Abstract
Background
Matrix metalloproteinases (MMPs), which show a significant ability to cleave the components of extracellular matrix, and tissue inhibitors of metalloproteinases (TIMPs), which slow down the activity of those enzymes, may be implicated in the pathogenesis and spread of psoriatic disease. This study aims to analyze plasma levels of MMP-2 and TIMP-2 in plaque psoriasis patients before and after the course of narrowband ultraviolet-B (NBUVB) therapy with respect to disease advancement.
Patients and methods
A total of 49 patients suffering from plaque psoriasis and 40 healthy volunteers were enrolled into the study. Plasma levels of MMP-2 and TIMP-2 were determined using enzyme-linked immunosorbent assay, while Psoriasis Area and Severity Index (PASI) was used to define the disease advancement.
Results
The results showed increased plasma levels of MMP-2 and TIMP-2, but this change was significant only in case of MMP-2 in total psoriatic group compared to healthy subjects. Moreover, there was an increase in the concentrations of chosen factors with an increase in the severity of the disease. The NBUVB therapy causes a decline in the concentration of the analyzed enzyme and its inhibitor, although this change was statistically significant in the total psoriatic group only in case of MMP-2. There was also a positive correlation between MMP-2, TIMP-2, and PASI score value.
Conclusion
Our study highlights a possible important role of MMP-2 in the activity of psoriasis and clearance of disease symptoms. Moreover, plasma MMP-2 seems to be a valuable psoriasis biomarker.
Keywords: gelatinase A, matrix metalloproteinases, tissue inhibitor of metalloproteinases, NBUVB therapy
Background
Psoriasis is characterized as a chronic, inflammatory skin disease driven by disturbed function of immunological system.1 The analyzed condition affects ~3% of global population, depending on geographical region and ethnicity.2–3 What is important, although psoriasis in not a life-threatening condition, is it has a profound negative impact on the patient’s quality of life.4
The most characteristic histopathological symptoms of this disease include abnormal differentiation and hyperproliferation of keratinocytes, leukocyte infiltrations to dermis, and expansion of vessels.5 Despite intensive research on disease etiology, the key factor causing psoriasis is still unknown. Scientists assumed that the occurrence of the described condition can be associated with genetic predisposition and environmental triggering factors as well as other disorders.3
Numerous studies have demonstrated a variety of factors which play a potential role in the pathogenesis of psoriasis. They include metalloproteinases (MMPs) – enzymes responsible for the integrity of extracellular matrix (ECM).6 The appropriate level of these parameters is fundamental for the maintenance of tissue homeostasis and skin cell functioning in extreme conditions.7 MMP-2 (gelatinase A), stimulated by interleukin-18 (IL-18), has been found to be very important in early progression of psoriasis.8 The remarkable role of the MMP-2 in this process includes the modification of ECM and basal membrane (BM), stimulation of cell migration, angiogenesis, and tissue remodeling activation.9
TIMP-1, -2, -3, and -4 form a group of tissue inhibitors of metalloproteinases (TIMPs). The main function of these proteins is inhibiting adverse effects caused by MMP overexpression.10 Furthermore, it was found that in physiological conditions, the MMP:TIMP ratio is highly regulated. The imbalance in the MMP:TIMP ratio value may result in pathological production and cleaving of ECM and BM components.11
NBUVB therapy for psoriasis was first used in 1988. Since then, it has become the most popular treatment with one of the highest satisfaction rates in psoriasis lesion improvement. NBUVB phototherapy as the second-line treatment for psoriasis, recommended when the topical therapy fails, is contraindicated or impractical. UVB radiations of wavelength 311 nm may result in the clearance of disease symptoms after just 5–8 weeks.12
The primary objective of this article was to determine the plasma levels of MMP-2 and TIMP-2 in psoriatic patients and the influence of NBUVB phototherapy course on the concentration of these parameters. We also conducted this study to examine the association between the levels of the tested enzyme, its inhibitor and psoriasis severity.
