Abstract
Gold nanovesicles contain multiple nanocrystals within a polymeric coating. The strong plasmonic coupling between adjacent nanoparticles in their vesicular shell makes ultrasensitive biosensing and bioimaging possible. In our laboratory, multifunctional plasmonic vesicles are assembled from amphiphilic gold nanocrystals (such as gold nanoparticles and gold nanorods) coated with mixed hydrophilic and hydrophobic polymer brushes or amphiphilic diblock co-polymer brushes. To fulfill the different requirements of biomedical applications, different polymers that are either pH=responsive, photoactive or biodegradable can be used to form the hydrophobic brush, while the hydrophilicity is maintained by polyethylene glycol (PEG). This protocol covers the preparation, surface functionalization and self-assembly of amphiphilic gold nanocrystals grafted covalently with polymer brushes. The protocol can be completed within 2 d. The preparation of amphiphilic gold nanocrystals, coated with amphiphilic diblock polymer brushes using a ‘grafting to’ method or mixed hydrophilic and hydrophobic polymer brushes using tandem ‘grafting to’ and ‘grafting from’ methods, is described. We also provide detailed procedures for the preparation and characterization of pH-responsive plasmonic gold nanovesicles from amphiphilic gold nanocrystals using a film-rehydration method that can be completed within ~3 d.
INTRODUCTION
Vesicle nanostructures, including polymersomes and liposomes, have been widely investigated as drug delivery platforms. These include polymersomes and liposomes that have made use of amphiphilic block co-polymers and lipids, respectively. It is useful to be able to trace the drug carrier, and plasmonic vesicles with tunable plasmonic coupling and a hollow cavity are attractive candidates for this application. Plasmonic vesicles each contain multiple gold nanoparticles. Light of a wavelength much longer than the diameter of the gold nanoparticle can produce coherent oscillations in the nanoparticle’s electron cloud relative to its stationary core1–5. Localized surface plasmon resonance (LSPR) is a coherent oscillation of the surface conduction electrons excited by electromagnetic radiation4,6,7. When gold nanoparticles form assemblies or aggregates, the surface plasmon absorption is strongly dependent on their interparticle distances, which cause more red-shift at smaller interparticle distance8–12. On the basis of this theory, plasmonic dimers, trimers, chains and clusters have been widely reported in regard to their potential use in tracing a drug carrier11,13–22. Vesicular nanostructures, such as liposomes, with a hollow cavity and thin shell layer, have shown broad biomedical application23. Similar to liposomes that are formed by amphiphilic lipid, polymer vesicles can also be prepared by self-assembly of amphiphilic block co-polymers24,25. With the development of functional polymers, various stimuli (e.g., pH, light, thermal, enzyme)-responsive polymersomes were developed25–27. We and others have applied this principle to design and prepare plasmonic nanocrystal-based hybrid vesicles by coassembly of a mixture of diblock polymers and gold nanoparticles coated with diblock polymers28–32, which showed distinctly different optical and physical properties from those of single nanocrystals (Table 1)33–38.
TABLE 1.
Plasmonic vesicles assembled from amphiphilic gold nanoparticles coated with different hydrophobic polymer brushes.
| Gold nanocrystals (nm) | Hydrophilic polymer | Hydrophobic polymer | Responsiveness | SPR peak (nm) | Reference |
|---|---|---|---|---|---|
| AuNP (14) | PEG | PMMA | Laser | 520–620 | 33 |
| AuNP (14) | PEG | PMMAVP | pH and laser | 520–550 | 36 |
| AuNP (14) | PEG | PNBA | UV light or laser | 530–745 | 34 |
| AuNR (14 × 50) | PEG | PLA | Enzyme and laser | 780–940 | 35 |
| AuNP (12–60) | PEG | PS | Laser | 550–670 | 37 |
| AuNP (26) | PEG | PCL | Laser | 600–990 | 38 |
AuNP, gold nanoparticle; AuNR, gold nanorod; PCL, poly(ε-caprolactone); PEG, polyethylene glycol; PLA, polylactide; PMMA, poly(methyl methacrylate); PMMAVP, 25% 4-vinyl pyridine in PMMA; PNBA, poly(2-nitrobenzyl acrylate); PS, polystyrene; SPR, surface plasmon resonance.
In the vesicle, the LSPR can be easily adjusted from the visible to the near-infrared region by tuning the interparticle distance of the gold nanocrystals in the vesicular shell34–36. Interparticle distance is highly dependent on the relative ratio of the hydrophilic and hydrophobic polymer chains on the gold nanocrystal surface. For instance, with a decreased amount of hydrophilic polymer brushes, the hydrophobicity of the gold nanoparticles was increased and the interparticle distance of the vesicle membrane was decreased, inducing a larger red shift in LSPR34–36. Furthermore, the inter-particle distance decreases with the decrease of the molecular weight of the hydrophobic polymer brush, leading to stronger plasmonic coupling and a larger red shift in LSPR34–36.
To fulfill the different requirements of drug delivery, various types of plasmonic vesicles have been prepared by using amphiphilic gold nanocrystals coated with pH-responsive36, photoactive34 or biodegradable polymers35 such as the hydrophobic brush; hydrophilicity was maintained by PEG, as displayed in Table 1. Of particular interest is that the disruption of the plasmonic vesicles can be triggered by different stimulus responses of the pH-, photo- or enzyme-sensitive polymers or the light-sensitive nanocrystals34–36. These plasmonic vesicles with hollow cavities allow high drug loading inside for cancer-specific targeting and drug delivery. More importantly, the Raman tag–embedded plasmonic vesicles with ultrastrong interparticle plasmonic coupling exhibit a greatly enhanced scattering cross-section as compared with single gold nanocrystals, which have been used to detect cancer cells.
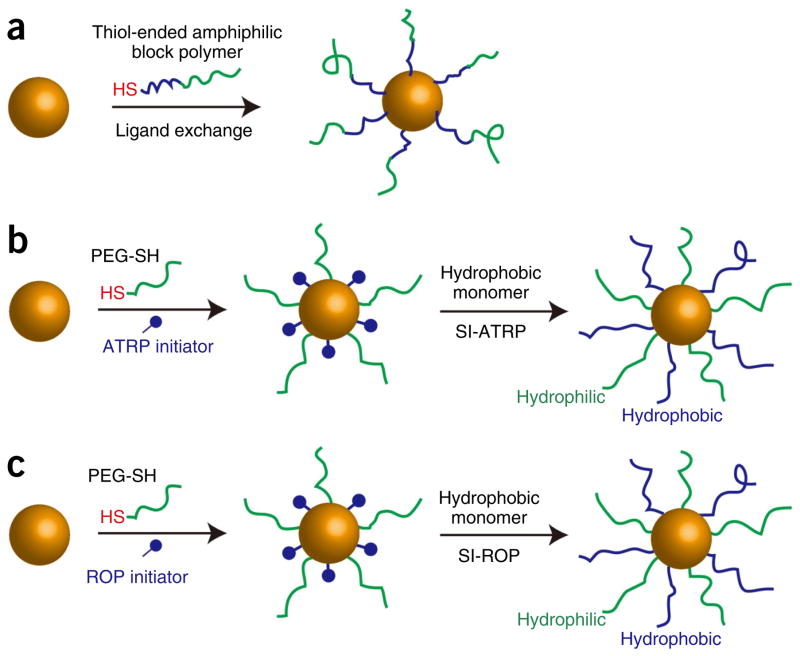
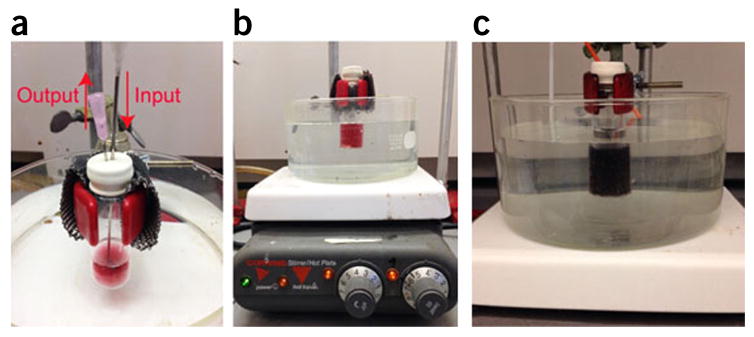
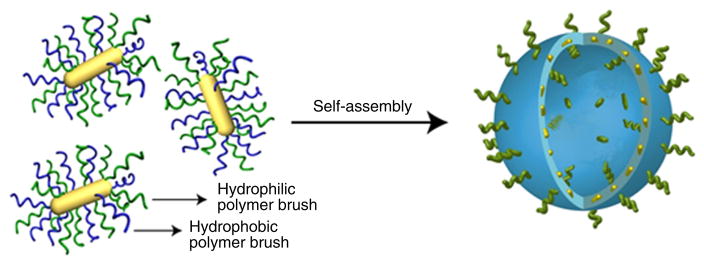
In this protocol, we describe the procedures and conditions required to prepare amphiphilic gold nanocrystals coated with polymer brushes using a one-step ‘grafting to’ method or a two-step method including ‘grafting to’ and ‘grafting from’ methods. In the one-step method, polymer chains are covalently attached to the gold nanoparticle by forming a covalent Au–S bond. In the two-step method, thiolated PEG and an atom-transfer radical-polymerization (ATRP) or ring-opening polymerization (ROP) initiator are attached to the gold nanoparticle surface in the ‘grafting to’ step, and then hydrophobic polymer brushes are grown on the gold nanoparticle surface through surface ATRP and ROP methods. For the ‘grafting from’ step, we developed two approaches, including surface-initiated (SI) ATRP (SI-ATRP) and SI-ROP, to grow hydrophobic polymer brushes (Figs. 1 and 2)35,39. The SI-ATRP allows for controlled growth of hydrophobic polymer brushes with hydrocarbon main chains (Fig. 3). The SI-ROP method enables growth of a broad spectrum of cyclic monomers such as biodegradable hydrophobic polymer grafts on the gold nanocrystal surface (Fig. 4)39. The amphiphilic gold nanocrystals coated with amphiphilic polymers can be further self-assembled into plasmonic vesicles using a film rehydration method (Fig. 5)33. In multiple systems, we have shown that the polymer grafts impart amphiphilicity-driven self-assembly to the hybrid nanoparticles, leading to well-defined vesicles in which plasmonic nanocrystals are embedded in the shell of collapsed hydrophobic polymers and the hydrophilic brushes extend into the external and interior aqueous environments to stabilize the vesicular structure (Fig. 5)36. More importantly, the interparticle distance of the gold nanocrystals in the vesicular shell can be tuned by the grafting density and molecular weight of the polymer brushes, leading to controllable optical properties (scattering, photothermal conversion and Surface enhanced Raman scattering) of the vesicle. The disassembly of the vesicle can be induced by changing the hydrophobicity of the responsive hydrophobic polymer brush induced by pH, light or enzyme. Herein, we used pH-responsive gold nanovesicles as a model to describe the preparation of a vesicle (Step 3). The preparation of amphiphilic gold nanocrystals coated with amphiphilic polymers, particularly mixed hydrophilic and hydrophobic polymer brushes, is the main focus of this protocol. The major goal of this article is to provide reliable and standard procedures to guide researchers in this field toward developing gold nanocrystal–based surface function and fabricating assemblies with high stability and controllability, which are often lacking in nanotechnology and nanomedicine. Recent advances in nanocrystal preparation and functional biopolymer synthesis open a wealth of possibilities to expand this concept to other types of nanocrystals and to integrate different types of nanocrystals into diverse multifunctional assemblies. This protocol could be helpful to researchers interested in or working in the fields of nanoparticle surface function, polymer science and self-assembly.
