 | |
| 9 | 28.4, CH2 |
2.05, t (8.4) | H-8 | C-8, 10 | |
| 10 | 142.5, C | ||||
| 11 | 41.3, CH2 |
3.33, s | C-9, 10, 12, 1′, 2′ |
||
| 12 | 114.0, CH2 |
5.79, s | C-9, 10 | ||
| 13 | 21.0, CH3 |
0.92, d (6.6) | H-7 | C-6, 7, 8 | H-3 |
| 14 | 11.9, CH3 |
1.57, d (1.2) | C-4, 5, 6 | H-7 | |
| 1’ | 138.4, C | ||||
| 2’ | 129.1, 2CH |
7.13, d (7.5) | C-11 | ||
| 3’ | 128.6, 2CH |
7.27, dd (7.5, 7.5) | |||
| 4’ | 126.7, CH |
7.20, dd (7.5, 7.5 |
Assignment based on HSQC and HMBC NMR data (150 MHz).
Assignment based on COSY and HMBC NMR data (600 MHz).
Figure 1.
1H–1H COSY ( ) and key HMBC (→) correlations of compound 1.
) and key HMBC (→) correlations of compound 1.
The relative configurations of the two stereogenic centers at C-4 and C-7 were initially proposed by comparing 1H NMR data with synthetic diastereomers.8 In common partial structures from C-3 to C-8 including C-13 and C-14, the most distinguishable feature of 1H NMR data between the two diastereomers is a coupling constant (J) of H-4. While H-4 has two J values as 3.5 and 8.9 Hz which appeared to be a doublet of doublets in a syn conformation, J is 6.0 Hz as a triplet in an anti conformation which corresponds to compound 1 (Fig. S1).8 Also, every possible rotamer was carefully considered by virtue of the 3JH,H value, as well as the 1D and 2D NOE correlations (Fig. 2). The large 3J(H-6, H-7) value (9.6 Hz) showed the anti orientation of H-6/H-7,9 which was in agreement with the rotamers A-1 and B-1, in addition to the NOE correlations of H-7/H-14 and H-4/H-6. However, cross-peaks between H-3 and H-13 in the NOESY and ROESY data (Fig. 2, Figs. S6 and S7), which were consistent with the 1D NOE analysis (Fig. S8), demonstrated rotamer A-1 in which the relative configurations of C-4 and C-7 were R* and S*, respectively.
Figure 2.
Newman projections of all possible rotamers for C-4–C-7 are shown. Predicted coupling constant values are labeled below the projections, and corresponding values with the observation are box highlighted. The NOE correlations are depicted as double-headed arrows (↔).
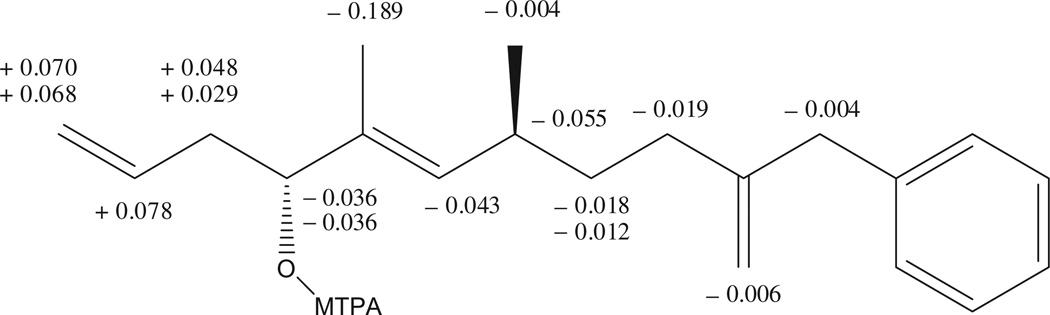
The absolute configuration of 1 was assigned by high-field FT NMR application of Mosher’s method.10 The R-(−) and S-(+)-α-methoxy-α-(trifluoromethyl)phenyl acetyl chloride (5 µL) were added to two portions (each 0.8 mg) of 1 in dry pyridine (200 µL) under N2 gas stream and respectively afforded the (S)- and (R)-α-methoxy-α-(trifluoromethyl)phenyl acetic acid (MTPA) esters at room temperature after 48 h. The 1H NMR chemical shifts were assigned by the analysis of the 1H–1H COSY NMR data for each MTPA ester (Figs. S11 and S12).11,12 The calculated ΔδS−R values were positive for the C-1–C-3 segment but negative for the C-4–C-14 segment (Fig. 3), which implied the absolute configuration of C-4 was R. Considering the relative configuration of C-4 and C-7, the absolute configuration of C-7 was assigned to be S. Consequently, the chemical structure of compound 1 was elucidated as (4R, 7S, E)-10-benzyl-5,7-dimethylundeca-1,5,10-trien-4-ol.
