Abstract
Background & Aims
Portal vein embolization (PVE) is a standard technique for patients not amenable to liver resection due to small future liver remnant ratio (FLR). Radiation lobectomy (RL) with 90Y-loaded microspheres (Y90) is hypothesized to induce comparable volumetric changes in liver lobes, while potentially controlling the liver tumor and limiting tumor progression in the untreated lobe. We aimed at testing this concept by performing a comprehensive time-dependent analysis of liver volumes following radioembolization.
Methods
83 patients with right unilobar disease with hepatocellular carcinoma (HCC; N = 67), cholangiocarcinoma (CC; N = 8) or colorectal cancer (CRC; N = 8) were treated by Y90 RL. The total liver volume, lobar (parenchymal) and tumor volumes, FLR and percentage of FLR hypertrophy from baseline (%FLR hypertrophy) were assessed on pre- and post-Y90 CT/MRI scans in a dynamic fashion.
Results
Right lobe atrophy (p = 0.003), left lobe hypertrophy (p <0.001), and FLR hypertrophy (p <0.001) were observed 1 month after Y90 and this was consistent at all follow-up time points. Median %FLR hypertrophy reached 45% (5–186) after 9 months (p <0.001). The median maximal %FLR hypertrophy was 26% (−14→86). Portal vein thrombosis was correlated to %FLR hypertrophy (p = 0.02). Median Child-Pugh score worsening (6→7) was seen at 1 to 3 months (p = 0.03) and 3 to 6 months (p = 0.05) after treatment. Five patients underwent successful right lobectomy (HCC N = 3, CRC N = 1, CC N = 1) and 6 HCCs were transplanted.
Conclusions
Radiation lobectomy by Y90 is a safe and effective technique to hypertrophy the FLR. Volumetric changes are comparable (albeit slightly slower) to PVE while the right lobe tumor is treated synchronously. This novel technique is of particular interest in the bridge-to-resection setting.
Keywords: Liver resection, Future liver remnant, Hypertrophy, Radiation lobectomy, Radioembolization
Introduction
Portal vein embolization (PVE) is a standard technique for patients with primary or secondary liver malignancies not amenable to liver resection due to small future liver remnant expressed as a percentage ratio of the whole liver volume (FLR). The aim of the procedure is to induce contralateral hypertrophy by redirecting the portal blood flow, thereby leading to an increased ratio of FLR. The range of cut-off ratios of the remnant liver varies from 20% (normal) to 40% (cirrhotic) [1–3]. However, some authors highlight the limitations of PVE, citing a concern for progression of untreated disease and an increased rate of contralateral metastases while time elapses during the hypertrophy process; this is potentially related to pro-angiogenic factors [4–8].
Promisingly, radiation lobectomy (RL), defined as the transarterial lobar infusion of 90Y-loaded microspheres (Y90), is suspected to induce similar or superior volumetric changes in liver lobes, but potentially offer the concomitant advantage of controlling the liver tumor and limiting tumor progression in the tumor-naïve (and untreated) left lobe by limiting the rate of portal blood flow diversion [9]. This “atrophy-hypertrophy complex” suggests lobar Y90 radioembolization as an alternate procedure to PVE as bridge to liver resection.
The goal of this study was to confirm the changes of embolized and unembolized liver volumes and FLR after lobar radioembolization. Additional goals included assessing long-term sequelae of RL (change in Child-Pugh score), assessing clinical factors predictive of %FLR hypertrophy, control of tumor in the treated lobe, and development of new tumor in the untreated radiation-naïve left lobe.
Materials and methods
Between 2003 and 2012, 700 patients were treated with radioembolization for hepatocellular carcinoma (HCC), colorectal cancer (CRC) liver metastases or cholangiocarcinoma (CC) in a lobar manner. In general, HCC, CC, and CRC patients with unilobar right lobe disease and no metastases were evaluated for surgical options during weekly multidisciplinary tumor board. Patients not candidates for immediate resection were considered for RL given the 3 theoretical advantages of this concept over PVE: treatment of the cancer, an embedded biologic test-of-time and contralateral hypertrophy. Hence, we studied patients with: (1) HCC, CRC or CC and (2) right lobe tumor(s) and (3) no extrahepatic metastases and (4) right lobar infusion of Y90 (non-selective). Exclusion criteria consisted of: (1) other malignancies, (2) any left lobe radioembolization, (3) segmental/subsegmental radioembolization, (4) prior liver-directed therapies or (5) any extrahepatic metastases. The conceptual definition of RL included the intentional lobar infusion of Y90 microspheres even in the setting where segmental injections could be performed, thereby optimizing tumor and normal parenchymal coverage. This retrospective study was approved by the Northwestern University Institutional Review Board and was compliant with the Health Insurance Portability and Accountability Act.
Eighty-eight patients fulfilled the study criteria with isolated right lobe disease and could (by imaging) be considered for right surgical hepatectomy. Five patients were further excluded because of missing baseline scans (3 patients), unacceptable motion artifact (1 patient) or non-contrast scans (1 patient). The analysis is therefore based on 83 patients fulfilling all study criteria.
Y90 treatment and imaging
Radioembolization treatment was preceded by a simulation procedure during which 99Tc-macroaggregated albumin was injected into the hepatic arterial vasculature simulating Y90 microspheres distribution in order to estimate the degree of extrahepatic deposition. Coiling of extrahepatic arteries was performed when required to avoid inadvertent deposition. Glass microspheres loaded with 90Yttrium (TheraSphere, Nordion, Canada) were used in this study per standard methodology. Patients were observed for 2 hours (arterial closure device) and subsequently discharged [10,11].