Figure 1.
Schematic illustration of the synthesis of amphiphilic gold nanocrystals grafted with polymer brushes. (a) ‘Grafting to’ amphiphilic diblock polymers ended with a thiol end group. (b) Tandem ‘grafting to’ (co-adsorption of hydrophilic homopolymers and ATRP or ROP initiators) and (c)‘grafting from’ (surface-initiated ATRP or ROP polymerization) hydrophobic polymers. The hydrophilic polymer brush is in green and the hydrophobic polymer brush is in blue.
Figure 2.
Experimental setup for the preparation of amphiphilic gold nanoparticles. (a) Experimental setup used to degas the solution of Au@PEG/DTBE in DMF using N2. (b) Equipment used to synthesize hydrophobic polymer brushes on the surface of Au@PEG/DTBE or AuNR@PEG/MUD using the surface-initiated polymerization method. The glass reaction bottle must be sealed and placed in a water or oil bath to control the reaction temperature. (c) Aggregation of the gold nanoparticles and color change from red to blue.
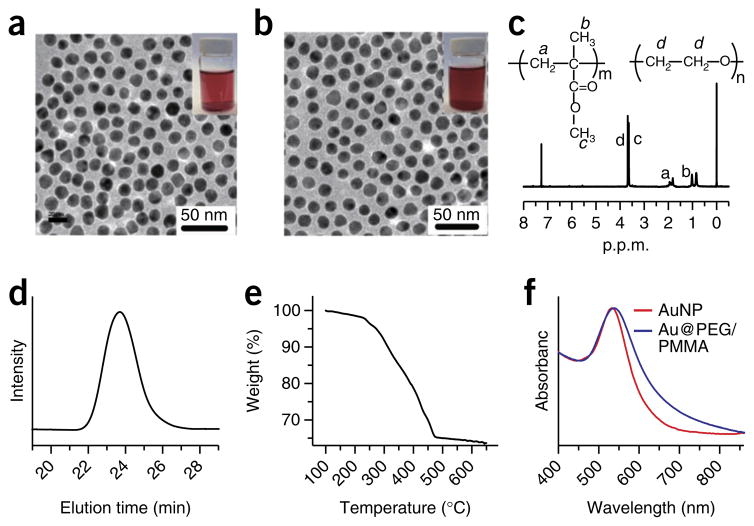
Figure 3.
Characterization of amphiphilic gold nanoparticles. (a) TEM images of citric acid–coated 14-nm gold nanoparticles (AuNPs) (inset: photographs of the AuNP solution in water). (b) TEM image of amphiphilic AuNPs coated with mixed PEG and poly(methyl methacrylate) (PMMA) (Au@PEG/PMMA) (inset: photograph of the Au@PEG/PMMA solution in chloroform). (c) 1H-NMR (300 MHz, δ, p.p.m., CDCl3) of Au@PEG/PMMA: 3.65 (-CH2-CH2-), 3.60 (-OCH3), 1.71–2.08 (-CH2-), 0.75–1.05 (-CH3). (d) GPC trace of the PMMA detached from the gold nanoparticles (Mn = 24 kDa, polydispersity index = 1.21). (e) TGA analysis of the gold nanoparticles grafted with mixed polymer brushes of PEG and PMMA (the weight fraction of the polymer brushes is 27.5%). (f) UV-visible spectra of citric acid–coated 14-nm AuNPs in water (red line) and amphiphilic Au@PEG/PMMA in chloroform (blue line).
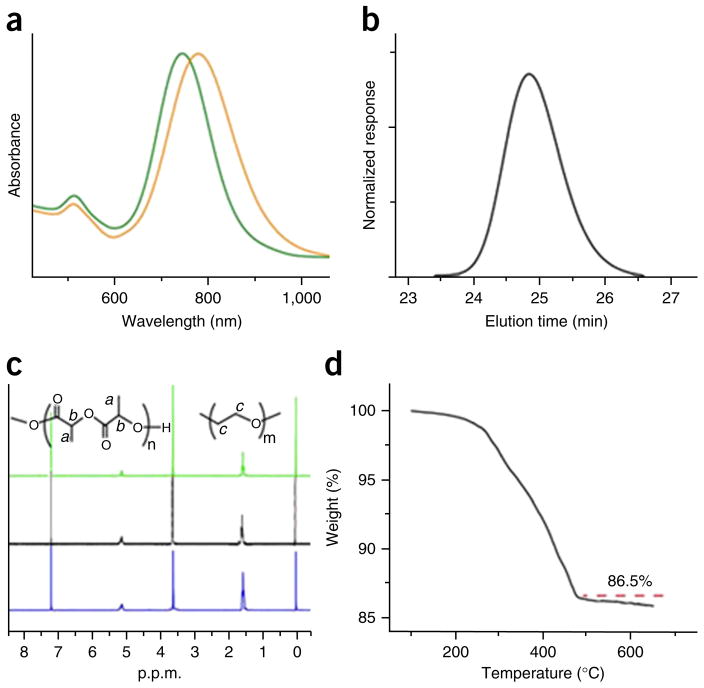
Figure 4.
Characterization of amphiphilic gold nanorods. (a) UV-visible spectra of gold nanorods (AuNRs) stabilized by cetyltrimethylammonium bromide (CTAB) in water (green line) and AuNRs coated with PEG and polylactide (AuNR@PEG/PLA) (Mn (PEG) = 5,000, Mn (PLA) = 13,000) in chloroform (orange line). (b) GPC traces of the PLA brush detached from amphiphilic AuNR@PEG/PLA, with a molecular weight of Mn = 13,000. (c) 1H-NMR of AuNR@PEG/PLA with PEG:PLA molecular ratios of 1:3 (green line), 1:4 (black line) and 1:5 (blue line). (d) TGA characterization of AuNR@PEG/PLA (the weight fraction of the organic polymer is 13.5%). Image adapted with permission from ref. 35, American Chemical Society.
Figure 5.
Schematic illustration of the self-assembly of amphiphilic gold nanorods with mixed polymer brushes into vesicular structures.
Experimental design
Assembly behaviors of amphiphilic polymers in solution phases have been intensively investigated, including well-known assemblies such as micelles, liposomes and vesicles40. The balance of attractive force between hydrophilic and hydrophobic interactions guides the assembly of amphiphilic polymers40. By tuning the structural parameters (ratio, molecular weight and so on) of the hydrophilic and hydrophobic parts of amphiphilic polymers, the assembled structures can be well controlled. On the basis of this observation, we developed a new kind of amphiphilic gold nanocrystals coated with diblock amphiphilic or mixed hydrophilic and hydrophobic polymer brushes as building blocks, and further assembled them into vesicular structures.
This protocol first describes the rationale and experimental procedures for the synthesis of gold nanocrystals coated with amphiphilic diblock polymer brushes using a one-step ‘grafting to’ method (Fig. 1) (Step 1A). We further describe the synthesis of amphiphilic gold nanocrystals coated with mixed hydrophilic and hydrophobic polymer brushes by a tandem two-step method (Fig. 1), including ‘grafting to’ and ‘grafting from’ methods. For the two-step method, we introduce two ‘grafting from’ approaches including SI-ATRP to grow polymer brushes with hydrocarbon main chains (Step 1B) and SI-ROP to grow biodegradable polyester brushes (Step 1C). We also give a detailed procedure for self-assembling them into plasmonic vesicles using a film-rehydration method that is commonly used to prepare polymer vesicles.