Figure 3.
ΔδS−R values in ppm for S- and R-MTPA esters of compound 1 in CDCl3
Due to limited quantities, compound 1 was only evaluated for cytotoxic activity against the cancer cell lines HL-60 and MCF-7 in the MTT (3-(4,5-dimethylythiazol-2-yl)-2,5-diphenyl-2H-tetrazolium hydrobromide) assay. Compound 1 was cytotoxic toward HL-60 cancer cells with an IC50 value of 8.1 µM. No significant cytotoxicity was observed against MCF-7 cancer cells.
Eribulin is a tubulin-inhibiting chemotherapeutic drug effective on multiple cancer cell types including leukemia, breast, and colon cancers.13 Eribulin is a structurally simplified synthetic analogue of halichondrin B, which was initially isolated from the marine sponge Halichondria okadai14 and has also been isolated from three other sponge species.15 To test whether compound 1 shares the cytotoxic pharmacophore of eribulin, conformational searches and consecutive flexible alignment analyses were conducted employing the MacroModel and Phase programs (Schrödinger LLC)(Tables Figs. S1, S2 and S16).16 The conformers of compound 1 which demonstrated partial structural similarity with eribulin were further docked to the β-tubulin protein following the previously reported approach to eribulin docking.17 Compound 1 and eribulin exhibited a stereoelectronic fit to the glycine-rich helix H4 based on the observed hydrophobic interactions with Gly144 and Gly148 (Fig. 4 and Ref.17a). According to the proposed docking pose, stabilizing factors for eribulin such as the hydrophobic side chains of Val182 and Leu189 in helix H5 and Leu70 from T2-loop were detected in the docking pose of compound 1 as well (Fig. 4 and Ref.17a). This suggests that the two ligands pack into a similar binding pocket generated by the hydrophobic residues of helixes H4 and H5, and T2-loop (Fig. 4 and Ref.17a). In addition to these lipophilic interactions, a favorable hydrogen bonding interaction of Asn186 to the methoxy group of eribulin and to the hydroxy group of compound 1 further stabilized the docking poses (Fig. 4, bottom). Even though eribulin bears more oxygen atoms than its counterpart, only one oxygen atom of each ligand participates in hydrogen bonding in the proposed docking poses. Based on the similar interactions of the two ligands with the above-mentioned residues of the β-tubulin protein, compound 1 seems to match the pharmacophore of eribulin required to exert cytotoxic activity through inhibition of microtubule activity.
Figure 4.
Overlaid image of compound 1 and eribulin docked to the β-tubulin protein (top) and ligand interaction diagrams (bottom). Colors stand for different types of interactions: gray (glycine interactions), green (hydrophobic interactions), cyan (polar interactions), purple (positively-charged interactions), and pink dots (hydrogen-bonding interaction with a side chain of a residue).
The synthetic approach will be undertaken to explore the anticancer potential and mechanism of action for the compound.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-C1ABA001-2010-0020484). This investigation was conducted in a facility constructed with support from research facilities improvement program C06 RR-14503-01 from the NIH National Center for Research Resources. The technical supports provided by Schrödinger are greatly appreciated.
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet.2013.05.032.