Patients and tumor characteristics
Age, gender, tumor type, diagnosis method, performance status (ECOG), Child- Pugh score (CP), cirrhosis, and underlying liver disease, prior liver-directed therapy/resection and baseline imaging were obtained. Following Y90 treatment, patients were scanned at 1 month, and every 3 months thereafter.
Liver function and clinical outcomes
The Child-Pugh score combining functional (total bilirubin, albumin, International Normalized Ratio (INR)), and clinical/imaging findings (encephalopathy, ascites), was calculated at each follow-up time-point. Adverse events post-Y90, left lobe tumor occurrence, right lobe resection, and orthotopic liver transplant post-Y90 radioembolization were assessed. Alpha-fetoprotein serum level (AFP) was recorded for HCC patients at each time-point.
Liver volumes
A detailed volume analysis was performed at each time point (baseline and all follow-ups). All liver volumes were measured assuming a potential extended trisegmentectomy (segments 6 + 7, 5 + 8, 1 + 4). In total, 292 scans were evaluated (205 magnetic resonance imaging (MRI), 87 computed tomography (CT)).
The volumetric delineation was performed at different time points (baseline, 1, 3, 3–6, and >9 months) by the primary investigators on VITAL IMAGES VITREA® medical imaging software from the Northwestern Memorial Hospital’s General Electric CENTRICITY Picture Archiving & Communications System software.
We performed a computer-assisted manual volumetric drawing of hepatic lobes on the portal-venous phase of T1 post-gadolinium MRI sequences (SHARP or VIBE) or on CT by contouring the right (right + medial left) and left (lateral) lobes, separated by the left hepatic vein in the upper left lobe and a virtual line joining the IVC and the insertion of the falciform ligament in the inferior left lobe (Couinaud methodology) [12]. The hepatic hilum (main biliary ducts, vessels [PV, hepatic artery]), gallbladder and IVC were excluded from the segmentation. The volumetric tumor burden (viable and necrotic portions) in each lobe was sculpted by manual volumetric segmentation, using arterial phase series. Steatotic areas were included in the parenchymal volume estimation.
The measured and calculated volumes were defined as follows:
Right lobe volume (RLV): segments 1 + 4 + 5 + 6 + 7 + 8
Left lobe volume (LLV): segments 2 + 3
Total liver volume (TLV): RLV + LLV
Right lobe tumor burden (RLTB): total tumor volume in the right lobe
Left lobe tumor burden (LLTB): total tumor volume in the left lobe
Right lobe parenchymal volume (RLPV): RLV-RLTB
Left lobe parenchymal volume (LLPV): LLV-LLTB
Total liver parenchymal volume (TLPV): RLPV + LLPV
In the literature, there is some confusion behind the use of the term “FLR”. In some series, it refers to the volume of the future liver remnant; in others, it refers to the future liver remnant over the total liver volume. We chose to define FLR as the ratio, expressed as a percentage, of the future liver remnant (segments 2/3) over the total liver parenchymal volume, this definition being more appropriate for surgical practice. Furthermore, we defined the %FLR hypertrophy as the percentage of hypertrophy of the FLR from baseline.
| [ 13] |
Statistics
Baseline patient/tumor characteristics were compared using the Fisher’s exact (categorical variables) and Kruskal-Wallis tests (continuous variables). Volumetric measurements values were expressed as median/range due to non-normal distribution. Volumetric measurements changes (lobes, tumor, FLR) and AFP changes were compared to baseline using the Wilcoxon test for non-normal distributions. Baseline/follow-up FLRs were compared using the Mann-Whitney test. The correlation between maximal %FLR hypertrophy and time post-treatment was tested by Pearson correlation coefficient. Uni/multivariate analysis looking for predictive variables of %FLR hypertrophy was conducted using Cox proportional regression model.
Results
Patient sample
83 patients without extrahepatic disease were treated by right radiation lobectomy (non-selective) using Y90 microspheres for unilobar HCC, CRC or CC. There were 66 males and 17 females with a median age of 68 (range: 36–89). The primary disease was HCC (N = 67, 9 infiltrative), CRC (N = 8, 4 synchronous, 3 metachronous, 1 unknown) and CC (N = 8). The underlying liver disease in cirrhotic patients (N = 47) was attributed to HCV (N = 24), HBV (N = 7), alcohol (N = 6), autoimmune (N = 1) or primary biliary cirrhosis (N = 1) and cryptogenic (N = 8). Baseline characteristics are listed in Table 1.
Table 1.
Baseline characteristics.