Patients and methods
Table 1 presents the details of the tested groups. This prospective study included 49 plaque psoriasis patients whose condition was diagnosed and treated in the Department of Dermatology, Division Outpatient Care, University Hospital, Bialystok, Poland, between 2013 and 2015. Assigning the participants to specific groups of disease advancement was carried out based on Psoriasis Area and Severity Index (PASI) formulated by Fredriksson and Pettersson.13 Furthermore, patients with mild advancement of the disease were divided into two subgroups: Ia (PASI <5) and Ib (PASI 5–10).
Table 1.
Characteristics of psoriatic patients and control group
| Study group | Number of patients |
|---|---|
| Psoriasis patients | |
| Plaque type | 49 (23 females, 26 males) |
| Median age (range) | 39 (18–76) |
| Severity | |
| Mild | 34 |
| PASI <5 | 15 |
| PASI 5–10 | 19 |
| Medium | 15 |
| PASI 10–30 | 15 |
| Control group | |
| Healthy subjects | 40 (19 females, 21 males) |
| Median age (range) | 36 (18–75) |
Abbreviation: PASI, Psoriasis Area and Severity Index.
All patients underwent a course of NBUVB phototherapy composed of 20 sessions. Considering the fact that this kind of treatment is suitable for patients with mild and medium psoriasis advancement, the study included the patients with PASI score between 2 and 30.
The control group consisted of 40 healthy volunteers who had never suffered from any autoimmunological or chronic dermatological diseases.
Moreover, a key exclusion criterion in both groups was not undergoing any additional pharmacotherapy.
All patients gave their written informed consent for the participation in the study. The research was approved by the Biochemical Committee of the Medical University of Bialystok, number R-I-002/74/2014.
Radiation source
The whole-body irradiation was performed in a Cosmedico GP-36 phototherapy cabinet (Cosmedico Medical System, Stuttgart, Germany), equipped with NBUVB 311 nm TL-01 100 W tubes (HEINE.MED, Baden-Württemberg, Germany). The energy output was measured with a standard intrinsic UV-meter.
Phototherapy protocol
Individual minimum erythema doses of NBUVB were determined before starting the exposure to prevent burns. The patients underwent 2–3 treatments weekly (to the total of 20), starting with a NBUVB dose of 0.018–0.025 J⁄cm2, and increasing by ~10%–20% per week, up to a maximum dose of 2.015 J⁄cm2.
Biochemical analyses
Venous blood samples were collected twice from each patient – before the beginning and after the last (20th) NBUVB phototherapy treatment – into a heparin sodium tube, centrifuged for 15 minutes at 1,000× g to obtain plasma samples and stored at −85°C until further analysis. The tested parameters (MMP-2 and TIMP-2) were measured using the enzyme-linked immunosorbent assay (Quantikine Human HGFs Immunoassay, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. Duplicate samples were assessed for each patient.
The intra-assay coefficient of variation (CV [%]) of MMP-2 was reported to be 3.6% at a mean concentration of 22.8 ng/mL (standard deviation [SD] =0.828), and for TIMP-2 was found to be 3.0% at a mean concentration of 6.09 ng/mL (SD =0.181).
The inter-assay coefficient of variation (CV [%]) of MMP-2 was reported to be 7.0% at a mean concentration of 21.5 ng/mL (SD =1.51), and for TIMP-2 was found to be 7.3% at a mean concentration of 6.38 ng/mL (SD =0.467).
The assay showed no significant cross-reactivity or interference with numerous human cytokines and other growth factors.