References and notes
- 1.(a) Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Nat. Prod. Rep. 2011;28:196–268. doi: 10.1039/c005001f. [DOI] [PubMed] [Google Scholar]; (b) Faulkner DJ. Nat. Prod. Rep. 2002;19:1–48. doi: 10.1039/b009029h. and earlier reports in the series. [DOI] [PubMed] [Google Scholar]
- 2.(a) Djura P, Stierle DB, Sullivan B, Faulkner DJ, Arnold E, Clardy J. J. Org. Chem. 1980;45:1435–1441. [Google Scholar]; (b) Hu J-F, Schetz JA, Kelly M, Peng J-N, Ang KKH, Flotow H, Leong CY, Ng SB, Buss AD, Wilkins SP, Hamann MT. J. Nat. Prod. 2002;65:476–480. doi: 10.1021/np010471e. [DOI] [PubMed] [Google Scholar]; (c) Kondracki M-L, Guyot M. Tetrahedron. 1989;45:1995–2004. [Google Scholar]; (d) Venkateswarlu Y, Faulkner DJ, Steiner JLR, Corcoran E, Clardy J. J. Org. Chem. 1991;56:6271–6274. [Google Scholar]; (e) Bourguet-Kondracki M-L, Guyot M. Tetrahedron Lett. 1999;40:3149–3150. [Google Scholar]; (f) Bourguet-Kondracki M-L, Martin M-T, Guyot M. Tetrahedron Lett. 1992;33:8079–8080. [Google Scholar]; Song J, Jeong W, Wang N, Lee H-S, Sim CJ, Oh K-B, Shin J. J. Nat. Prod. 2008;71:1866–1871. doi: 10.1021/np8003694. [DOI] [PubMed] [Google Scholar]; (h) Segraves NL, Crews P. J. Nat. Prod. 2005;68:1484–1488. doi: 10.1021/np0501334. [DOI] [PubMed] [Google Scholar]; Rho J-R, Lee H-S, Shin H-J, Ahn J-W, Kim J-Y, Sim CJ, Shin J. J. Nat. Prod. 2004;67:1748–1751. doi: 10.1021/np040103l. [DOI] [PubMed] [Google Scholar]
- 3.(a) Ciminiello P, Dell’Aversano C, Fattorusso E, Magno S, Pansini M. J. Nat. Prod. 2000;63:263–266. doi: 10.1021/np990343e. [DOI] [PubMed] [Google Scholar]; (b) Mierzwa R, King A, Conover MA, Tozzi S, Puar MS, Patel M, Coval SJ, Pomponi SA. J. Nat. Prod. 1994;57:175–177. doi: 10.1021/np50103a029. [DOI] [PubMed] [Google Scholar]; (c) Kochanowska AJ, Rao KV, Childress S, El-Alfy A, Matsumoto RR, Kelly M, Stewart GS, Sufka KJ, Hamann MT. J. Nat. Prod. 2008;71:186–189. doi: 10.1021/np070371u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcos IS, Conde A, Moro RF, Basabe P, Diez D, Urones JG. Mini Rev. Org. Chem. 2010;7:230–254. [Google Scholar]
- 5.Sponge material: S. aurea, S. cerebriformis, and V. rigida were collected from the Key Largo, FL in October 2008. The sponges were collected from a shallow coral reef habitat between 3 and 24 m depth.
- 6.Compound 1: colorless oil; (c 0.1, MeOH); UV (MeOH) λmax 205 nm; 1H NMR (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) see Table 1; HRFABMS m/z 285.2215 [M+H]+ (calcd for C20H29O: 285.2218).
- 7.Oh D-C, Gontang EA, Kauffman CA, Jensen PR, Fenical W. J. Nat. Prod. 2008;71:570–575. doi: 10.1021/np0705155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teruya T, Sasaki H, Kitamura K, Nakayama T, Suenaga K. Org. Lett. 2009;11:2421–2424. doi: 10.1021/ol900579k. [DOI] [PubMed] [Google Scholar]
- 9.Matsumori N, Kaneno D, Murata M, Nakamura H, Tachibana K. J. Org. Chem. 1999;64:866–876. doi: 10.1021/jo981810k. [DOI] [PubMed] [Google Scholar]
- 10.Ohtani I, Kusumi T, Kashman Y, Kakisawa H. J. Am. Chem. Soc. 1991;113:4092–4096. [Google Scholar]
- 11.S-MTPA ester of 1: 1H NMR (CDCl3, 500 MHz) δ 5.79 (2H, s), 5.63 (1H, m), 5.39 (1H, dd, J = 7.5, 6.0 Hz), 5.25 (1H, br d), 5.07 (1H, br d, J = 17.0 Hz), 5.01 (1H, br d, J = 10.5 Hz), 3.32 (2H, s), 2.47 (1H, m), 2.32 (1H, m), 2.25 (1H, m), 2.01 (2H, m), 1.373 (3H, d, J = 1.5 Hz), 1.372 (1H, m), 1.22 (1H, m), 0.91 (3H, d, J = 6.5 Hz) (Fig. S9).
