ANTIBODIES AND BISPECIFIC ANTIBODIES
Antibodies are widely used in biochemistry, molecular biology, and medical research, and one of their innovative uses has been as therapeutic agents for the treatment of a variety of diseases, including cancer. At least 45 antibody-based products are currently marketed for therapy or imaging in the United States and Europe with approximately 63 billion US dollars in total worldwide sales in 2013.1 Important advances have improved the engineering, safety, and efficacy of the first generation of therapeutic antibodies. These developments, along with a greater understanding of the immunomodulatory properties of antibodies, have paved the way for the next generation of novel and improved antibody-based therapeutics, such as bispecific antibodies (BsAbs).2-4
BsAbs combine the specificity of two antibodies so that they can simultaneously bind to different antigens.5 Most BsAbs involve specificities that bind to an antigen on a cancer cell and to T-cell surface glycoprotein CD3 ε-chain (CD3) on T cells. As with many of the strategies that are based the on use of monoclonal antibodies, BsAbs have benefited from a steady improvement in technology and lessons learned from earlier clinical and preclinical studies.6 The engineering of monospecific antibodies to be bispecific opens up a variety of potential therapeutic applications as evidenced by the more than 30 BsAbs currently in clinical development.7
BISPECIFIC T-CELL ENGAGER
A bispecific T-cell engager (BiTE) is a unique BsAb that has two linked, single-chain variable fragments constructed to be flexible and have a 1 + 1 antigen-binding valency.8 BiTEs are a class of bispecific monoclonal antibodies currently under investigation as anticancer therapeutics. They bind CD3 on T cells and an antigen on tumor cells to activate T cells to kill tumor cells. BiTEs direct a host’s immune system, more specifically the T cells’ cytotoxic activity, against cancer cells. The BiTE blinatumomab specifically targets CD19 on B cells, which is expressed throughout most of B-cell development and in corresponding B-cell malignancies. However, CD19 is not expressed on plasma cells or plasma cell neoplasias. Blinatumomab is used as a second-line treatment of Philadelphia chromosome–negative relapsed or refractory acute lymphoblastic leukemia and was approved by the US Food and Drug Administration in December 2014.
HOW DO BiTEs WORK?
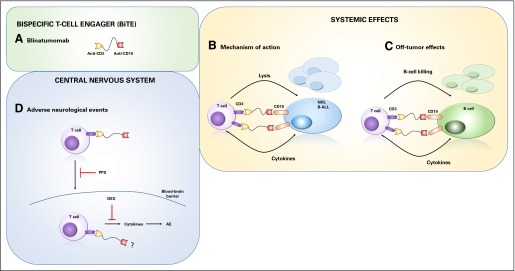
BiTEs are small, flexible molecules that bring together T cells and tumor cells (Fig 1).9 They only trigger T-cell cytotoxicity and cytokine production when both binding sites are occupied.10 BiTEs activate T cells without the apparent need for costimulation, and data suggest that BiTEs preferentially activate memory T cells.11-13 Because of their small size, they are rapidly cleared through the kidneys, so continuous dosing may be needed.14-16 However, their small size may also allow more rapid tumor and tissue penetration. BiTEs are unique in that they lack an Fc-binding portion, so they do not activate Fc-bearing immune cells such as macrophages, neutrophils, or natural killer (NK) cells. Other bispecific formats may trigger NK cell cytotoxicity of tumor cells through binding to CD16a (FcRγIIIa) on NK cells instead of binding to T cells through CD3, and these are referred to as BiKEs (bispecific killer engagers).17-19
Fig 1.