Statistical analysis
The statistical analysis was performed using Program STATISTICA 12.0 PL. A preliminary statistical analysis revealed that MMP-2 and TIMP-2 failed to follow a normal distribution. Consequently, the Mann–Whitney U test was used for statistical analysis between the psoriatic patients and the control group. Additionally, statistical analysis between the groups with different degrees of psoriasis was performed with the use of Bonferroni correction. Moreover, the Wilcoxon matched-pair test was used for statistical analysis of changes in parameters between the beginning and the end of the NBUVB phototherapy treatment. The data were presented as median, mean, and range. The Spearman rank correlation was used in the correlation analysis. Statistically significant differences were defined as comparisons resulting in P<0.05.
Results
The mean PASI score of patients enrolled in the study was 8.53 (range 2.0–25.0). After the completion of the treatment, the mean PASI decreased to 4.74 (range 0–18.2). The mean PASI improvement was 52%.
Table 2 presents the median, mean, and range of plasma levels for the investigated parameters in the tested groups. The median of MMP-2 and TIMP-2 in the total psoriatic group before the beginning of NBUVB phototherapy was increased when compared to healthy individuals, but this difference was statistically significant only in case of MMP-2.
Table 2.
Plasma levels of the tested parameter in psoriatic patients and in control group
| Groups tested | MMP-2 (ng/mL)
|
TIMP-2 (ng/mL)
|
||
|---|---|---|---|---|
| Before treatment | After the treatment | Before treatment | After the treatment | |
| Psoriatic patients | ||||
| Total psoriatic group |
aP=0.000075 dP=0.01125 |
|||
| Median | 185.52 | 176.94 | 81.83 | 80.95 |
| Mean | 213.86 | 189.56 | 86.77 | 85.65 |
| Range | 122.75–458.06 | 109.10–330.13 | 51.37–169.32 | 44.36–182.03 |
| Mild | bP=0.000754 | bP=0.021454 | bP=0.018598 | |
| Median | 171.23 | 168.58 | 75.06 | 77.04 |
| Mean | 188.72 | 173.88 | 81.50 | 84.51 |
| Range | 122.75–398.72 | 109.10–330.13 | 51.37–134.64 | 44.36–182.03 |
| Mild – subgroup Ia | cP=0.000397 | cP=0.008131 | cP=0.004204 | |
| Median | 142.05 | 144.09 | 72.72 | 73.07 |
| Mean | 165.77 | 153.46 | 75.02 | 89.48 |
| Range | 122.75–347.37 | 109.10–270.98 | 51.37–117.77 | 48.52–165.45 |
| Mild – subgroup Ib | ||||
| Median | 183.59 | 176.94 | 78.94 | 78.01 |
| Mean | 206.84 | 190.01 | 86.62 | 80.59 |
| Range | 165.31–398.72 | 115.80–330.13 | 53.03–134.64 | 44.36–182.03 |
| Medium |
aP=0.000005 dP=0.010594 |
aP=0.001811 |
aP=0.02378 dP=0.026757 |
|
| Median | 246.72 | 237.35 | 93.16 | 89.23 |
| Mean | 270.84 | 225.09 | 98.71 | 88.22 |
| Range | 176.78–458.06 | 119.83–290.16 | 66.26–169.32 | 60.52–150.30 |
| Control group | ||||
| Healthy subjects | ||||
| Median | 181.45 | 80.44 | ||
| Mean | 160.44 | 77.87 | ||
| Range | 32.50–270.40 | 42.50–105.00 | ||
Notes:
Statistically significant when psoriatic patients are compared with healthy subjects (P<0.05);
statistically significant when patients with mild scales are compared to subjects with medium lesions (P<0.05);
statistically significant when subgroup Ia is compared to medium cases (P<0.05);
statistically significant when pretreatment concentration is compared to posttreatment levels (P<0.05).
Abbreviations: MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinases.
After the division of the total group based on disease severity, we found significantly higher concentrations of MMP-2 and TIMP-2 in the patients with a moderate advancement of the disease compared to those with mild scales and those in control group. Additionally, after the division of mild group based on PASI score, we detected significantly decreased levels of MMP-2 and TIMP-2 in the subgroup Ia when compared to the individuals with moderate advancement of psoriasis.