| HCC (n = 67) | CRC (n = 8) | CC (n = 8) | Total (n = 83) | p value | |
|---|---|---|---|---|---|
| Age (yr) | 0.44 | ||||
| Median | 66 | 73 | 73 | 68 | |
| Range | 42–87 | 36–87 | 55–89 | 36–89 | |
| Sex | 0.89 | ||||
| Male | 53 | 7 | 6 | 66 | |
| Female | 14 | 1 | 2 | 17 | |
| Tumor volume (ml) | 0.90 | ||||
| Median | 157 | 90 | 118 | 134 | |
| Interquartile range | 48–305 | 51–469 | 64–234 | 51–297 | |
| Range | 8–1756 | 21–952 | 20–432 | 8–1756 | |
| Method of diagnosis | 0.03 | ||||
| Imaging | 39 | 8 | 2 | 49 | |
| Biopsy | 28 | 1 | 6 | 35 | |
| ECOG | 1.0 | ||||
| 0, 1 | 59 | 7 | 7 | 73 | |
| 2, 3 | 8 | 1 | 1 | 10 | |
| Child-Pugh score | 0.90 | ||||
| <7 | 36 | 4 | 3 | 43 | |
| 7, 8 | 23 | 1 | 2 | 26 | |
| 9, 10 | 8 | 0 | 0 | 8 | |
| Cirrhosis | <0.001 | ||||
| Absent | 20 | 8 | 8 | 36 | |
| Present | 47 | 0 | 0 | 47 | |
| Dose to treatment site (Gy) | 0.44 | ||||
| Median | 112 | 107 | 120 | 112 | |
| Range | 74–215 | 75–144 | 95–150 | 74–215 | |
Sixty-nine patients had not been previously treated by systemic chemotherapy. Six patients with CRC had received one or more treatment lines of FOLFOX or FOLFIRI (folinic acid, 5-FU, and oxaliplatin or irinotecan), 5-FU alone, bevacizumab, capecitabine, irinotecan alone, sorafenib or experimental drugs (monoclonal antibodies 17-1A and oral VEGF inhibitor). Two patients with CC were previously treated with gemcitabine ± cisplatin and one patient with HCC with sorafenib.
After Y90 radioembolization, left lobe (segments 2 or 3) tumors occurred in 13/67 cases of HCC, 2/8 CRC, and 2/8 CC. Eleven patients underwent hepatic surgery: 5 surgical right lobectomy (3 for HCC, 1 for CRC and 1 for CC) and 6 orthotopic liver transplant (all for HCC) (Table 2).
Table 2.
Time-dependent volumetric, left lobe tumor occurrence, and Child Pugh score changes.
| Baseline n = 83 | 1 mo (± 15 d) n = 80 | 45 d-3 mo n = 34 | 3–6 mo n = 42 | 6–9 mo n = 28 | >9 mo n = 25 | p value baseline to1 mo | p value baseline to 1–3 mo | |
|---|---|---|---|---|---|---|---|---|
| Liver volume (ml) | 1745 (698–3673) | 1718 (688–4133) | 1734 (866–4051) | 1592 (851– 3062) | 1509 (781– 3059) | 1425 (775– 2534) | 0.16 | 0.03 |
| Right lobe volume (ml) | 1302 (504–3081) | 1280 (494– 3308) | 1255 (560– 3172) | 1033 (559–2030) | 942 (467–1919) | 887 (479–1551) | 0.003 | <0.001 |
| Left lobe volume (ml) | 371 (126–1022) | 391 (95–946) | 438 (213–1107) | 505 (120–1033) | 476 (153–1139) | 512 (173–985) | <0.001 | <0.001 |
| Right lobe tumor burden (ml) | 134 (8–1756) | 117 (4–1755) | 99 (4–1158) | 61 (1–1280) | 70 (0–1148) | 56 (0–425) | 0.22 | 0.02 |
| Left lobe tumor burden (ml)* | 0 (0–0) | 0 (0–106) | 0 (0–5) | 0 (0–126) | 0 (0–49) | 0 (0–33) | n.a. | n.a. |
| Total liver parenchymal volume (ml) | 1520 (674–3029) | 1447 (684–4110) | 1572 (837–3978) | 1452 (840–2618) | 1389 (768–2711) | 1406 (736–2270) | 0.32 | 0.22 |
| Right lobe parenchymal volume (ml) | 1075 (450–2537) | 1029 (477–3286) | 1093 (531–3099) | 882 (566–1787) | 853 (454–1572) | 827 (474–1452) | 0.012 | 0.003 |
| Left lobe parenchymal volume (ml) | 371 (126–1022) | 391 (95–946) | 438 (213–1107) | 505 (120–1033) | 475 (153–1139) | 508 (171–985) | <0.001 | <0.001 |
| FLR (LLPV/TLPV*100) | 26 (9–57) | 28 (9–58) | 31 (11–57) | 36 (14–64) | 36 (15–59) | 37 (15–55) | <0.001 | <0.001 |
| %FLR hypertrophy from baseline (%) | 0 (0–0) | 7 (−22–51) | 24 (−6–98) | 35 (0–109) | 36 (−10–139) | 45 (5–186) | <0.001 | <0.001 |
| Left tumor occurrence | Recurrences | |||||||
| HCC | 0/67 | 4/65 | 2/29 | 3/33 | 5/23 | 6/18 | 13/67 | |
| CRC | 0/8 | 0/7 | 0/2 | 1/6 | 0/4 | 1/5 | 2/8 | |
| CC | 0/8 | 0/8 | 2/3 | 2/3 | 0/1 | 0/2 | 2/8 | |
| Child-Pugh score** | n = 77 | n = 69 | n = 26 | n = 32 | n = 16 | n = 13 | ||
| 6 (5–10) | 6 (5–12) | 7 (5–11) | 7 (5–13) | 6.5 (5–10) | 6 (5–10) | 0.34 | 0.03 | |
The median (range) maximal volume of left lobe tumors was 10 ml (1–126 ml).
Missing CP scores are mostly in CRC and CC patients where INR was unavailable.
n.a., not available.
Adverse events following Y90 were fatigue (N = 51), abdominal pain (N = 19), nausea (N = 12), vomiting (N = 6), fever/chills (N = 9), weight loss (N = 5), hyperbilirubinemia, diarrhea and pseudoaneurysm at the puncture site (N = 1).