The successful preparation of amphiphilic gold nanocrystals and plasmonic vesicles with excellent stability and narrow size distribution is the major task of this protocol. Because of the steric hindrance of the polymer brush on the gold nanoparticle (AuNP) surface during the polymerization process, only a certain amount of the surface initiator is active in the polymer growth, as shown in Table 2. In comparison with the method of modifying gold nanoparticles by coating with amphiphilic diblock co-polymer based on hydrophobic interactions41, our approach of modifying gold nanoparticles using covalent gold–thiol bonds exhibits several advantages and also has some limitations, as shown in Table 3.
TABLE 2.
Initiator efficiency of SI-ATRP and SI-ROP.
| Method | SI-ATRP | SI-ROP | ||||
|---|---|---|---|---|---|---|
| PEG/initiator | 1:5 | 1:10 | 1:20 | 1:23 | 1:28 | 1:37 |
| PEG/hydrophobic brush | 1:1 | 1:2 | 1:4 | 1:2.5 | 1:3.5 | 1:4.5 |
| Initiation efficiency (%) | 5 | 5.8 | 6.7 | 10.8 | 12.5 | 12.1 |
TABLE 3.
Comparison of polymer brush–coated AuNPs and amphiphilic block co-polymer–encapsulated AuNPs.
| Amphiphilic polymer brush attached to the AuNP surface | Amphiphilic block co-polymer encapsulation of AuNPs | |
|---|---|---|
| Conjugation method | Covalent gold–thiol (Au–S) bond | Hydrophobic–hydrophobic interactions41,47 |
| Advantages | Stable in organic solvent or water | Polymer thickness can be easily tuned |
| Both hydrophilic and hydrophobic polymer brushes can be attached to the AuNP surface | The polymer layer can cover the whole AuNP | |
| Can be self-assembled into various nanostructures, such as dimers and vesicles | Only amphiphilic block co-polymers are needed to encapsulate AuNPs | |
| The ratio of hydrophilic to hydrophobic polymer brushes can be easily tuned | ||
| Limitations | The polymer graft density is limited because of the steric hindrance of the polymer brush | The AuNPs should be modified with hydrophobic ligand first before encapsulation by the polymer |
| The reaction method needs longer time to grow hydrophobic polymer brushes | The AuNPs cannot disperse in solvents that can dissolve the hydrophobic polymer |
Amphiphilic gold nanoparticles grafted with amphiphilic diblock polymer brushes using the ‘grafting to’ method
A large-scale synthesis of uniform 14-nm AuNPs can be realized by reduction of HAuCl4 in the aqueous phase using sodium citrate6. The gold nanoparticles that are made this way contain citric acid molecules on the surface that can be easily replaced by small molecules or polymers with thiol-end functionalized groups. We developed a ‘grafting to’ method to prepare amphiphilic gold nanoparticles coated with amphiphilic diblock polymers38. To do this, thiolated diblock polymers are prepared using a reversible addition–fragmentation chain transfer method, and they are attached to the gold nanocrystal surface through Au–S covalent bonds37. For example, the thiol-ended polystyrene-b-PEG (SH-PS-b-PEG), can be grafted onto the surface of AuNPs using a one-step ‘grafting to’ method42. To do this, the citric acid on the AuNP surface is replaced with SH-PS-b-PEG after 12 h of ligand exchange reaction at room temperature (25 °C).
Amphiphilic gold nanoparticles grafted with mixed hydrophilic and hydrophobic polymer brushes using the SI-ATRP method
To adjust the ratio and molecular weight of the hydrophobic polymer brushes, we developed a tandem two-step method to prepare amphiphilic gold nanoparticles with mixed polymer brushes (Fig. 1)33–36. In the first (‘grafting to’) step, hydrophilic thiolated-PEG (PEG-SH) and an ATRP initiator with a disulfide group (2, 2′-dithiobis[1-(2-bromo-2-methyl-propionyloxy)] ethane (DTBE))33 are co-attached to the gold nanoparticle surface (Au@PEG/DTBE) through covalent Au–S bonds. In the following (‘grafting from’) step, Au@PEG/DTBE is used to initiate the polymerization of monomers to grow hydrophobic polymer brushes such as poly(methyl methacrylate) (PMMA)33.
For biomedical applications, we have reported the generation of pH-responsive amphiphilic gold nanoparticles coated with PEG and pH-responsive polymer brushes of poly(methyl methacrylate-co-4-vinylpyridine) (PMMAVP) by introducing 25% 4-vinylpyridine (4VP; pKa = 5.4) in the hydrophobic PMMA grafts. Compared with the one-step ‘grafting to’ method, the two-step approach can increase the graft density of the polymer brushes and control the molecular weight of the hydrophobic polymer brush. The ratio of hydrophilic to hydrophobic polymer brushes can be easily tuned by changing the feeding ratio of PEG-SH to DTBE in the first ‘grafting to’ step. The reaction vessel and equipment are shown in Figure 2.
Amphiphilic gold nanorods grafted with mixed hydrophilic and hydrophobic biodegradable polymer brushes using the SI-ROP method
Nontoxic, biocompatible and bioabsorbable materials are increasingly studied for biomedical purposes43. To further explore the biomedical applications of gold nanovesicles, we developed a new SI-ROP method to grow biodegradable polylactide (PLA) brushes on the gold nanorod (AuNR) surface35. In the first ‘grafting to’ step, PEG-SH and the SI-ROP initiator 11-mercapto-1-undecanol (MUD) are co-attached to the AuNR surface to form AuNR@PEG/MUD. In the following ‘grafting from’ step, the PLA brushes are grown on the AuNR surface using a SI-ROP method to polymerize lactic acid. Previously, stannous octanoate was widely used for the surface-initiated polymerization of lactide (LA) from iron oxide or silica nanoparticles at temperatures >100 °C. However, the Au–S bond is unstable over 60 °C, so growing well-ordered PLA brushes from gold nanocrystal surface is a challenge35. We introduced an organocatalytic ROP method to initiate the polymerization of LA; the process can be performed at 40 °C with high efficiency. The molecular weight of PLA can be controlled by the initial feeding amount of LA and the reaction time. The ratio of PEG to PLA on the AuNR surface can be easily adjusted by tuning the feeding ratio of PEG to MUD in the first ‘grafting to’ step. This method provides the possibility of tuning the plasmonic coupling and the size of vesicles by adjusting the polymer weight of PLA and the PEG-to-PLA ratio35.
Once the coated amphiphilic gold nanoparticles have been obtained, it is important to fully characterize the amphiphilic polymer–functionalized gold nanocrystals (Step 2) before they are assembled into hybrid vesicles (Step 3).
Quality control
The composition and graft density of the polymer brushes on the gold nanocrystal surface are determined based on a series of characterization methods (Figs. 3 and 4). 1H-NMR characterization of amphiphilic gold nanocrystals coated with polymers can be carried out to determine the composition of the polymer brushes (Step 2B). We use gel permeation chromatography (GPC) to analyze the molecular weight of the obtained hydrophobic polymer brushes (Step 2C). Thermogravimetric analysis (TGA) should be used to determine the weight fraction of the polymer brushes grafted to the gold nanocrystal surface (Step 2D). UV-visible spectra can be used to determine the successful growth of hydrophobic polymer brushes, SPR peak and stability of the amphiphilic gold nanocrystals (Step 2E).
Preparation of plasmonic vesicles (Step 3)
As we previously described, by tuning the structural parameters (ratio and molecular weight) of the hydrophilic and hydrophobic parts of the amphiphilic polymers, the optical properties of the assembled vesicles can be precisely tuned34,44,45. On the basis of this observation, we prepared amphiphilic gold nanocrystals coated with mixed hydrophilic and hydrophobic polymers or amphiphilic diblock polymers as building blocks, and further self-assembled them into vesicles. The vesicles were prepared using a film rehydration method that was usually used to prepare polymer vesicles (Fig. 3). This method is rather simple and can be completed within a short period of time with high yield. In our laboratory, we have prepared pH-responsive Au@PEG/PMMAVP and biodegradable AuNR@PEG/PLA vesicles to further explore their function and biomedical applications.
MATERIALS
REAGENTS
Deionized water (Millipore, Milli Q POD).
-
N,N-Dimethylformamide (DMF; Sigma-Aldrich, cat. no. 270547)
! CAUTION It is a volatile, flammable and harmful organic chemical, and it can cause skin and eye irritation.
-
Dichloromethane (DCM; Sigma-Aldrich, cat. no. 650463)
! CAUTION This organic chemical is volatile and harmful. It can cause serious skin and eye irritation and respiratory sensitization. Handle it carefully.
DMSO (Sigma-Aldrich, cat. no. D8148) ▲ CRITICAL This chemical should be kept in desiccator to avoid moisture.
Methoxy-PEG-thiol with a molecular weight of 5 kDa (PEG-SH; Laysan Bio, cat. no. MPEG-SH-5000-1g)
Hydrogen tetrachloroaurate (III) trihydrate (HAuCl4·3H2O; Sigma-Aldrich, cat. no. 520918) ▲ CRITICAL This chemical should be kept in desiccator to avoid moisture.
N,N,N′,N′,N″-pentamethyldiethylenetriamine (PMDETA; Sigma-Aldrich, cat. no. 369497).
Methyl methacrylate (MMA; Sigma-Aldrich, cat. no. W400201).
Copper(I) bromide (CuBr; Sigma-Aldrich, cat. no. 254185).
2, 2′-dithiobis[1-(2-bromo-2-methyl-propionyloxy)] ethane (DTBE; synthesized according to our previous report6)
-
Chloroform-d (CDCl3; Sigma-Aldrich, cat. no. 151858)
▲ CRITICAL This chemical should be kept in a desiccator to avoid moisture, and it should be stored at 4 °C ! CAUTION It is a volatile, flammable and harmful organic chemical that may cause skin and eye irritation.