The Spearman rank correlation was used for the dependence analyses of the investigated parameters. We observed a positive correlation between baseline concentrations of MMP-2 and TIMP-2 in patients suffering from psoriasis (Figure 1). Moreover, there was a positive correlation between baseline levels of MMP-2 and PASI score in these individuals (Figure 2). Furthermore, the same dependency was found between baseline TIMP-2 concentration and PASI value (Figure 3) in psoriatic patients. We also detected a negative correlation between MMP-2 concentration and the age of participants (R=−0.31).
Figure 1.
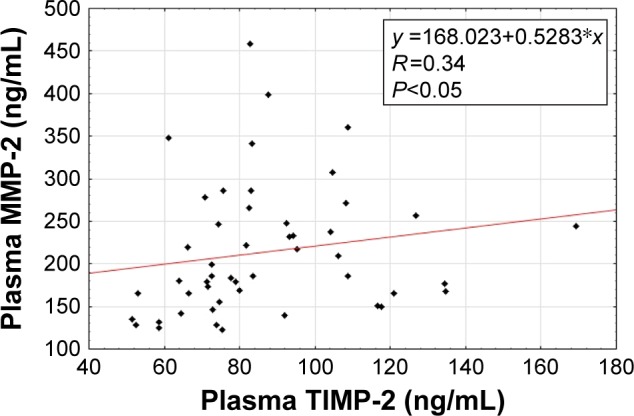
Correlation of baseline plasma metalloproteinase-2 (MMP-2) with baseline plasma tissue inhibitor of metalloproteinases-2 (TIMP-2) in patients with psoriasis.
Figure 2.
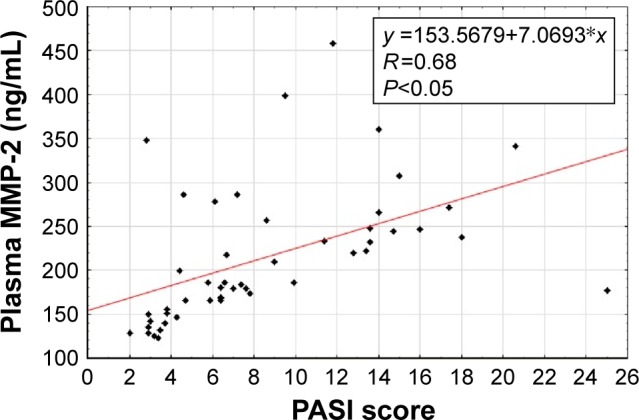
Correlation of baseline plasma metalloproteinase-2 (MMP-2) with baseline Psoriasis Area and Severity Index (PASI) in patients with psoriasis.
Figure 3.
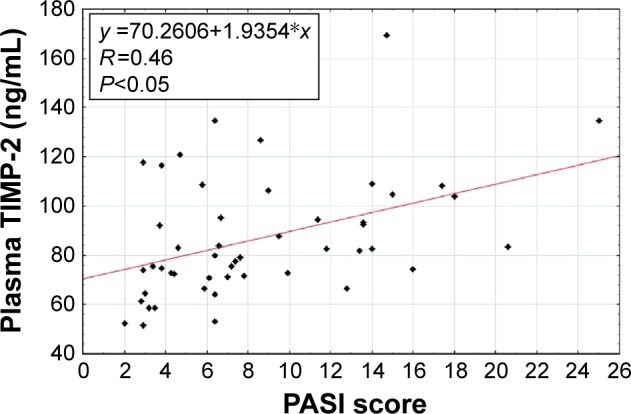
Correlation of baseline plasma tissue inhibitor of metalloproteinases-2 (TIMP-2) with baseline Psoriasis Area and Severity Index (PASI) in patients with psoriasis.
After the completion of the NBUVB therapy, following dependences were noted: positive correlation between MMP-2 levels and PASI score (P<0.05, R=0.60) and between TIMP-2 levels and PASI score (P<0.05, R=0.30); however, no correlation between concentrations of the investigated parameters was observed.