Volumetric changes
At baseline, median (range) total liver, right lobe and left lobe volumes were 1745 ml (698–3673), 1302 ml (504–3081), and 370 ml (126–1022), respectively; the right lobe tumor burden was 134 ml (8–1756) and the FLR was 26% (9–57).
Compared to baseline, a significant decrease in the total liver volume was observed from 1 to 3 months (1734 ml; 866–4051; p = 0.03) post Y90. In parallel, a significant decrease in right lobe volume (1280 ml; 494–3308; p = 0.003) and hypertrophy of the left lobe (391 ml; 95–946; p <0.001) were observed after only one month. These changes were maintained at all future time points (Table 2).
The %FLR hypertrophy was also significantly increased one month after therapy (7%; −22→51; p <0.001) and reached 45% (5–186) after more than 9 months (p <0.001) (Table 2). The median maximal %FLR hypertrophy was 26% (−14→186). A strong correlation between the maximal percentage of hypertrophy and the time (days) after treatment was observed (r = 0.57, p <0.001). A %FLR increase of >35% was noted in 25/67 (37%) of HCC patients; 4 patients exhibited >100% increase in %FLR.
A significant reduction in median tumor volume from 134 cc to 99 cc at 3 months (p = 0.02) and 56 cc at the >9 month time point was observed. Furthermore, median serum AFP levels (N, range), in patients treated for HCC, drop from 870 ng/ml (48, 14–270,339) to 197 (48, 8–135,566, p <0.001) at 1 month, to 301 (16, 5–10,173, p = 0.006) at 1–3 months, to 130 (21, 3–83,589, p <0.001) at 3–6 months, to 81 (12, 2–30,000, p = 0.001) at 6–9 months and to 28 (11, 4–21,485, p = 0.03) at >9 months. Hence, the antitumoral effect of Y90 was confirmed, as expressed by reduction in AFP and/or tumor volume. Figs. 1 and 2 illustrate examples where patients subsequently underwent curative resection following RL.
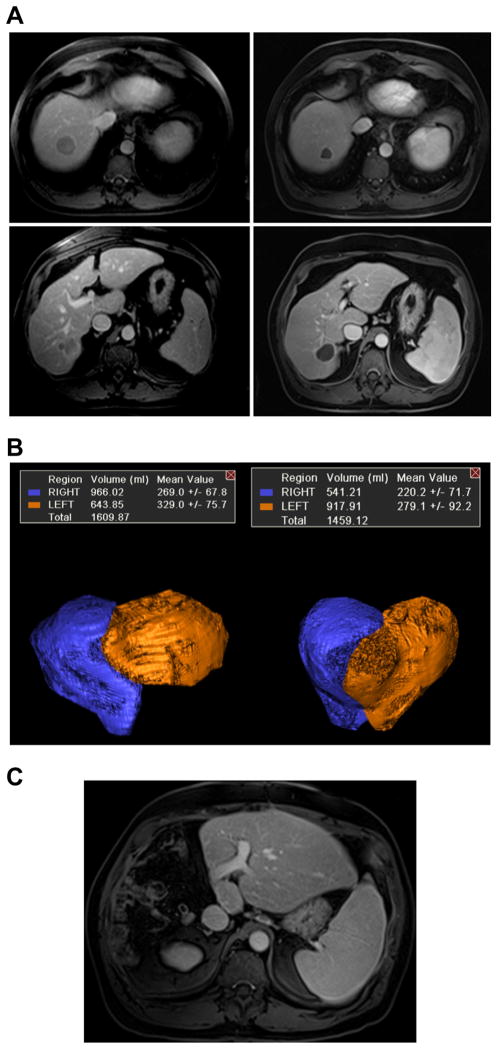
Case 1. Radiation lobectomy of the right lobe for multifocal hepatocellular carcinoma and hepatitis C prior to consideration for surgery.
(A) Pre and post contrast enhanced MRI showing complete response to treatment by mRECIST criteria. Subjectively, segments 2/3 appeared to have hypertrophied significantly. (B) 3D volume reconstructions of the right lobe (including 4) and segment 2/3 demonstrate 43% post treatment hypertrophy of segment 2/3. (C) The patient underwent successful right trisegmentectomy and is free of disease with normal liver functions two years after surgery. (This figure appears in colour on the web.)
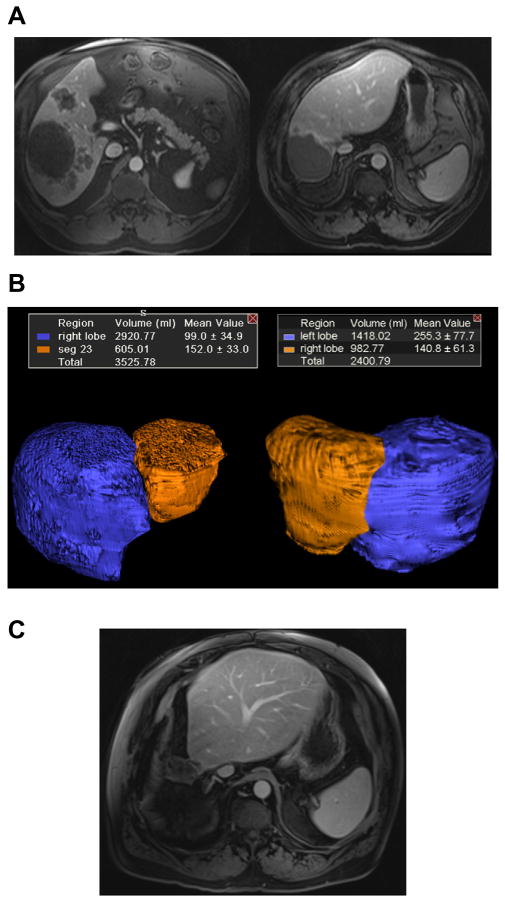
Case 2. Radiation lobectomy of the right lobe for colorectal cancer metastases.