Iodine (I2; Sigma-Aldrich, cat. no. 207772) ! CAUTION It is a volatile, flammable and harmful organic chemical that may cause eye irritation. It is harmful on contact with skin or if inhaled.
Methanol (CH3OH; Sigma-Aldrich, cat. no. 34860)
4-(Dimethylamino)pyridine (DMAP; Sigma-Aldrich, cat. no. 107700)
3,6-Dimethyl-1,4-dioxane-2,5-dione (D,L-lactide (LA); Sigma-Aldrich, cat. no. 303143)
11-Mercapto-1-undecanol (MUD; Sigma-Aldrich, cat. no. 447528)
Cetyltrimethylammonium bromide (CTAB; Sigma-Aldrich, cat. no. 1102974)
2-(2-aminoethoxy)ethanol (NH2CH2CH2OCH2CH2OH; Sigma-Aldrich, cat. no. A54059)
Tetrahydrofuran (THF; Sigma-Aldrich, cat. no. 401757)
4-Vinylpyridine (4VP; Sigma-Aldrich, cat. no. V3204)
Gold nanorods (AuNRs; Sigma-Aldrich, cat. no. 716820-25ML)
Cell Counting Kit-8 (CCK-8; Sigma-Aldrich, cat. no. 96992)
Polystyrene standards (Waters)
Aqua regia
EQUIPMENT
Transmission electron microscope (TEM; Jeol, model no. JEM 2010)
UV/visible light spectrophotometer (Genesys 10S UV-Vis, Thermo Scientifics, cat. no. 840-208100)
Benchtop centrifuge (Eppendorf centrifuge 5424, cat. no. 5424 0000.410)
Water bath sonicator (Branson, cat. no. 5510)
Scanning electron microscope (FESEM; Jeol, model no. JSM-6700F)
HPLC system (Shimadzu, model no. CBM-20A)
Spectrometer (Bruker AV300)
Particle measurement system (Malvern NANO-ZS90 Zetasizer)
Thermal analysis system (e.g., Perkin-Elmer Diamond TG/DTA)
Teflon-coated, rod-shaped magnetic stirring bars (dimensions: 12 × 3 mm; PTFE Labware, cat. no. 001.2912).
EASYpure RoDi system (Thermo Fisher Scientific)
Stopwatch
Two micropipettes (ranges: 20–200 μl and 100–1,000 ml) with appropriate disposable plastic tips (e.g., Eppendorf, cat. no. 022575296)
Indium-tin-oxide-coated glass slide (Sigma-Aldrich, cat. no. 636916)
Harrick Plasma cleaner and sterilizer (Harrick Plasma, cat. no. PDC-32G)
REAGENT SETUP
AuNRs
Prepare AuNRs by using a seed-mediated method46; purify the obtained CTAB-coated AuNRs by centrifuging (at 9,000g for 20 min at 25 °C) three times.
Ultrapure water
Prepare ultrapure water by purifying deionized (DI) water using the EASYpure RoDi system (Thermo) according to the standard protocol.
HAuCl4 solution
Dissolve 50 mg of HAuCl4·3H2O in 10 ml of ultrapure water. ▲ CRITICAL The HAuCl4 solution is light-sensitive; keep the solution bottle wrapped with aluminum foil in the dark at 4 °C until use (for up to 10 h).
I2 solution
Dissolve 6.3 mg of I2 in 5 ml of dichloromethane.
▲ CRITICAL The solution can be stored in a brown glass bottle for up to 2 d.
Lactide
LA should be recrystallized from dry ethyl acetate three times. Briefly, place 5 g of LA in a 20-ml glass bottle with a cap, and then add 7 ml of dry ethyl acetate. Place the bottle in an oil bath at 70 °C under stirring for 20 min to dissolve the LA completely. Cool the solution to 25 °C and leave it at 4 °C for 1 h. Remove the ethyl acetate from the bottle using a pipette. Dry the LA in a vacuum oven at 40 °C for 12 h.
▲ CRITICAL The obtained LA should be stored in a glass bottle with a cap at 25 °C for up to 1 d.
EQUIPMENT SETUP
TEM
In our laboratory, TEM characterization is conducted on a Jeol JEM 2010 electron microscope at an acceleration voltage of 300 kV.
GPC
In our laboratory, GPC is measured on a Shimadzu HPLC system using THF as the eluent, and the molecular weight is calibrated with polystyrene standards.
TGA
In our laboratory, samples are placed in platinum sample pans and heated under a nitrogen atmosphere at a rate of 10 °C/min to 100 °C and held for 40 min to completely remove residual solvent. Samples are then heated to 700 °C at a rate of 15 °C/min.
1H-NMR
In our laboratory, 1H-NMR spectra are obtained with a Bruker AV300 spectrometer, using CDCl3 as the solvent.
PROCEDURE
Preparation of coated amphiphilic gold nanoparticles
! CAUTION Handling of reagents and chemical reactions should be carried out with caution in designated fume hoods. Most of the reagents in this protocol are toxic or harmful, and they require the use of lab goggles, gloves and a lab coat. In some conditions, a face mask will be needed for protection.
-
1|
Prepare the coated amphiphilic gold nanoparticles as described in options A, B or C.
In option A, the preparation of amphiphilic gold nanoparticles coated with diblock polymer brushes is based on a ligand exchange method. If the polymer synthesis requires a temperature higher than 60 °C, the polymer should first be synthesized with an end-functional thiol group and then be attached to the gold nanoparticle surface using option A. The reason is that the Au–S bond is not stable at temperatures higher than 60 °C. Option B can be used to surface-initiate polymerization of monomers containing carbon–carbon double bonds that can be processed at a relatively low temperature (<60 °C) using the SI-ATRP method. Option C is a surface organocatalytic ROP method that can be used to synthesize biodegradable polyesters and polycarbonates.
-
Preparation of amphiphilic gold nanoparticles coated with diblock polymer brushes ● TIMING 12 h
-
Dissolve 5 mg of amphiphilic thiol-ended polystyrene-b-PEG (SH-PS275-b-PEG45) in 10 ml of DMF:THF or DMSO:THF (1:1, vol/vol) in a 20-ml glass bottle, and add a magnetic stir bar. The polymer should be synthesized using a reversible addition–fragmentation chain transfer polymerization method37.
▲ CRITICAL STEP Make sure that the polymers are dissolved completely.
▲ CRITICAL STEP The bottle should be a new one or it should be very clean in order to avoid contamination or aggregation of the gold nanoparticle solution.
-
To prepare 14-nm AuNPs, add sodium citrate water solution (205 mg in 4 ml DI water) to a boiling aqueous HAuCl4 solution (60 mg in 400 ml of DI water) under vigorous stirring. There should be a color change from colorless to red after 5 min. Boil the mixture for another 30 min, and then cool the resulting solution to 25 °C.
▲ CRITICAL STEP The sodium citrate solution should be added to the boiling water quickly (all at once), which is vital to the process of obtaining uniform gold nanoparticles.
■ PAUSE POINT The gold solution can be stored at 4 °C for ~6 months.
-
Centrifuge 15 ml of AuNP (14 nm) solution at 10,000g for 10 min at 25 °C, and then add 3.5 ml of DI water to dissolve the AuNPs at the bottom of the centrifuge tube. The expected concentration of the obtained AuNP solution is ~2 mg/ml.
▲ CRITICAL STEP Select a suitable centrifugal speed and time to concentrate the AuNP solution. A higher centrifugation speed may cause aggregation of the citric acid–coated AuNPs.
-
Slowly add 2 ml of concentrated AuNP solution (~2 mg/ml) to the polymer solution prepared in Step 1(A)i. This should take just less than 10 min under vigorous stirring.
▲ CRITICAL STEP The concentration of the polymer solution used here should be lower than 2 mg/ml to avoid precipitation of the polymer.
? TROUBLESHOOTING
-
Sonicate the mixture for 1 h and stir the solution for 12 h at room temperature.
? TROUBLESHOOTING
-
Purify the amphiphilic diblock polymer–modified AuNPs by centrifuging (12,000g, 10 min, 25 °C) five times, and then disperse the mixture in chloroform at a concentration of 80 nM.
▲ CRITICAL STEP Make sure that the free polymer has been removed completely.
? TROUBLESHOOTING
■ PAUSE POINT The obtained amphiphilic Au@PS-b-PEG can be stored in a glass bottle at 4 °C for over 6 months.
-
-
Preparation of amphiphilic AuNPs coated with mixed PEG and PMMA polymer brushes ● TIMING 3 d
Transfer 50 ml of as-prepared 14-nm Au nanoparticles (3.5 nM, in water) to a 100-ml glass bottle and add one new or clean stir bar.
-
Make a solution of PEG-SH (Mn = 5,000; 25 mg) and DTBE (12 mg) in DMF (2 ml; 100 μl/min) and pipette this into the AuNP solution. Stir the solution for 24 h to obtain AuNPs coated with PEG and DTBE (Au@PEG/DTBE). Magnetic stirring must be maintained during the experimental process.
▲ CRITICAL STEP Select a suitable injection speed for the mixed PEG-SH and DTBE solution. A fast injection speed may cause aggregation of the citric acid–coated AuNPs. The reaction solution should be stirred continuously.
-
Recover and purify the Au@PEG/DTBE in 5 ml of DMF by centrifuging (10,000g, 20 min, 25 °C) three times to remove the free PEG and DTBE. Disperse the obtained sample into 5 ml of DMF after each centrifugation.