The course of NBUVB phototherapy resulted in changes in the levels of MMP-2 and TIMP-2. After 20 radiations, the plasma value of MMP-2 in the total psoriatic group was significantly lower compared to pretreatment values. The concentration of TIMP-2 in the total psoriatic group also dropped, but this change was not statistically significant. Furthermore, we noticed significantly declined levels of the chosen enzyme and its inhibitor in patients with moderate disease advancement, but not in the mild group or mild subgroups after successful treatment.
Posttreatment values of MMP-2 and TIMP-2 in the total psoriatic group were not significantly different from those detected in the healthy subjects. Similar to the concentrations of the analyzed parameters present before NBUVB radiation, we detected significantly higher concentrations of MMP-2 and TIMP-2 in patients with a moderate disease advancement compared to healthy individuals.
Discussion
MMP-2 is a member of MMPs subfamily, known as gelatinases. This enzyme is produced by fibroblasts, endothelial cells, keratinocytes, infiltrating immune cells, and also by cancer cells in pathological conditions. It presents a very broad specificity. Gelatinase A is able to degrade ECM and BM components, such as collagen type I, IV, V, VII, X, XI, gelatins, gelectin 3, elastin, laminin 1, and fibronectin.9,14 The studies performed so far underline an important role for MMP-2 in a number of physiological processes, which include angiogenesis, embryogenesis, and wound healing.6 On the other hand, increased concentrations of this enzyme were detected in patients suffering from different kinds of cancers, Alzheimer disease, and rheumatic or cardiovascular diseases.15–18
The overexpression of MMPs is impeded by TIMPs. Available literature indicates that TIMP-2 is the most effective inhibitor of membrane-type MMPs.19 The functions of this protein also include matrix binding, angiogenesis downregulation and apoptosis induction. Furthermore, it was demonstrated that TIMPs are able to stimulate keratinocyte growth.9 Importantly, maintenance of the correct balance between concentrations and activities of the components of MMPs subfamily as well as between MMPs and TIMPs is the key factor that determines ECM and BM stability.11
During the course of psoriasis, eruption of 20 million of the total 28–30 million body lymphocytes, which migrate to the sites of the damage, significantly modifies epidermal cellular composition. The dermis comprises an inflammatory infiltrate made of lymphocytes, macrophages, mast cells, and neutrophils.5,20 The synthesis of MMPs, which was earlier described in large number of studies, may result from the abnormal cytokine production (eg, tumor necrosis factor-alpha, IL-1, interferon-g [IFN-g], IL-6, transforming growth factor-alpha, epidermal growth factor) by the above-mentioned cells.21
Feliciani et al were the first scientists to detect that significant overexpression of MMP-2 was not accompanied by an upregulated expression of TIMP-1 or TIMP-2 in psoriatic skin. According to this information, they suspect a potential role for the analyzed enzyme in psoriatic skin remodeling.22 With regard to the available literature, a number of research groups also reported MMP-2 overexpression in psoriatic epidermis, but the value of this increased expression remains contradictory.9 In contrast, Sidhom et al23 showed a decreased, while Fleischmajer et al24 showed an increased expression of TIMP-2 in the skin affected by psoriasis disease.