(A) Baseline MRI scan demonstrates large hepatic metastases confined to the right lobe. Following treatment, significant response and dramatic hypertrophy of segments 2/3 are observed. (B) 3D volume reconstructions of the right lobe (including 4) and segments 2/3 demonstrate significant FLR increase from 17% to 41%. (C) The patient underwent an extended right trisegmentectomy and is free of liver disease three years following surgery. (This figure appears in colour on the web.)
Uni/multivariate analysis
Baseline FLR was significantly greater in patients with portal vein thrombosis (PVT) (especially right), in patients with HCC and CC compared to CRC, and in patients with CP scores ≥7 (Table 3). Those differences were no longer significant after a follow-up period of 1–3 months for the CP score, 6–9 months for the tumor type and >9 months for the PVT status. Baseline and post-treatment FLR was not found to be different in patients with documented cirrhosis (imaging or biopsy) (Table 3).
Table 3.
Time-dependent FLR change by baseline characteristics.
| Baseline | 1 mo | 1–3 mo | 3–6 mo | 6–9 mo | >9 mo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||
| n | FLR | n | FLR | n | FLR | n | FLR | n | FLR | n | FLR | |
| PVT | ||||||||||||
|
| ||||||||||||
| No | 46 | 24 (9–45) | 45 | 26 (9–45) | 15 | 26 (11–40) | 25 | 30 (14–44) | 20 | 32 (15–45) | 22 | 37 (15–55) |
|
| ||||||||||||
| Yes | 37 | 29 (15–57) | 35 | 30 (17–58) | 19 | 35 (19–57) | 17 | 41 (22–64) | 8 | 41 (36–59) | 3 | 37 (37–55) |
|
| ||||||||||||
| p value | <0.001 | 0.002 | 0.03 | 0.02 | 0.01 | 0.40 | ||||||
|
| ||||||||||||
| CP score | ||||||||||||
|
| ||||||||||||
| <7 | 49 | 24 (9–47) | 47 | 26 (9–58) | 21 | 26 (11–56) | 27 | 37 (14–64) | 21 | 37 (15–54) | 20 | 38 (15–55) |
|
| ||||||||||||
| ≥7 | 34 | 29 (10–57) | 33 | 29 (13–58) | 13 | 35 (24–57) | 15 | 34 (14–57) | 7 | 32 (30–59) | 5 | 37 (31–45) |
|
| ||||||||||||
| p value | 0.01 | 0.05 | 0.06 | 0.89 | 0.94 | 0.95 | ||||||
|
| ||||||||||||
| Tumor type | ||||||||||||
|
| ||||||||||||
| HCC | 67 | 27 (12–47) | 65 | 29 (9–58) | 29 | 33 (13–57) | 33 | 37 (14–64) | 23 | 36 (15–59) | 18 | 37 (15–55) |
|
| ||||||||||||
| CC | 8 | 25 (17–57) | 8 | 26 (20–58) | 3 | 26 (23–52) | 3 | 42 (30–57) | 1 | 38 | 2 | 37 (33–41) |
|
| ||||||||||||
| CRC | 8 | 16 (9–24) | 7 | 18 (12–35) | 2 | 16 (11–22) | 6 | 24 (14–39) | 4 | 33 (23–42) | 5 | 39 (23–43) |
|
| ||||||||||||
| HCC vs. CRC p value | <0.001 | 0.01 | 0.04 | 0.03 | 0.68 | 0.77 | ||||||
|
| ||||||||||||
| HCC vs. CC p value | 0.99 | 0.86 | 0.72 | 0.26 | n.a. | 1.00 | ||||||
|
| ||||||||||||
| CRC vs. CC p value | 0.01 | 0.02 | 0.08 | 0.07 | n.a. | 0.70 | ||||||
|
| ||||||||||||
| Cirrhosis (imaging or biopsy) | ||||||||||||
|
| ||||||||||||
| No | 36 | 24 (9–57) | 33 | 27 (9–58) | 14 | 30 (11–56) | 19 | 36 (14–64) | 15 | 36 (15–57) | 14 | 36 (15–55) |
|
| ||||||||||||
| Yes | 47 | 27 (14–45) | 45 | 28 (13–58) | 20 | 31 (22–57) | 23 | 37 (14–45) | 13 | 36 (19–48) | 11 | 39 (23–55) |
|
| ||||||||||||
| p value | 0.24 | 0.85 | 0.65 | 0.89 | 0.98 | 0.30 | ||||||
n.a., not available.
In the multivariate analysis (40% FLR hypertrophy as end-point), PVT was the only significant correlated variable (Table 4). Age, ECOG, CP score, primary disease, and irradiation were not correlated with %FLR hypertrophy.
Table 4.