By adjusting the amount of PEG and DTBE added, the ratio of PEG and hydrophobic polymer brushes can be well controlled. For instance, PEG and PNBA nanoparticles with molecular ratios of 1:1, 1:2 and 1:4 were prepared by adding weight ratios of PEG:DTBE of 4:1, 2:1 and 1:1.2, respectively.
▲ CRITICAL STEP The unbound PEG and DTBE should be removed completely. A higher centrifugation speed may cause aggregation of the Au@PEG/DTBE.
-
Add 0.14 ml of MMA and 0.6 ml of 4-vinylpyridine monomer to the initiator-coated Au@PEG/DTBE solution in DMF (4.0 ml) in a 20-ml glass bottle with a cap. Degas the mixture for 30 min with nitrogen. Figure 2a shows the equipment used for degassing with nitrogen. Nitrogen is flowed into the bottle through one plastic tube and flowed out through the other plastic tube. Vigorous stirring must be maintained during the experimental process.
▲ CRITICAL STEP Make sure that the air is completely replaced with nitrogen.
-
Mix 4 mg of CuBr and 15 mg of PMDETA in 1 ml of DMF; degas with nitrogen for 30 min, as described above. Immediately add the mixture to the Au@PEG/DTBE solution to initiate polymerization. Set the temperature to 40 °C and allow the reaction to progress (Fig. 6) for 10 h. Maintain vigorous stirring throughout the reaction, and make sure that the bottle is sealed.
▲ CRITICAL STEP The reaction temperature should not be >60 °C; otherwise, the Au–S bond will break.
? TROUBLESHOOTING
Inject 0.2 ml of methanol into the solution to terminate the reaction. Stir the mixed solution for another 30 min and then cool it to room temperature.
-
Remove the catalyst and unreacted monomer by mixing in 5 ml of DMF and centrifuging the mixture three times (12,000g, 20 min, 25 °C).
▲ CRITICAL STEP Select a suitable centrifugation speed and time to remove the catalyst, monomer and free polymer. A higher centrifugation speed may lead to aggregation of the AuNPs.
■ PAUSE POINT The purified amphiphilic gold nanoparticles coated with PEG and PMMAVP (25% 4VP in PMMAVP polymer brushes; Au@PEG/PMMAVP) can be stored in a chloroform solution at 4 °C for >2 months without changing the stability and properties of the vesicles.
-
Synthesis of amphiphilic gold nanorods grafted with mixed PEG and biodegradable PLA brushes. ● TIMING 2 d
Inject 0.2 ml of 2-(2-aminoethoxy)ethanol into 5 ml of AuNR dispersion (30 nM) under stirring. The mixed solution should be stirred for 12 h at room temperature.
-
Obtain the modified AuNRs by centrifuging the mixture twice at 9,000g for 10 min at 25 °C). Disperse the product in 5 ml of DMSO.
▲ CRITICAL STEP Select a suitable centrifugation speed and time to remove unbound 2-(2-aminoethoxy)ethanol.
-
Prepare a mixed solution of PEG and MUD. Dissolve 40 mg of PEG and 6.5 mg of MUD in 1 ml of DMSO and then add the solution to the as-prepared AuNR solution over 15 min. Allow the reaction to proceed under stirring for 12 h at room temperature.
▲ CRITICAL STEP Tune the ratio of PEG and PLA on the AuNR surface by adjusting the ratio of PEG and MUD added. To obtain amphiphilic AuNR@PEG/PLA with PEG:PLA ratios of 1:3, 1:4 and 1:5, we used PEG:MUD ratios of 5:24, 5:33 and 5:44, respectively, in the ligand exchange reaction.
Add the above mixed solution to 40 ml of chloroform to precipitate the AuNRs. Obtain the AuNRs coated with PEG and MUD (AuNR@PEG/MUD) by centrifuging the mixture twice at 3,000g for 10 min at 25 °C). Save the obtained AuNR@ PEG/MUD in 6 ml of anhydrous chloroform in a 20-ml glass bottle.
-
Add 12 mg of DMAP and 2 g of LA to the glass bottle. Degas the solution with nitrogen for 30 min.
▲ CRITICAL STEP Make sure that the air is replaced with nitrogen completely.
-
Put the bottle in a 40 °C oil bath (Fig. 6b) to initiate the polymerization of LA. Allow the reaction to proceed for 12 h.
? TROUBLESHOOTING
-
Centrifuge the solution three times at 8,000g for 10 min at 25 °C. The purified AuNR@PEG/PLA should be obtained and dispersed in chloroform.
▲ CRITICAL STEP Select a suitable centrifugation speed and time to remove the catalyst and free the polymer.
■ PAUSE POINT The obtained AuNR@PEG/PLA can be stored in 5 ml of chloroform at 4 °C for >6 months.
-
Figure 6.
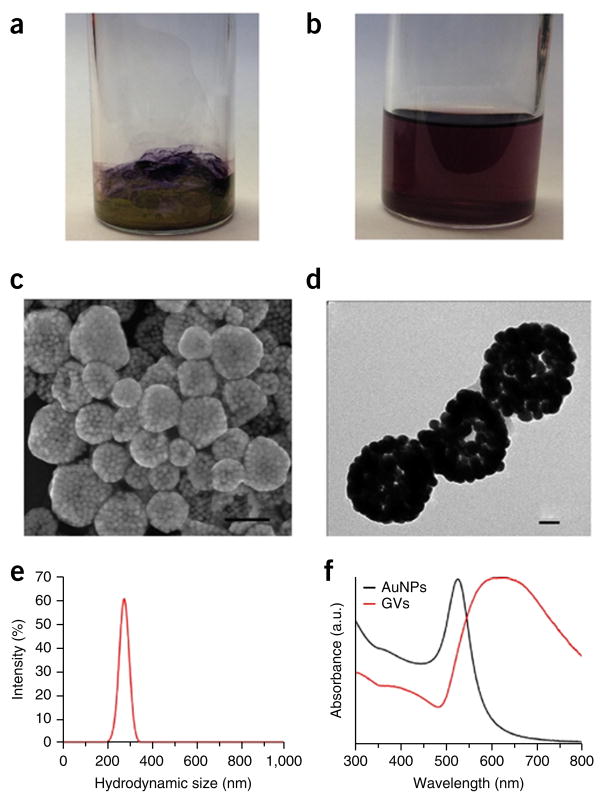
Self-assembly of vesicular structures. (a,b) Photographs of gold nanoparticles with mixed PEG and poly(methyl methacrylate) (PMMA) (Au@PEG/PMMA) film on the glass bottle surface (a) and plasmonic vesicles in water (b). (b,c) SEM (c) and TEM (d) images of the plasmonic vesicles (GVs) assembled from amphiphilic gold nanoparticles coated with PEG-b-polystyrene (Au@PEG-b-PS). Scale bars, 200 nm (c) and 50 nm (d). (e) Hydrodynamic diameter distribution of the Au@PEG-b-PS vesicles. (f) UV-visible spectra of Au@PEG-b-PS (black line) and vesicles (red line).
Quality control steps
-
2|
Before using polymer-coated gold nanocrystals for further self-assembly, it is important to ensure that they are of sufficient quality. Possible quality control tests include the stability test (option A), 1H-NMR characterization (option B), the GPC test (option C), the TGA test (option D) and UV-visible spectroscopy (option E). Perform at least two of these quality control tests before proceeding to Step 3. Because gold nanocrystals with poor surface functionalization will rapidly aggregate in chloroform or after several cycles of centrifugation, we usually use the UV-visible spectra as a quality-check approach to determine whether polymer modification is successful. Using 14-nm Au@PEG/PMMA as an example, the characterization and calculation of the mixed polymer brush density on the gold nanoparticle surface are briefly described.
-
Stability in chloroform ● TIMING 1 d
Mix 2 ml of Au@PEG/PMMA (20 nM) in chloroform. The color should be red.
The Au@PEG/PMMA should be highly stable and not show substantial aggregation after centrifugation at 10,000g for 10 min; the Au@PEG/PMMA should remain the same color (red) as citric acid–modified AuNPs that have not undergone polymer modification (Fig. 3a,b).
-
1H-NMR characterization ● TIMING 6 h
Centrifuge 20 ml of the sample (20 nM) at 12,000g for 10 min at room temperature, and remove the supernatant.
Dissolve the precipitate in 6 ml of CDCl3 and centrifuge the mixture twice at 12,000g for 10 min at 25 °C, and then remove the supernatant.
-
Use 0.8 ml of CDCl3 to dissolve the centrifuged sample under sonication for 10 min.
▲ CRITICAL STEP The sample must be completely dissolved and dispersed in CDCl3. The color of the solution should be red and no precipitation should be observed.
Use 1H-NMR to test the composition of the polymer grafts.
- Calculate the ratio of PEG to PMMA grafts on the nanocrystal surface. 1H-NMR measurement (Fig. 3c) will determine the resonance of the -CH2-CH2O- group (3.65 p.p.m.) in PEG and that of the -OCH3 group (3.60 p.p.m.) in PMMA. In our example, these have a ratio of 1:3, which leads to a molar ratio of 1:4 for ethylene glycol (EG) and the MMA monomer. Using the molecular weights of PMMA (Mn = 22 kDa) and PEG (5 kDa), the ratio of PEG to PMMA grafts can be calculated using equation 1, where MWMMA is the molecular weight of the MMA monomer and MWEG is the molecular weight of the EG monomer. In our example, the PEG:PMMA ratio is 1:2.