In this study, we detected significantly higher plasma concentrations of MMP-2, but not TIMP-2, in psoriatic patients compared to healthy subjects. Our results related to MMP-2 are in line with the data obtained by Guan et al in the serum of psoriatic patients.25 Moreover, there have also been a few studies concerning other MMPs and TIMPs in psoriatic patients. Flisiak et al showed significantly increased levels of MMP-1 and TIMP-1 in the plasma of psoriatic subjects compared to healthy controls.26 Significantly higher serum concentrations of MMP-3 and MMP-9 in psoriatic patients were also observed by other authors.27,28
Our analyses demonstrated a strong positive correlation of MMP-2 level with PASI score, which means that the spread of psoriatic scale is accompanied by an intensive increase in the concentration of gelatinase A. Furthermore, positive correlation between TIMP-2 concentration and PASI score was detected; however, its value was definitely lower. It was the first study to present this dependence. Other authors detected the analogous tendency between plasma levels of TIMP-1 and PASI score, while MMP-1 levels were negatively correlated with PASI value in psoriatic scales.26
Moreover, we observed positive correlation between MMP-2 and TIMP-2 concentrations, yet the value of this dependence was also low. These results can be a strong evidence for the fact that elevated concentration of MMP-2, which increases with the advancement of psoriasis with no adequate increase of its tissue inhibitor, promotes the formation of new vessels and, as a consequence, the spread of psoriatic lesions.
Psoriasis begins and develops through angiogenesis in the superficial dermal microvasculature. Dermal papillary capillaries become elongated and are characterized by tortuosity, dilatation, and permeability. These morphological disorders occur before the lesions become visible on the epidermis.29 As we mentioned earlier, MMP-2, as well as other MMPs, presents the ability to promote angiogenesis,30,31 while TIMP-2 shows antiangiogenic effects.32 In view of this information, our finding seems to be vital. Elevated concentration of gelatinase A, which increases with the advancement of psoriasis with no significant higher levels of its tissue inhibitor, promotes the formation of new vessels, and as a consequence of this process, the spread of the analyzed condition occurs.
Usefulness and effectiveness of NBUVB phototherapy in the treatment of psoriasis and many other skin disorders are determined by a multisite action of this kind of treatment, which includes alteration of the cytokine profile, induction of apoptosis, and immunosuppression promotion.12,33 Furthermore, clinical improvement of psoriasis by NBUVB is related to the downregulation of IL-17 pathway and also type I and type II IFN signaling pathway which is vital in the pathogenesis of the disease. In addition, clinically effective NBUVB therapy involves the suppression of numerous significant molecular pathways in psoriatic skin.34,35
In this study, we established that NBUVB phototherapy course decreased the concentrations of MMP-2 and TIMP-2, but this change was statistically significant in the total psoriatic group only in case of MMP-2. Similar effects of NBUVB treatment on MMP-2 level were detected in psoriatic lesions.36
There have been a few studies describing changes in MMP concentrations in psoriatic individuals following other kinds of treatment. Flisiak et al detected that typical application of 5% salicyl ointment resulted in a decrease in the plasma levels of MMP-1 and TIMP-1 in psoriatic patients.37 The same tendency has been demonstrated following UVA phototherapy on MMP-1 concentration.38
This is the first study to show that the levels of MMP-2 and TIMP-2 rise with the increase in disease advancement and decline analogously after a successful treatment. The changes occurring after the course of NBUVB therapy are most visible in case of mild advancement of psoriasis in most expanded healing process.
Conclusion
Elevated concentration of MMP-2 during the course of psoriasis eruption and its significant decrease after the clearance of disease symptoms following a successful NBUVB treatment indicate that this enzyme is implicated in the pathogenesis and expansion of the analyzed condition. Consequently, plasma MMP-2 seems to be a good candidate for a biomarker of psoriasis.