Multivariate analysis by Cox proportional regression model.
| Covariate | p value | Exp (b) | 95% CI of Exp (b) |
|---|---|---|---|
| Age >70 | 0.88 | 1.10 | 0.33 to 3.66 |
| Female sex | 0.90 | 1.09 | 0.29 to 4.08 |
| ECOG 2–3 | 0.70 | 1.39 | 0.27 to 7.24 |
| No cirrhosis | 0.94 | 0.95 | 0.25 to 3.57 |
| Child-Pugh score ≥7 | 0.81 | 1.17 | 0.33 to 4.08 |
| Cholangiocarcinoma | 0.48 | 2.05 | 0.28 to 15.00 |
| CRC | 0.40 | 2.02 | 0.40 to 10.10 |
| M PVT | 0.18 | 3.16 | 0.60 to 16.47 |
| R PVT | 0.02 | 6.39 | 1.40 to 29.06 |
| Dose ≤100 Gy | 0.56 | 1.55 | 0.36 to 6.72 |
| Dose >120 Gy | 0.35 | 1.68 | 0.57 to 5.00 |
End point of regression model was %FLR hypertrophy ≥40%.
Liver function
A slight median worsening of the Child-Pugh score from 6 (5–10) to 7 (5–11) points was seen 1 to 3 months (p = 0.03) and 3 to 6 months (p = 0.05) after treatment but not after 6 to 9 months (p = 0.46) or >9, where a score of 6 was maintained (p = 0.85) (Table 2).
Survival
Median survival of BCLC B and C patients was 34.4 (CI: 15.8, 55.8) and 9.6 (CI: 6.9, 18.1) months, respectively.
Discussion
Volumetric analysis and predictive variables
This is the first description of a dynamic and time-dependent analysis of the volumetric changes in liver lobes and FLR after radioembolization. The right lobe shrinkage and the left lobe hypertrophy confirm, in this analysis, the linear time-dependent hypertrophy of the FLR, not related to tumor burden volumetric change (“atrophy-hypertrophy complex”). The FLR was found to be greater in patients with HCC and CC compared to CRC, and in patients with CP scores ≥7. Those observations could possibly be explained by the underlying liver dysfunction predisposing to primary liver disease (such as HCC) and CC even though patients with documented cirrhosis did not show higher left lobe/right lobe ratios. Due to the preponderance of viral hepatitis in patients exhibiting HCC, a selection bias by early biopsy detection of mildmoderate cirrhosis without anatomical change could have occurred in the studied population. A subgroup analysis by degree of fibrosis on biopsy, or limiting the cirrhosis categorization to imaging-detected cirrhotic patterns, could potentially increase the accuracy of baseline FLR estimation but was limited by the number of patients in our study.
In patients with PVT (all in HCC patients), the baseline FLR was higher, and the degree of %FLR hypertrophy was found to be greater, particularly when PVT was limited to the right portal (vs. main) branch. These observations may be related to the physiological portal flow redirection by “natural PVE” due to bland or tumoral thrombus invasion of the portal veins.
Progressive disease of untreated radiation naïve left lobe
In our study, the incidence of disease progression in the untreated left lobe was 2/8 in CRC and 2/8 in CC patients at any time during follow-up. As comparison, the literature reports 25% of metastatic recurrence in the FLR 3 weeks after PVE (CRC) and a disease-free survival of 46%, 1-year after hepatic resection for CC [14,15]. In contrast to the time-dependent reporting of FLRs in this report, comparison with the literature is difficult, since authors usually report a one-time static FLR following PVE.
Left lobe tumor occurred in 13/67 (19%) patients with HCC after a median follow-up of 224 days (23–549), comparable to natural history in patients treated by radiofrequency ablation for early-stage HCC without any other treatment (15% after 1 year) [16]. No difference in the frequency of left lobe progression was observed between patients with (7/37) or without PVT (6/30) (p = 1.0). While new lesions developed in the untreated left lobe (natural history of cirrhosis), in no case it was massive tumor progression, postulated to occur following treatments where pro-angiogenic factors are released, in the untreated tumor-naïve left lobe observed after RL. Grouping all tumor types, the maximal left-sided tumor burden (new disease) was found to be small (median: 10 ml, range: 1–126 ml). Moreover, right lobe tumor volume (in all patients, p = 0.02) and AFP serum levels (in HCC patients, p <0.001) were found to be significantly decreased from baseline at each successive time point, confirming adequate tumor control by radioembolization. Patients expressing such positive tumor biology following this test-of-time are ideally suited for resection. Hence, no dramatic left lobe tumor progression could be documented following RL.
Clinical outcomes and comparison to portal vein embolization
First, the median maximal %FLR hypertrophy was 26% (−14→186). The median %FLR hypertrophy (+7%, 24%, 35%, 36%, 45% after, respectively 1, 1–3, 3–6, 6–9, and >9 months) was found to be comparable to similar studies reported in the literature with PVE. However, comparison to other seminal series is complex, since reporting is heterogeneous, often associated with differing methodologies and volume calculation methods [5,13,17,18]. However, the kinetics of %FLR hypertrophy may be slower with lobar Y90 than with PVE. For instance, compared to Farges et al., the FLR (termed %FFLR in their study) increased from 31 ± 6% to 47 ± 11% in patients with normal liver function and from 35 ± 13% to 44 ± 13% in those with chronic liver disease, 4 to 8 weeks after PVE. This translates to approximate hypertrophies of 50% and 26%. Compared to Pamecha et al., the median FLR increased from 22% to 32% (approximate hypertrophy of 45%) after a median follow-up of 12 weeks. In our series, an FLR of 30–35%, 35–40 and >40 was reached in 5 (6%), 9 (11%), and 25/83 (30%) patients. A goal of 40% FLR was reached in 13/47 (28%) cirrhotic patients (median: 35%, range: −7→58).