(1)
-
GPC characterization ● TIMING 2 d
For GPC characterization, disperse the purified 2 ml of Au@PEG/PMMA in chloroform. To make sure that the free polymer is completely removed, the chloroform solution should be collected after centrifugation and dispersed in water. If no precipitate is found after centrifugation at 1,000g for 10 min at 25 °C, the free polymer has been completely removed.
Add 0.1 ml of 5 mM iodine solution in dichloromethane to 20 ml of Au@PEG/PMMA in chloroform (30 nM). Stir the solution at room temperature for 5 h. This procedure is used to remove the polymer from the gold nanoparticle surface.
Centrifuge the solution at 5,000g for 5 min to remove the gold nanoparticles.
Add the supernatant to 80 ml of methanol under stirring.
Centrifuge the solution at 5,000g for 5 min and remove the supernatant. The PMMA should now be on the bottom of the tube. This procedure is used to remove the PEG.
Dry the PMMA using a vacuum oven at 50 °C for 24 h.
Dissolve the PMMA in THF (1 mg/ml) for the GPC test (see EQUIPMENT SETUP).
-
TGA characterization ● TIMING 2 d
Centrifuge 50 ml of Au@PEG/PMMA in chloroform (30 nM) at 12,000g for 20 min.
Remove chloroform and obtain Au@PEG/PMMA at the bottom of the centrifuge tube.
Dry the sample using an oven at 50 °C for 12 h.
-
Place the samples in platinum sample pans and heat them under a nitrogen atmosphere at a rate of 10 °C/min to 100 °C and hold for 1 min.
▲ CRITICAL STEP Make sure that the residual solvent is completely removed.
? TROUBLESHOOTING
-
Heat the sample to 700 °C at a rate of 15 °C/min.
! CAUTION The temperature is very high. Wear a lab coat, goggles and gloves during the experiment.
- Calculate the graft density of the polymer brushes on the gold nanoparticles. Given the volume of a gold atom as 0.017 nm3, the number of gold atoms (NAu atom) in a 14-nm Au nanoparticle can be calculated using equation 2, where r is the radius of the gold nanoparticles. In this equation Mn is the average molecular weight. In our example there were 84,472 gold atoms per nanoparticle, and therefore the molar mass (MAu nanoparticle) of a gold nanoparticle was 197 NAu Atom. On the basis of the molar mass of the gold nanoparticle, the ratio of PEG to PMMA, and the weight fraction obtained in the TGA analysis, the average number of polymer grafts can be calculated by equation 3, where Wpolymer is the weight fraction (21%) of the organic part, WAu nanoparticle is the weight fraction of the gold nanoparticle and MPEG+2PMMA is the sum of the molar mass of one PEG and two PMMA grafts. The result is 246 grafts per nanoparticle, which includes 82 PEG chains and 164 PMMA chains, and the graft density is ~0.4 chain per nm2.
(2) (3) Dry the sample using an oven at 50 °C for 12 h to remove the chloroform completely.
-
UV-visible absorbance spectra test ● TIMING 1 h
Measure the UV-visible spectra of the polymer-modified AuNPs. Make sure that shape of the spectrum of the polymer-modified AuNPs is consistent with that of the unmodified AuNPs, as shown in Figure 3f.
-
Look at the resulting spectrum. The peak of the polymer-modified AuNPs should have an ~10-nm red shift as compared with that of the unmodified AuNPs, because of the increased refractive index of the covalently attached polymer brushes over water.
▲ CRITICAL STEP The shape of the UV-visible spectrum of Au@PEG/MMA is similar to that of the citric-acid-modified gold nanoparticles, indicating that the Au@PEG/MMA is not aggregated.
-
Preparation of plasmonic vesicles ● TIMING 2 d
▲ CRITICAL We describe the preparation of pH-responsive plasmonic vesicles using the film rehydration method as an example. It helps to further prepare different responsive vesicles. Choose the method that is most appropriate for your application.
-
3|
Add a 0.5 ml-solution (80 nM for gold nanospheres or 20 nM for gold nanorods) of Au@PEG/PMMA, Au@PEG/PMMAVP, Au@PEG-b-PS or AuNR@PEG/PLA in chloroform to a 10-ml glass vial.
-
4|
In a model system, dry the amphiphilic Au@PEG/PMMAVP slowly on the wall of a glass vial under a gentle flow of N2 gas. A gold film on the glass vial surface should be seen (Fig. 6a).
! CAUTION The flow speed of the N2 should be controlled to let the Au@PEG/PMMA slowly adsorb on the surface of the glass vial.
-
5|
Add 2 ml of DI water to the glass vial. Sonicate at 30 °C to rehydrate the film. In 2–5 min, the solution should become clear, indicating that vesicles have formed (Fig. 6b).
▲ CRITICAL STEP Make sure that the vial is at the best position in the water bath sonicator to ensure the most efficient sonication. The temperature of the water bath should be adjusted between 30 and 35 °C.
? TROUBLESHOOTING
-
6|
Save the as-prepared vesicles in water at 4 °C for further use. The vesicles can also be dispersed in basic buffer or neutral solutions.
■ PAUSE POINT The obtained plasmonic gold nanovesicles can be stored at 4 °C for at least 2 months without obvious change in their structure and properties. The vesicles have shown excellent colloidal stability in basic buffer or neutral solutions. Although they settle to the bottom of the storage vial in a week, which is commonly observed for heavy gold colloids, a homogeneous dispersion can be readily recovered with gentle shaking for a few seconds.
Characterization of plasmonic vesicles ● TIMING 3 d
-
7|
Acquire SEM image of the vesicles. Samples for SEM are prepared by casting 5–10 μl of vesicle aqueous solution onto an indium-tin-oxide-coated glass slide and drying it at room temperature. Image the sample using your instrument’s operating procedures (typically at 10 kV). See Figures 6c, 7 and 8 for typical SEM images of three kinds of vesicles.
-
8|
Acquire TEM images of the vesicles. TEM grids are first treated with oxygen plasma in a Harrick plasma cleaner and sterilizer for 1 min to improve their surface hydrophilicity. A drop of vesicle solution is carefully added to the surface of the TEM grid. After drying in air, it is used for TEM characterization. See Figures 6d, 7a and 8b for representative TEM images of the vesicles.
-
9|
Record UV-visible spectra of the as-prepared vesicles. Follow your instrument’s operating procedures. See Figures 6f, 7c and 8c for the UV-visible spectra of the vesicles.
-
10|
Record the diameter and size distribution of the as-prepared vesicles in water. Follow your instrument’s operating procedures. See Figures 6e, 7d and 8d for the dynamic light scattering results of the vesicles.
Figure 7.
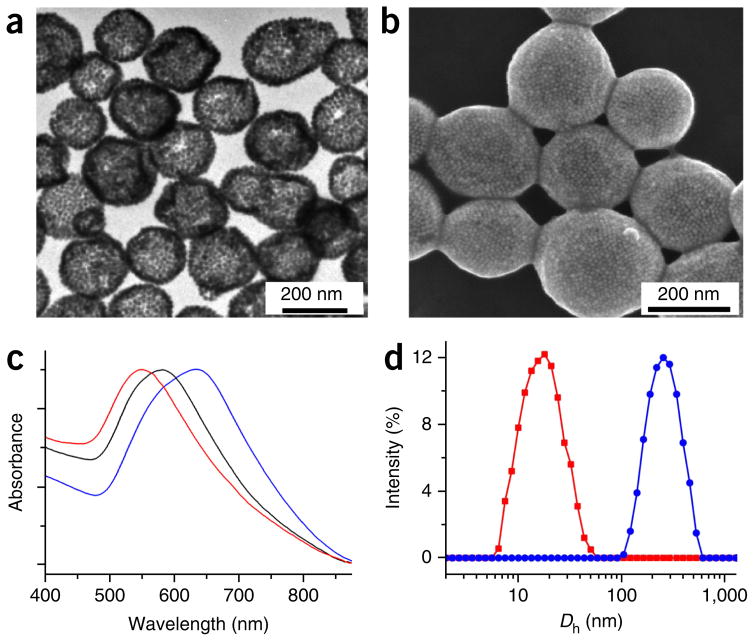
Characterization of gold nanovesicles. (a,b) SEM (a) and TEM (b) images of the Au@PEG/PMMA vesicles. (c) UV-visible spectra of the amphiphilic Au@PEG/PMMA in chloroform (red line) and the vesicles in water with different ratios of PEG and PMMA (black and blue lines). (d) Hydrodynamic diameter distribution (Dn) of the gold nanoparticles (red line) and Au@PEG/PMMA vesicles (blue line).
Figure 8.