Acknowledgment
This work was supported by grants from the Medical University of Białystok (153-30590F and 153-30591F).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665–675. doi: 10.1016/j.rdc.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Queiro R, Tejón P, Alonso S, Coto P. Age at disease onset: a key factor for understanding psoriatic disease. Rheumatology. 2014;53:1178–1185. doi: 10.1093/rheumatology/ket363. [DOI] [PubMed] [Google Scholar]
- 3.Huerta C, Rivero E, Rodríguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143:1559–1565. doi: 10.1001/archderm.143.12.1559. [DOI] [PubMed] [Google Scholar]
- 4.Kim GE, Seidler E, Kimball AB. A measure of chronic quality of life predicts socioeconomic and medical outcomes in psoriasis patients. J Eur Acad Dermatol Venereol. 2015;29:249–254. doi: 10.1111/jdv.12503. [DOI] [PubMed] [Google Scholar]
- 5.Ayala-Fontánez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis: Targets Ther. 2016;6:7–32. doi: 10.2147/PTT.S64950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravi Kanth VV, Nageshwar Reddy D. Role of matrix metalloproteinases in physiological processes & disease. Indian J Med Res. 2014;140:585–587. [PMC free article] [PubMed] [Google Scholar]
- 7.Moore CS, Crocker SJ. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am J Pathol. 2012;180:12–16. doi: 10.1016/j.ajpath.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Vasku V, Bienertova Vasku J, Slonková V. Matrix metalloproteinase-2 promoter variability in psoriasis. Arch Dermatol Res. 2009;301:467–473. doi: 10.1007/s00403-009-0947-5. [DOI] [PubMed] [Google Scholar]
- 9.Mezentsev A, Nikolaev A, Bruskin S. Matrix metalloproteinases and their role in psoriasis. Gene. 2014;540:1–10. doi: 10.1016/j.gene.2014.01.068. [DOI] [PubMed] [Google Scholar]
- 10.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 11.Giannelli G, Erriquez R, Fransvea E, et al. Proteolytic imbalance is reversed after therapeutic surgery in breast cancer patients. Int J Cancer. 2004;109:782–785. doi: 10.1002/ijc.20009. [DOI] [PubMed] [Google Scholar]
- 12.Racz E, Prens EP. Phototherapy and photochemotherapy for psoriasis. Dermatol Clin. 2015;33:79–89. doi: 10.1016/j.det.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Fredriksson T, Pettersson U. Severe psoriasis oral therapy with a new retinoid. Dermatologica. 1978;157:238–244. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 14.Lipka D, Boratyński J. Metalloproteinases: structure and function. Postepy Hig Med Dośw. 2007;62:328–336. Polish. [PubMed] [Google Scholar]
- 15.Fink K, Boratyński J. The role of metalloproteinases in modification of extracellular matrix in invasive tumor growth, metastasis and angiogenesis. Postepy Hig Med Dośw. 2012;66:609–628. doi: 10.5604/17322693.1009705. Polish. [DOI] [PubMed] [Google Scholar]
- 16.Olewicz-Gawlik A, Hrycaj P, Łącki JK. Metalloproteinases in the pathogenesis of systemic inflammatory rheumatic diseases. Ru. 2005;43:280–285. [Google Scholar]
- 17.Mieszało K, Ławicki S, Szmitkowski M. The utility of metalloproteinases (MMPs) and their inhibitors (TIMPs) in diagnostics of gynecological malignancies. Pol Merkur Lekarski. 2016;40:193–197. Polish. [PubMed] [Google Scholar]
- 18.Giannelli G, Erriquez R, Iannone F, Marinosci F, Lapadula G, Antonaci S. MMP-2, MMP-9, TIMP-1 and TIMP-2 levels in patients with rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol. 2004;22:335–338. [PubMed] [Google Scholar]
- 19.Guinea-Viniegra J, Zenz R, Scheuch H, et al. TNFalpha shedding and epidermal inflammation are controlled by Jun proteins. Genes Dev. 2009;23:2663–2674. doi: 10.1101/gad.543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidenreich R, Röcken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol. 2009;90:232–248. doi: 10.1111/j.1365-2613.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eysteinsdóttir JH, Sigurgeirsson B, Ólafsson JH, et al. The role of Th17/Tc17 peripheral blood T cells in psoriasis and their positive therapeutic response. Scand J Immunol. 2013;78:529–537. doi: 10.1111/sji.12114. [DOI] [PubMed] [Google Scholar]