Second, right lobar radioembolization should now be considered a safe technique: usual complications of radioembolization are now well-known and easily managed (pain, flu-like symptoms) or avoided (gastro-intestinal or lung deposition of microspheres) by appropriate patient selection, treatment planning and dosimetry. All adverse events reported in our cohort were minor.
A moderate and transient increase of the median Child-Pugh score from 6 to 7 was observed after one to three months; this minor change could be attributed to natural fluctuation of Child-Pugh score. Conceptually, as a transarterial technique, radioembolization also offers the advantage of avoiding the transhepatic puncture of the portal system necessary for PVE. As opposed to PVE, PVT does not constitute a contraindication to right lobe radioembolization. In fact, it was demonstrated in our study to be the only variable correlated with greater hypertrophy of the FLR and is thus suspected to provide a synergistic effect [19].
Although one could argue that PVT in HCC should have been exclusion criteria for our study since hepatic resection is not usually performed on such patients in the West, it is routinely performed in Asia (limited transplant availability) [20,21]. In fact, one of our patients, with right lobe PVT treated by RL, subsequently underwent (in Hong Kong) a right trisegmentectomy and exhibited 4-year survival.
Surgical resection post Y90
In this study, 5 patients ultimately underwent right lobe resection (with or without segment 4) and 6 received liver transplantation. The surgical concepts following Y90 in this setting deserve discussion. From a purely technical perspective, the surgical issues encountered during resection following Y90 treatment consist primarily of increased inflammation and induration both with respect to surgical planes and with the liver parenchyma itself. Our surgical team has learned easily how to adjust the approach, by changing the settings on the dissecting instruments, and by using more sharp dissection. One cautionary note is that the diaphragm is often adhered to the liver; great care must be exercised in order not to cause injury. Nonetheless, we have not encountered hostile or prohibitive surgical conditions during either hepatectomy or explantation. One common misconception has been the reason why the specimen needs to be sequestered and this is important to address given that pathological staging might be delayed. While there may be a sense that this has to do with the risk of radiation to the pathologist, in fact this risk does not exist, and this applies to the surgeon as well. The reason why the specimen is quarantined has to do strictly with environmental concerns regarding final disposition. Therefore, arrangements should be made between pathology and the radiation personnel such that pathological assessment precedes quarantine.
Postulated physiology
Direct portal flow redirection is probably not the primum movens of the right lobe/left lobe ratio after glass microsphere radioembolization. Physiologically, even though microspheres of approximately 25 ± 10 microns of diameter are known to be entrapped in the arterial side of the capillary bed and could act like other embolic particles of the same size range, material for standard PVE, such as polyvinyl alcohol particles, ranging from 300 to 500 μm, and microcoils, is more likely to induce complete vascular occlusion [22]. However, radiation-induced parenchymal lesions such as loss of cellularity, fibrosis and retraction, with concomitant decreased blood supply could be a part of the shrinkage process on the irradiated side, inducing a slow redirection of the portal flow to the non-irradiated side. This is contrast to PVE, where the embolization of the right portal vein is complete and sudden; it is unlikely that the left portal vein perfusing the small liver remnant can accommodate the entire portal diversion resulting from acute right PVE. Quantitative perfusion imaging studies or anatomic imaging studies evaluating the left and right portal veins diameter before and after radioembolization could support or reject such a physiological hypothesis.
Strengths and limitations
Amongst the limitations of this study we recognize the limited sample size. However, as a focused analysis, this is one of the most robust analyses incorporating the time-dependent component of liver volume changes. By contrast, most studies on PVE provide a static 4–6 week singular hypertrophy rate. This study was thorough and comprehensive, limiting the studied population to clinical scenarios where right lobe resection would be indicated (unilobar right CRC/HCC/CC, no extrahepatic metastasis). We also recognize that measurements were performed with computer-assisted manual drawings and that we did not use the FLR formula based on BSA. These latest observations could however be considered strengths rather than limitations because of the strict anatomical landmarks used for the liver segmentation described in our methodology and the complexity of the parenchymal and tumor delineation, which would unlikely be improved by automated segmentation. Urata’s BSA-based formula was developed to simplify the liver graft volume need estimation and derived from volumetric measurements on abdominal computed tomography [23]. We counteracted that limitation by measuring exactly normal and tumor parenchyma at all time points. The advent of 3-D volume software has permitted more advanced volume analyses. Furthermore, while Couinaud-based segmentation is not perfect, it is widely accepted in the surgical literature [12,24].
As a counter for these limitations, we believe volumetric measurements presented herein are underestimates since the irradiated liver parenchyma, introduced as the denominator in the FLR formula, is probably less functional than the non-irradiated parenchyma. Moreover, we measured hepatic volumes assuming the extreme scenario of extended trisegmentectomy. Nevertheless, depending on the tumor confinement and size, surgical partial hepatectomy can often be limited to a right hepatectomy (segments 5/6/7/8) or less, increasing the FLR estimation. Future concepts of RL as a bridge-to-resection in clinical practice should extend to cases of massive tumor burden limited to the right lobe (right lobe and segment 4 only). The potential benefit of Y90 over PVE may be in the inherent biologic test-of-time waiting for contralateral growth while simultaneously assessing tumor response (surrogate biologic test-of-time). Particularly in cirrhotic livers, Y90 is a strong stress test on cirrhotic/fibrotic livers assessing regeneration capacity; this is of importance in making the ultimate decision for resection. Finally, we also consider that a future comparison to a control group treated by standard PVE will be necessary to confirm the benefits of the RL concept.