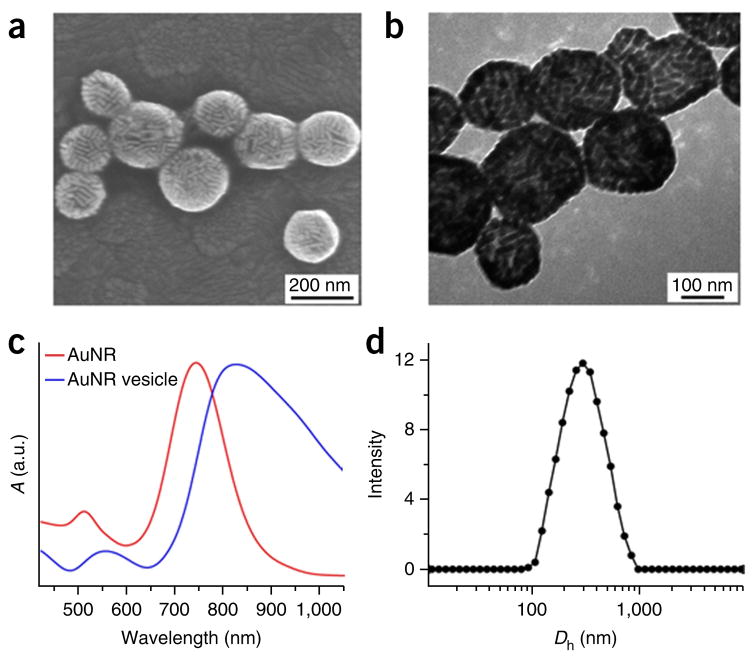
Characterization of AuNR@PEG/PLA vesicles. (a,b) SEM (a) and TEM (b) images of the plasmonic vesicles assembled from amphiphilic gold nanorods coated with PEG and polylactide (AuNR@PEG/PLA). (c) UV-visible spectra of the amphiphilic AuNR@PEG/PLA in chloroform (red line) and the plasmonic AuNR@PEG/PLA vesicles in water (blue line). (d) Hydrodynamic diameter distribution (Dn) of the plasmonic AuNR@PEG/PLA vesicles. c adapted with permission from ref. 35, American Chemical Society.
In vitro cytotoxicity evaluation of plasmonic vesicles ● TIMING 2–3 d
-
11|
In a model system, use a standard Cell Counting Kit-8 (CCK-8) to analyze the cytotoxicity of Au@PEG/PMMAVP vesicles following a general protocol. To do this using an MDA-MB-435 cell, seed the cells in the central 60 wells of the 96-well plate at a concentration of 5 × 104 cells per well. Incubate at 37 °C for 24 h.
-
12|
Add 100 μl of Au@PEG/PMMAVP vesicles at a final concentration of 0.25, 0.5, 1 or 2 nM and incubate the cells for 2, 4, 8, 16 and 24 h.
-
13|
Add 10 μl of CCK-8 solution to each well of the 96-well plate and incubate for another 4 h. Determine cell viability by measuring the absorbance of each well at 450 nm using a microplate reader.
Plasmonic vesicles for bioimaging application ● TIMING 2 d
-
14|
Seed and allow cancer cells to adhere to glass slides in the cell culture medium for 24 h.
-
15|
Incubate 100 μl of Au@PEG/PMMAVP vesicles (0.2 nM) in 4 ml of culture medium with the cells for 30 min.
-
16|
Pipette the unbound sample out. Add 100 μl of PBS to the well.
-
17|
Use an Olympus IX71 inverted microscope with an oil-immersion dark-field condenser for dark-field imaging.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 4.
TABLE 4.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 1A(iv) | Gold nanoparticle aggregation | The injection speed for the polymer solution is too high | Add the solution at a speed of 50 μl/min, and sonicate the solution for 1 h after the addition. The glass bottle must be cleaned with aqua regia for 2 h and with DI water three times |
| 1A(v) | Polymer precipitation on the glass bottom | The volume of AuNP solution is too small | The volume of the AuNP solution should be >4 ml |
| 1A(vi) | AuNPs aggregate, and the solution color changes to blue | The centrifugation speed is too high or the centrifugation time is too long | The centrifuge speed cannot be >12,000g, and the centrifugation time cannot be >20 min. Sonication can be used to assist the dispersion of the AuNPs |
| 1B(v) | The solution color changes to blue | The air in the glass reaction bottle is not completely removed, as shown in Figure 2c | Make sure that the glass reaction bottle is tightly capped, or change to a new cap |
| 1C(vi) | AuNRs are not dispersed well | The reaction temperature is too low or the solution was not sonicated during the reaction process | Adjust the reaction temperature to 40 °C to initiate the polymerization of LA. The reaction bottle should be sonicated for 1 min every 10 min during the first 1 h of the reaction |
| 2D(iv) | The sample cannot be removed from the tube | The centrifugation speed is too low, and the sample adheres to the tube wall | Increase the centrifugation speed and the time to precipitate the sample to the bottom of the centrifuge tube |
| 5 | The solution is not clear | Vesicles are not formed, or the sonication time is not long enough | Shake the bottle during the sonication. If the film is still on the wall of the glass bottle after 2 min of sonication, the water should be removed completely. Add 5 μl of chloroform to the glass bottle to rinse the film, followed by 1 ml of water and sonication |
● TIMING
Step 1A, preparation of amphiphilic gold nanoparticles coated with diblock polymer brushes: 12 h
Step 1B, preparation of amphiphilic AuNPs coated with mixed PEG and PMMA, polymer brushes: 3 d
Step 1C, synthesis of amphiphilic gold nanorods grafted with mixed PEG and biodegradable PLA brushes: 2 d
Step 2A, chloroform stability test: 1 d
Step 2B, 1H-NMR characterization: 6 h
Step 2C, GPC characterization: 2 d
Step 2D, TGA characterization: 2 d
Step 2E, UV-visible absorbance spectra test: 1 h
Steps 3–6, preparation of plasmonic vesicles: 2 d
Steps 7–10, characterization of plasmonic vesicles: 3 d
Steps 11–13, in vitro cytotoxicity evaluation of plasmonic vesicles: 2–3 d
Steps 14–17, plasmonic vesicles for bioimaging application: 2 d
ANTICIPATED RESULTS
Amphiphilic gold nanoparticles coated with polymer brushes
All amphiphilic polymer–modified gold nanocrystals prepared in Steps 1–3 can be dissolved in chloroform and DMF (Fig. 3b), and stored for >2 months at 4 °C. Compared with AuNPs coated with citric acid or AuNRs coated with CTAB (Step 1C(i)), the polymer-modified gold nanocrystals resulting from Step 1C have UV-visible absorbance at longer wavelengths (Figs. 3f and 4a).
For the amphiphilic gold nanocrystals coated with mixed polymer brushes, the ratio of hydrophilic and hydrophobic polymer brushes can be easily tuned by changing the amounts of PEG and ATRP initiator DTBE or ROP initiator MUD added in the ‘grafting to’ step. The molecular weight of the hydrophobic polymers can be easily controlled by tuning the reaction time and the amount of added monomers (Fig. 4c). Using the two-step method, different kinds of responsive hydrophobic polymer brushes with carbon chains or biodegradable chains can be grown on the gold nanocrystal surface, as displayed in Table 1.
1H-NMR was used to confirm the successful attachment and composition of the polymer brushes (Figs. 3c and 4c). GPC was used to further test the polymer weight of the polymer brush (Figs. 3d and 4b). TGA was used to measure the polymer weight fraction of the amphiphilic AuNPs (Figs. 3e and 4d). The combined tests can be used to calculate the graft density of the polymer brushes. For the amphiphilic Au@PEG/PMMA, the graft density of the polymer is 0.4 chain per nm2.
The high drug-loading efficiency and excellent photothermal effect of the plasmonic vesicles enable effective imaging-guided integrated chemo-thermal cancer therapy.
Plasmonic gold nanovesicles
In the vesicle, hydrophobic polymer–coated AuNP forms the vesicle shell and the hydrophilic polymer extends to both the interior and exterior of this vesicle (Fig. 5). Thus, the vesicles can be well dispersed in water. Because the vesicular shell is composed of hydrophobic polymers, the relative ratio of hydrophilic to hydrophobic polymers, and the molecular weight can affect the interparticle distance. Because of the strong plasmonic coupling of the AuNPs in the vesicular shell, the LSPR peak of the vesicle shows a red shift as compared with solutions containing individual AuNPs (Fig. 6f). Thus, by tuning the interparticle distance in the shell, the LSPR peak of the amphiphilic gold nanoparticle vesicles can be easily adjusted from the visible to the near-infrared region38. The adjustable ratio and molecular weight of the hydrophilic and hydrophobic polymer brushes provide the possibility of tuning the LSPR peak of the plasmonic vesicle to fit the requirements of different applications.
TEM and SEM are used to determine whether the vesicles have a hollow structure (Figs. 6c,d, 7a,b and 8a,b), determined by observations of less contrast of the interior as compared with the shell of the vesicle in TEM images and holes observed in disrupted vesicles in SEM images. Dynamic light scattering is used to measure the size of the vesicles (Figs. 6e, 7d and 8d); this protocol generates vesicles with a narrow size distribution of ~220 nm. UV-visible spectra are used to examine the absorption maximum of the vesicles, which shows a substantial red shift of the SPR peak as compared with that of single gold nanocrystals34–36 (Figs. 6f, 7c and 8c).
Acknowledgments
This work was supported by the intramural research program (IRP) of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the US National Institutes of Health (NIH) and the National Science Foundation of China (grant 81401465 to P. Huang).