Activity of bispecific T-cell engager (BiTE) blinatumomab. (A) Blinatumomab consists of two single-chain variable fragments where one binds to CD3 and the other binds to CD19, with a flexible linker between them. This BiTE protein can connect a T cell and a CD19+ tumor cell (B) or a CD19+ B cell (C) by simultaneously binding CD3 and CD19. When both single-chain variable fragments bind to their target antigens, T-cell activation is triggered, which leads to the release of cytotoxic granules, cytokines (eg, interferon gamma, tumor necrosis factor α, interleukin-2), and T-cell proliferation. Lysis of the tumor cell or B cell involves membrane perforation followed by programmed cell death induced by granzymes. BiTEs trigger serial killing by activated T cells. (D) Approaches to limit adverse neurologic events caused by blinatumomab treatment include the blocking of activated T-cell migration into the CNS through pentosan polysulfate (PPS) treatment or the reduction of cytokine activity through the use of corticosteroids, such as dexamethasone (DEX). What cells blinatumomab may recognize in the CNS (eg, tumor cells) is unknown, but the adverse effects resolve when treatment is discontinued. AE, adverse event; B-ALL, B-cell acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma.
CLINICAL FINDINGS
The BiTE blinatumomab has demonstrated clinical responses at very low doses in patients with non-Hodgkin lymphoma. Because of the small protein size and rapid clearance, a continuous infusion can be used effectively, and a maximum tolerated dose of 60 μg/m2/day with an overall response rate of 69% across non-Hodgkin lymphoma subtypes has been achieved with a median response duration of 404 days.16 In contrast, intact antibodies, such as rituximab (anti-CD20), are given at doses of 375 mg/m2/day. Thus, the BiTE format allows for efficacy against tumors at very low doses.
In many patients treated with blinatumomab, mild inflammatory symptoms related to T-cell activation at initiation of therapy develops, whereas in some patients, cytokine release syndrome, a severe condition characterized by flu-like symptoms, develops.20,21 Although symptom severity varies, grade 3 or higher cytokine release syndrome has been observed in a small percentage of adult patients treated with blinatumomab.21 Release of inflammatory cytokines, such as interleukin-2 (IL-2), IL-6, IL-10, interferon gamma, and tumor necrosis factor α, has been demonstrated in both adult and pediatric patients.14,20 In the current study, the authors noticed transient proinflammatory cytokine elevations in the serum during the first 48 hours of treatment.22 Patients receiving higher doses were more likely to experience dose-limiting toxicities, primarily neurologic events.
Frequent adverse events observed include lymphopenia, pyrexia, and increased C-reactive protein concentrations, which are consistent with the mode of action of a T-cell–activating therapy that also depletes the CD19+ B cells. Another common adverse event is neurologic findings, which are believed to be due to increased cytokine release of activated T cells within the CNS, even though tumor cells may not be present in the CNS. In the study by Goebeler et al,16 corticosteroids and pentosan polysulfate were tested as methods to limit T-cell cytokine activity or trafficking of T cells into the CNS, respectively. All adverse events detected were associated with blinatumomab treatment, were time and dose dependent, and generally resolved when treatment was discontinued. Stepwise dosing of BiTEs and the addition of molecules to limit T-cell cytokine production resulted in an improved ability for patients to complete full BiTE treatment.
FUTURE DIRECTIONS
Many different BsAbs are in development. Their ability to target and activate specific immune effector cells and responses against tumors holds great promise as cancer therapy. Blinatumomab represents the first-in-class BiTE antibody in clinical use and provides a novel therapeutic option for patients with relapsed/refractory B-cell acute lymphoblastic leukemia.22-26 Protein engineering and design will be used to create molecules with powerful efficacy, and we will need to learn how to deliver doses and manage adverse events to use them effectively.
Supplementary Material
Acknowledgment
Supported in part by National Institutes of Health grant CA164178 and funds from the Center for Synthetic Immunity.