Conclusions
Radiation lobectomy by Y90 is a safe and effective technique to increase FLR. Volumetric changes (atrophy-hypertrophy complex) are comparable to (albeit at a slower kinetic) PVE, while the right lobe tumor is treated synchronously. From a bridge-to-resection perspective, these combined effects could optimize patient selection, maximize FLR, and incorporate a valuable test-of-time before resection. These would help identify patients that would benefit most from surgery, thereby improving post-surgical outcomes and minimizing recurrence rates.
Acknowledgments
Financial support
There was no funding provided for this study. RS is supported in part by NIH grant CA126809.
Abbreviations
- AFP
alpha-fetoprotein
- CC
cholangiocarcinoma
- CP
Child-Pugh
- CRC
colorectal cancer
- CT
computed tomography
- FLR
ratio (expressed as percentage) of the future liver remnant (segments 2/3) to the total liver parenchymal volume
- %FLR hypertrophy
percentage increase in FLR hypertrophy from baseline
- HCC
hepatocellular carcinoma
- INR
international normalized ratio
- MRI
magnetic resonance imaging
- PVE
portal vein embolization
- PVT
portal vein thrombosis
- RL
radiation lobectomy
- Y90
90Y-loaded glass microspheres radioembolization
Footnotes
Conflict of interest
LK, RJL, MFM, and RS are advisors to Nordion. None of the other authors have any conflict of interest.
The underlying research reported in the study was funded by the NIH Institutes of Health.
References
- 1.Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 2.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 3.Shindoh J, Tzeng C-WD, Aloia TA, Curley SA, Zimmitti G, Wei SH, et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol. 2013;20:2493–2500. doi: 10.1245/s10434-012-2864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belghiti J, Benhaim L. Portal vein occlusion prior to extensive resection in colorectal liver metastasis: a necessity rather than an option! Ann Surg Oncol. 2009;16:1098–1099. doi: 10.1245/s10434-009-0379-7. [DOI] [PubMed] [Google Scholar]
- 5.Pamecha V, Glantzounis G, Davies N, Fusai G, Sharma D, Davidson B. Longterm survival and disease recurrence following portal vein embolisation prior to major hepatectomy for colorectal metastases. Ann Surg Oncol. 2009;16:1202–1207. doi: 10.1245/s10434-008-0269-4. [DOI] [PubMed] [Google Scholar]
- 6.Pamecha V, Levene A, Grillo F, Woodward N, Dhillon A, Davidson BR. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer. 2009;100:617–622. doi: 10.1038/sj.bjc.6604872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoekstra LT, van Lienden KP, Verheij J, van der Loos CM, Heger M, van Gulik TM. Enhanced tumor growth after portal vein embolization in a rabbit tumor model. J Surg Res. 2013;180:89–96. doi: 10.1016/j.jss.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587–1596. doi: 10.1245/s10434-009-0454-0. [DOI] [PubMed] [Google Scholar]
- 10.Salem R, Lewandowski RJ, Gates VL, Nutting CW, Murthy R, Rose SC, et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol. 2011;22:265–278. doi: 10.1016/j.jvir.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 1: technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 12.Couinaud C. Errors in the topographic diagnosis of liver diseases. Ann Chir. 2002;127:418–430. doi: 10.1016/s0003-3944(02)00802-7. [DOI] [PubMed] [Google Scholar]
- 13.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 14.Hoekstra LT, van Lienden KP, Doets A, Busch OR, Gouma DJ, van Gulik TM. Tumor progression after preoperative portal vein embolization. Ann Surg. 2012;256:812–817. doi: 10.1097/SLA.0b013e3182733f09. discussion 817–818. [DOI] [PubMed] [Google Scholar]
- 15.Sulpice L, Rayar M, Boucher E, Pracht M, Meunier B, Boudjema K. Treatment of recurrent intrahepatic cholangiocarcinoma. Br J Surg. 2012;99:1711–1717. doi: 10.1002/bjs.8953. [DOI] [PubMed] [Google Scholar]
- 16.Kudo M. Early detection and curative treatment of early-stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2005;3:S144–S148. doi: 10.1016/s1542-3565(05)00712-3. [DOI] [PubMed] [Google Scholar]
- 17.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2012;137:675–680. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 19.Madoff DC, Hicks ME, Vauthey JN, Charnsangavej C, Morello FA, Jr, Ahrar K, et al. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics. 2002;22:1063–1076. doi: 10.1148/radiographics.22.5.g02se161063. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Park MS, Park YN, Kim KS, Choi JS, Ahn SH, et al. Preoperative radiologic and postoperative pathologic risk factors for early intra-hepatic recurrence in hepatocellular carcinoma patients who underwent curative resection. Yonsei Med J. 2009;50:789–795. doi: 10.3349/ymj.2009.50.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim JH, Jang HJ, Kim EY, Park CK, Joh JW, Kim YI. Early recurring hepatocellular carcinoma after partial hepatic resection: preoperative CT findings. Korean J Radiol. 2000;1:38–42. doi: 10.3348/kjr.2000.1.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness–study in 26 patients. Radiology. 2003;227:251–260. doi: 10.1148/radiol.2271012010. [DOI] [PubMed] [Google Scholar]
- 23.Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 24.Fischer L, Thorn M, Neumann JO, Schobinger M, Heimann T, Grenacher L, et al. The segments of the hepatic veins-is there a spatial correlation to the Couinaud liver segments? Eur J Radiol. 2005;53:245–255. doi: 10.1016/j.ejrad.2004.02.003. [DOI] [PubMed] [Google Scholar]