- 22.Feliciani C, Vitullo P, D’orazi G, et al. The 72-kDa and the 92-kDa gelatinases, but not their inhibitors TIMP-1 and TIMP-2, are expressed in early psoriatic lesions. Exp Dermatol. 1997;6:321–327. doi: 10.1111/j.1600-0625.1997.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 23.Sidhom E, Pilmane M, Kisis J. The distribution of matrix metalloproteinase-2, tissue inhibitor of metalloproteinase-2 and tissue inhibitor of metalloproteinase-4 in psoriatic skin. Br J Med Med Res. 2015;8:883–890. [Google Scholar]
- 24.Fleischmajer R, Kuroda K, Hazan R, et al. Basement membrane alterations in psoriasis are accompanied by epidermal overexpression of MMP-2 and its inhibitor TIMP-2. J Invest Dermatol. 2000;115:771–777. doi: 10.1046/j.1523-1747.2000.00138.x. [DOI] [PubMed] [Google Scholar]
- 25.Guan Q, Xiong ZG, Li HJ. Serum concentration of MMP-2, MMP-9, and IL-18 in the patients with Psoriasis Vulgaris and its clinical significance. Chin J Lab Diag. 2012;7:011. [Google Scholar]
- 26.Flisiak I, Porebski P, Chodynicka B. Effect of psoriasis activity on metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in plasma and lesional scales. Acta Derm Venereol. 2006;86:17–21. doi: 10.1080/00015550510011600. [DOI] [PubMed] [Google Scholar]
- 27.Altrichter S, Boodstein N, Maurer M. Matrix metalloproteinase-9: a novel biomarker for monitoring disease activity in patients with chronic urticaria patients? Allergy. 2009;64:652–656. doi: 10.1111/j.1398-9995.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- 28.Chandran Y, Cook RJ, Edwin J, et al. Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology (Oxford) 2010;49:1399–1405. doi: 10.1093/rheumatology/keq105. [DOI] [PubMed] [Google Scholar]
- 29.Gronski TJ, Martin RL, Kobayashi DK, et al. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem. 1997;272:12189–12194. doi: 10.1074/jbc.272.18.12189. [DOI] [PubMed] [Google Scholar]
- 30.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo SY, Kwon SM. Angiogenesis and its therapeutic opportunities. Mediators Inflamm. 2013;2013:e127170. doi: 10.1155/2013/127170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stetler-Stevenson WG, Seo DW. TIMP-2: an endogenous inhibitor of angiogenesis. Trends Mol Med. 2005;11:97–103. doi: 10.1016/j.molmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Reich A, Mędrek K. Effects of narrow band UVB (311 nm) irradiation on epidermal cells. Int J Mol Sci. 2013;14:8456–8466. doi: 10.3390/ijms14048456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rácz E, Prens EP, Kurek D. Effective treatment of psoriasis with narrow-band UVB phototherapy is linked to suppression of the IFN and Th17 pathways. J Invest Dermatol. 2011;131:1547–1558. doi: 10.1038/jid.2011.53. [DOI] [PubMed] [Google Scholar]
- 35.Richard EG, Hönigsmann H. Phototherapy, psoriasis, and the age of biologics. Photodermatol Photoimmunol Photomed. 2014;30:3–7. doi: 10.1111/phpp.12088. [DOI] [PubMed] [Google Scholar]
- 36.FuMin L, Xue W, XiLing D, et al. Action mechanism of narrow-band ultraviolet B on vascular regulatory factors in psoriasis vulgaris. Chin J Dermatol. 2009;42:163–166. [Google Scholar]
- 37.Flisiak I, Myśliwiec H, Chodynicka B. Effect of psoriasis treatment on plasma concentrations of metalloproteinase-1 and tissue inhibitor of metalloproteinases-1. J Eur Acad Dermatol Venereol. 2005;19:418–421. doi: 10.1111/j.1468-3083.2005.01199.x. [DOI] [PubMed] [Google Scholar]
- 38.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]