Footnotes
AUTHOR CONTRIBUTIONS J.S. and X.C. conceived and designed the research; J.S. performed the experiments and contributed new reagents and analytical tools; J.S. and P.H. analyzed the data; and J.S. and X.C. wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Graham D, Thompson DG, Smith WE, Faulds K. Control of enhanced Raman scattering using a DNA-based assembly process of dye-coded nanoparticles. Nat Nanotechnol. 2008;3:548–551. doi: 10.1038/nnano.2008.189. [DOI] [PubMed] [Google Scholar]
- 2.Halas NJ. Plasmonics: an emerging field fostered by nano letters. Nano Lett. 2010;10:3816–3822. doi: 10.1021/nl1032342. [DOI] [PubMed] [Google Scholar]
- 3.Halas NJ, Lal S, Chang WS, Link S, Nordlander P. Plasmons in strongly coupled metallic nanostructures. Chem Rev. 2011;111:3913–3961. doi: 10.1021/cr200061k. [DOI] [PubMed] [Google Scholar]
- 4.Hutter E, Fendler JH. Exploitation of localized surface plasmon resonance. Adv Mater. 2004;16:1685–1706. [Google Scholar]
- 5.Skrabalak SE, Au L, Li X, Xia Y. Facile synthesis of Ag nanocubes and Au nanocages. Nat Protoc. 2007;2:2182–2190. doi: 10.1038/nprot.2007.326. [DOI] [PubMed] [Google Scholar]
- 6.Cheng L, Liu AP, Peng S, Duan HW. Responsive plasmonic assemblies of amphiphilic nanocrystals at oil-water interfaces. ACS Nano. 2010;4:6098–6104. doi: 10.1021/nn101685q. [DOI] [PubMed] [Google Scholar]
- 7.Giljohann DA, et al. Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim DK, et al. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat Nanotechnol. 2011;6:452–460. doi: 10.1038/nnano.2011.79. [DOI] [PubMed] [Google Scholar]
- 9.Morton SM, Silverstein DW, Jensen L. Theoretical studies of plasmonics using electronic structure methods. Chem Rev. 2011;111:3962–3994. doi: 10.1021/cr100265f. [DOI] [PubMed] [Google Scholar]
- 10.Rosi NL, Mirkin CA. Nanostructures in biodiagnostics. Chem Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 11.Klinkova A, Choueiri RM, Kumacheva E. Self-assembled plasmonic nanostructures. Chem Soc Rev. 2014;43:3976–3991. doi: 10.1039/c3cs60341e. [DOI] [PubMed] [Google Scholar]
- 12.Kneipp J, Kneipp H, Kneipp K. SERS-a single-molecule and nanoscale tool for bioanalytics. Chem Soc Rev. 2008;37:1052–1060. doi: 10.1039/b708459p. [DOI] [PubMed] [Google Scholar]
- 13.Cheng L, Song J, Yin J, Duan H. Self-assembled plasmonic dimers of amphiphilic gold nanocrystals. J Phys Chem Lett. 2011:2258–2262. [Google Scholar]
- 14.He J, et al. Self-assembly of amphiphilic plasmonic micelle-like nanoparticles in selective solvents. J Am Chem Soc. 2013;135:7974–7984. doi: 10.1021/ja402015s. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, et al. Regiospecific plasmonic assemblies for in situ Raman spectroscopy in live cells. J Am Chem Soc. 2011;134:1699–1709. doi: 10.1021/ja2088713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C, et al. Controlling assembly of paired gold clusters within apoferritin nanoreactor for in vivo kidney targeting and biomedical imaging. J Am Chem Soc. 2011;133:8617–8624. doi: 10.1021/ja200746p. [DOI] [PubMed] [Google Scholar]
- 17.Kim NH, Lee SJ, Moskovits M. Reversible tuning of SERS hot spots with aptamers. Adv Mater. 2011;23:4152–4156. doi: 10.1002/adma.201101847. [DOI] [PubMed] [Google Scholar]
- 18.Murthy VS, Cha JN, Stucky GD, Wong MS. Charge-driven flocculation of poly(L-lysine)gold nanoparticle assemblies leading to hollow microspheres. J Am Chem Soc. 2004;126:5292–5299. doi: 10.1021/ja038953v. [DOI] [PubMed] [Google Scholar]
- 19.Pornpattananangkul D, et al. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J Am Chem Soc. 2011;133:4132–4139. doi: 10.1021/ja111110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasch MR, et al. Hydrophobic gold nanoparticle self-assembly with phosphatidylcholine lipid: membrane-loaded and janus vesicles. Nano Lett. 2010;10:3733–3739. doi: 10.1021/nl102387n. [DOI] [PubMed] [Google Scholar]
- 21.Maye MM, Chun SC, Han L, Rabinovich D, Zhong CJ. Novel spherical assembly of gold nanoparticles mediated by a tetradentate thioether. J Am Chem Soc. 2002;124:4958–4959. doi: 10.1021/ja025724k. [DOI] [PubMed] [Google Scholar]
- 22.Han X, Liu Y, Yin Y. Colorimetric stress memory sensor based on disassembly of gold nanoparticle chains. Nano Lett. 2014;14:2466–2470. doi: 10.1021/nl500144k. [DOI] [PubMed] [Google Scholar]
- 23.Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc Chem Res. 2011;44:1094–1104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- 24.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297:967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 25.Tanner P, et al. Polymeric vesicles: from drug carriers to nanoreactors and artificial organelles. Acc Chem Res. 2011;44:1039–1049. doi: 10.1021/ar200036k. [DOI] [PubMed] [Google Scholar]
- 26.Discher BM, Hammer DA, Bates FS, Discher DE. Polymer vesicles in various media. Curr Opin Chem Biol. 2000;5:125–131. [Google Scholar]
- 27.Zhuang J, Gordon MR, Ventura J, Li L, Thayumanavan S. Multi-stimuli responsive macromolecules and their assemblies. Chem Soc Rev. 2013;42:7421–7435. doi: 10.1039/c3cs60094g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vigderman L, Khanal BP, Zubarev ER. Functional gold nanorods: synthesis, self-assembly, and sensing applications. Adv Mater. 2012;24:4811–4841. doi: 10.1002/adma.201201690. [DOI] [PubMed] [Google Scholar]
- 29.Shenhar R, Norsten TB, Rotello VM. Polymer-mediated nanoparticle assembly: structural control and applications. Adv Mater. 2005;17:657–669. [Google Scholar]
- 30.Tang Z, Kotov NA. One-dimensional assemblies of nanoparticles: preparation, properties, and promise. Adv Mater. 2005;17:951–962. [Google Scholar]
- 31.Amstad E, Kim SH, Weitz DA. Photo- and thermoresponsive polymersomes for triggered release. Angew Chem Int Ed Engl. 2012;124:12667–12671. doi: 10.1002/anie.201206531. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Smith AE, Lokitz BS, McCormick CL. In situ formation of gold-’decorated’ vesicles from a RAFT-synthesized, thermally responsive block copolymer. Macromolecules. 2007;40:8524–8526. [Google Scholar]
- 33.Song J, et al. Plasmonic vesicles of amphiphilic gold nanocrystals: self-assembly and external-stimuli-triggered destruction. J Am Chem Soc. 2011;133:10760–10763. doi: 10.1021/ja204387w. [DOI] [PubMed] [Google Scholar]
- 34.Song J, et al. Photolabile plasmonic vesicles assembled from amphiphilic gold nanoparticles for remote-controlled traceable drug delivery. Nanoscale. 2013;5:5816–5824. doi: 10.1039/c3nr01350b. [DOI] [PubMed] [Google Scholar]
- 35.Song J, Pu L, Zhou J, Duan B, Duan H. Biodegradable theranostic plasmonic vesicles of amphiphilic gold nanorods. ACS Nano. 2013;7:9947–9960. doi: 10.1021/nn403846v. [DOI] [PubMed] [Google Scholar]
- 36.Song J, Zhou J, Duan H. Self-assembled plasmonic vesicles of SERS-encoded amphiphilic gold nanoparticles for cancer cell targeting and traceable intracellular drug delivery. J Am Chem Soc. 2012;134:13458–13469. doi: 10.1021/ja305154a. [DOI] [PubMed] [Google Scholar]
- 37.Lin J, et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano. 2013;7:5320–5329. doi: 10.1021/nn4011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang P, et al. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew Chem Int Ed Engl. 2013;52:13958–13964. doi: 10.1002/anie.201308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nederberg F, Connor EF, Moller M, Glauser T, Hedrick JL. New paradigms for organic catalysts: the first organocatalytic living polymerization. Angew Chem Int Ed Engl. 2001;40:2712–2715. doi: 10.1002/1521-3773(20010716)40:14<2712::AID-ANIE2712>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Xu H, Zhang X. Tuning the amphiphilicity of building blocks: controlled self-assembly and disassembly for functional supramolecular materials. Adv Mater. 2009;21:2849–2864. [Google Scholar]
- 41.Kang Y, Taton TA. Controlling shell thickness in core–shell gold nanoparticles via surface-templated adsorption of block copolymer surfactants. Macromolecules. 2005;38:6115–6121. [Google Scholar]
- 42.He J, Liu Y, Babu T, Wei Z, Nie Z. Self-assembly of inorganic nanoparticle vesicles and tubules driven by tethered linear block copolymers. J Am Chem Soc. 2012;134:11342–11345. doi: 10.1021/ja3032295. [DOI] [PubMed] [Google Scholar]
- 43.Sinha Ray S. Polylactide-based bionanocomposites: a promising class of hybrid materials. Acc Chem Res. 2012;45:1710–1720. doi: 10.1021/ar3000376. [DOI] [PubMed] [Google Scholar]
- 44.Jouault N, Lee D, Zhao D, Kumar SK. Block-copolymer-mediated nanoparticle dispersion and assembly in polymer nanocomposites. Adv Mater. 2014;26:4031–4036. doi: 10.1002/adma.201305641. [DOI] [PubMed] [Google Scholar]
- 45.Zubarev ER, Xu J, Sayyad A, Gibson JD. Amphiphilic gold nanoparticles with V-shaped arms. J Am Chem Soc. 2006;128:4958–4959. doi: 10.1021/ja060782h. [DOI] [PubMed] [Google Scholar]
- 46.Nikoobakht B, El-Sayed MA. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater. 2003;15:1957–1962. [Google Scholar]
- 47.Kang Y, Taton T. Core/shell gold nanoparticles by self-assembly and crosslinking of micellar, block-copolymer shells. Angew Chem Int Ed Engl. 2005;44:409–412. doi: 10.1002/anie.200461119. [DOI] [PubMed] [Google Scholar]