Footnotes
See accompanying article on page 1104
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Bispecific T-Cell Engagers (BiTEs) as Treatment of B-Cell Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Nicole C. Smits
No relationship to disclose
Charles L. Sentman
Stock or Other Ownership: Celyad
Honoraria: Biogen
Consulting or Advisory Role: Celdara Medical, Celdara Medical (I)
Research Funding: Celdara Medical, Celdara Medical (I, Inst)
Patents, Royalties, Other Intellectual Property: Patents on bispecific molecules and chimeric antigen receptors, patents on bispecific molecules and chimeric antigen receptors (Inst)
Travel, Accommodations, Expenses: Celdara Medical, Celyad, Biogen
REFERENCES
- 1.Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7:9–14. doi: 10.4161/19420862.2015.989042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 3.Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4:182–197. doi: 10.4161/mabs.4.2.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne H, Conroy PJ, Whisstock JC, et al. A tale of two specificities: Bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol. 2013;31:621–632. doi: 10.1016/j.tibtech.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nisonoff A, Rivers MM. Recombination of a mixture of univalent antibody fragments of different specificity. Arch Biochem Biophys. 1961;93:460–462. doi: 10.1016/0003-9861(61)90296-x. [DOI] [PubMed] [Google Scholar]
- 6.Segal DM, Weiner GJ, Weiner LM. Bispecific antibodies in cancer therapy. Curr Opin Immunol. 1999;11:558–562. doi: 10.1016/s0952-7915(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 7.Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Mack M, Riethmüller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci U S A. 1995;92:7021–7025. doi: 10.1073/pnas.92.15.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93:290–296. doi: 10.1038/icb.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brischwein K, Parr L, Pflanz S, et al. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother. 2007;30:798–807. doi: 10.1097/CJI.0b013e318156750c. [DOI] [PubMed] [Google Scholar]
- 11.Brischwein K, Schlereth B, Guller B, et al. MT110: A novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Lutterbuese R, Raum T, Kischel R, et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc Natl Acad Sci U S A. 2010;107:12605–12610. doi: 10.1073/pnas.1000976107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torisu-Itakura H, Schoellhammer HF, Sim MS, et al. Redirected lysis of human melanoma cells by a MCSP/CD3-bispecific BiTE antibody that engages patient-derived T cells. J Immunother. 2011;34:597–605. doi: 10.1097/CJI.0b013e3182307fd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–6233. doi: 10.1182/blood-2012-01-400515. [DOI] [PubMed] [Google Scholar]
- 15.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 16.Goebeler ME, Knop S, Viardot A, et al. Bispecific T-cell engager (1 BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: Final results from a phase 1 study. J Clin Oncol. 34:1104–1111. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 17.Gleason MK, Verneris MR, Todhunter DA, et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11:2674–2684. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiernik A, Foley B, Zhang B, et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res. 2013;19:3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothe A, Sasse S, Topp MS, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:4024–4031. doi: 10.1182/blood-2014-12-614636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litzow MR. Monoclonal antibody-based therapies in the treatment of acute lymphoblastic leukemia. Am Soc Clin Oncol Educ Book. 2013:294–299. doi: 10.14694/EdBook_AM.2013.33.294. [DOI] [PubMed] [Google Scholar]
- 22.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 23.Buie LW, Pecoraro JJ, Horvat TZ, et al. Blinatumomab: A first-in-class bispecific T-cell engager for precursor B-cell acute lymphoblastic leukemia. Ann Pharmacother. 2015;49:1057–1067. doi: 10.1177/1060028015588555. [DOI] [PubMed] [Google Scholar]
- 24.Traynor K. Blinatumomab approved for rare leukemia. Am J Health Syst Pharm. 2015;72:90. doi: 10.2146/news150005. [DOI] [PubMed] [Google Scholar]
- 25.Zugmaier G, Topp MS, Alekar S, et al. Long-term follow-up of serum immunoglobulin levels in blinatumomab-treated patients with minimal residual disease-positive B-precursor acute lymphoblastic leukemia. Blood Cancer J. 2014;4:244. doi: 10.1038/bcj.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong R, Pepper C, Brennan P, et al. Blinatumomab induces autologous T-cell killing of chronic lymphocytic leukemia cells. Haematologica. 2013;98:1930–1938. doi: 10.3324/haematol.2012.082248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.