- 12.R-MTPA ester of 1: 1H NMR (CDCl3, 500 MHz) δ 5.80 (2H, s), 5.55 (1H, m), 5.43 (1H, dd, J = 7.5, 6.5 Hz), 5.30 (1H, br d), 5.00 (1H, br d, J = 17.0 Hz), 4.94 (1H, br d, J = 10.5 Hz), 3.32 (2H, s), 2.42 (1H, m), 2.30 (1H, m), 2.29 (1H, m), 2.03 (2H, m), 1.56 (3H, d, J = 1.5 Hz), 1.39 (1H, m), 1.23 (1H, m), 0.92 (3H, d, J = 6.5 Hz) (Fig. S10).
- 13.(a) Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, Welsh S, Zheng W, Seletsky BM, Palme MH, Habgood GJ, Singer LA, DiPietro LV, Wang Y, Chen JJ, Quincy DA, Davis A, Yoshimatsu K, Kishi Y, Yu MJ, Littlefield BA. Cancer Res. 2001;61:1013–1021. [PubMed] [Google Scholar]; (b) Dabydeen DA, Burnett JC, Bai R, Verdier-Pinard P, Hickford SJH, Pettit GR, Blunt JW, Munro MHG, Gussio R, Hamel E. Mol. Pharmacol. 2006;70:1866–1875. doi: 10.1124/mol.106.026641. [DOI] [PubMed] [Google Scholar]
- 14.Uemura D, Takahashi K, Yamamoto T, Katayama C, Tanaka J, Okumura Y, Hirata Y. J. Am. Chem. Soc. 1985;107:4796. [Google Scholar]
- 15.(a) Pettit GR, Herald CL, Boyd MR, Leet JE, Dufresne C, Doubek DL, Schmidt JM, Cerny RL, Hooper JNA, Rutzler KC. J. Med. Chem. 1991;34:3339. doi: 10.1021/jm00115a027. [DOI] [PubMed] [Google Scholar]; Pettit GR, Tan R, Gao F, Williams MD, Doubek DL, Boyd MR, Schmidt JM, Chapuis J-C, Hamel E, Bai R, Hooper JNA, Tackett LP. J. Org. Chem. 1993;58:2538. [Google Scholar]; (c) Gravalos DG, Lake RJ, Blunt JW, Munro MHG, Litaudon MSP. EP 0572109 A1. European Patent Appl. 1993 Dec.
- 16.All conformational searches were conducted using the macromodel program included in the Schrödinger software package (Schrödinger LLC). The searches were executed in water using the OPLS2005 force field, a 10 kcal/mol upper energy limit, and 0.001 convergence threshold. Alignment of eribulin with the generated conformers of compound 1 was conducted using the Flexible Alignment algorithm of the Phase program, Schrödinger LLC, but restricting the two aligned structures to be strictly rigid.
- 17. Bai R, Nguyen TL, Burnett JC, Atasoylu O, Munro MHG, Pettit GR, Smith AB, Gussio R, Hamel E. J. Chem. Inf. Model. 2011;51:1393–1404. doi: 10.1021/ci200077t. (b) The conformers having shape similarity scores >0.3 were chosen for a docking study. 1JFF (http://www.pdb.org) was prepared and minimized using the protein preparation wizard (Schrödinger LLC). Two loops (residues 94–119 and 399–414) were removed to accommodate the binding of eribulin and the apo-protein structure was subjected to a grid generation process using the glide program (Schrödinger LLC). The docking site was the centroid of the three active residues reported previously (Ref.17a) (Trp103, Leu189 and Tyr408). Given that Trp103 and Tyr408 are located in the removed loops, before their removal the xyz-coordinates of the active residues were selected and used to fashion a grid for further Glide docking experiments. The conformations of the ligands were retained during all docking procedures.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.