Abstract
Background
The neoplasm is still a potential threat for breast, Non-Small Cell Lung (NSCL) and cervix cancer patients. Those gradually invade into other body organs, inducing complex pathological complications. Whereas, the anticancer drugs suppress the bone marrow, resulting serious hematological toxicities. Thus, the monocytic toxicity may the chance of infections, particularly in AID’s patients.
Objective
We aimed this retrospective study to investigate the monocytopenia induced by vinorelbine following chemotherapy in cancer patients.
Patients and method
A total 60 adult cancer patients were divided into two groups; Group-1 patients received the treatment of Vinorelbine alone while group 2 patients received Vinorelbine based combination chemotherapy.
Result
The overall comparison of mean monocyte count (×103 per μl) with time showed a significant statistical difference (p value <0.001) for G-I and no significant difference for G-II (p value <0.08). The independent comparison of mean values for two groups at every week confirms the non-significant statistical difference during all of the five weeks (p values 0.551, 0.112, 0.559, 0.372, 0.468 respectively). In addition of that, the comparison of mean values observed before therapy with that of week 4 (after therapy) showed significant difference in G-I (p value <0.001) and non-significant in G-II (p value 0.053).
Conclusion
Monocytopenia is induced in both of the chemotherapy protocols allows the clinical oncologists and consultant physicians to select either of the chemotherapy protocol. The therapeutic efficacy should constitute the intervening consideration to treat the breast, cervix and NSCL (Non-Small Cell Lung’s) cancers.
Keywords: Monocytopenia, Vinorelbine, Cisplatin, Doxorubicin, Breast Cancer, NSCLC
Introduction
Cancer is one of the major life threatening diseases which is mysterious and often feared by mankind. The surprising advancement in applied therapeutics still cannot assure successful treatment of this condition. The chemotherapeutic agents used to treat cancer exert deleterious effects along with benefits. Monocytopenia is one of the unwanted side effects of chemotherapy. Monocyte is a type of white blood cell and part of the human body’s immune system. [1] Monocytes are stored in spleen in clusters in red pulp’s Cords of Billroth and classified as the classical monocyte (CD14++ monocyte) and the non-classical, pro-inflammatory monocyte. [2]
Monocytopenia is a form of leukocytopenia associated with a deficiency of monocytes. [2] The major causes of this condition include use of myelotoxic drugs, acute infectious stress, aplastic anemia, hairy cell leukemia and myeloid leukemia. [3] There are several types and stages of chemotherapy and chemotherapeutic agents used to treat cancer. Vinorelbine (anti-mitotic) drug is used for the treating a wide range of cancer including non-small cell lung cancer. It is a semi-synthetic vinca alkaloid extracted from Catharanthus roseus. Cisplatin, cisplatinum, or cis-diamminedichloroplatinum (II) (CDDP) is a platinum-based drug used for the treatment of sarcomas, carcinomas, lymphomas, and germ cell tumors. These platinum complexes bind to and cause cross linking of DNA which ultimately triggers apoptosis. Doxorubicin is an anthracycline antibiotic, which works by intercalating DNA. It was originally isolated from bacteria found in soil samples taken from Castel del Monte, an Italian castle, in 1950’s. It is commonly used in the treatment of a wide range of cancers, including hematological malignancies, many types of carcinoma, and soft tissue sarcomas. [4]
Thus, we aimed this study to determine effective treatment to minimize the toxicity in certain cancerous conditions such as NSCL cancer, metastatic breast cancer, and cervical cancer. The consultant oncologist use vinorelbine as alone and in combination to treat certain neoplasm. Hence, we aimed this project to investigate the monocytopenia induced pre & post chemotherapy to evaluate the most current clinical value of this protocol.
Materials and methods
The study was conducted at Shaukat Khanum Memorial Cancer Hospital & the Research Center (SKMCH&RC), M.A Johar town, Lahore, Pakistan to investigate the changes in monocyte count of adult cancer patients with Non-small cell lung cancer, metastatic breast cancer, and of the cervix, treated with Vinorelbine alone, Vinorelbine/Doxorubicin and Vinorelbine/Cisplatin treatment protocols.
Study Design
For this retrospective study the patients were selected from the outpatient department (OPD) of SKMCH&RC, diagnosed as breast cancer, NSCLC and cancer of cervix. An exclusion criterion is involvement of patients in any other study. A total 60 cancer patients were divided into two groups; Group-1 comprised of cancer patients who received vinorelbine as single therapy and Group-2 comprised of cancer patients on treatment protocol of vinorelbine based combinations i.e. Vinorelbine/ Cisplatin or vinorelbine/ Doxorubicin (Table 1).
Table 1.
The chemotherapy protocols follow up schedule and cancer site of experimental patients
| Group | Sample size | Chemotherapy protocol | Patient neoplasm type | Chemotherapy schedule (days) | Follow up schedule (days) |
|---|---|---|---|---|---|
| G-I | 45 | Vinorelbine | Metastatic breast cancer | 1, 7, 14, 21 | 6, 13, 20, 28 |
| NSCL Cancer | 1, 7, 14, 21 | 6, 13, 20, 28 | |||
| G-II | 15 | Vinorelbine/Doxurubicin | Metastatic breast cancer | 1, 8 | 7, 15 |
| Vinorelbine/Cisplatin | NSCL Cancer | 1, 8 | 7, 15 | ||
| Cervix Cancer | 1, 8 | 7, 15 |
Preparations of Standard Regimen of Chemotherapeutic Agents
The standard treatment regimen including dosing of vinorelbine, cisplatin and doxorubicin in either Group 1 or Group 2 were given appropriately. In brief, the vinorelbine was administered intravenously in the dose of 25 mg/m2 on day 1 (week-1) and day 21 (week 3), with 0.45% sodium chloride or 5% glucose solution as diluents and delivered over intravenous push (IVP) as reported by Taha et al. [5] The injected dose was infused over a short time period between 15 to 20 minutes. In combination therapy, the dose of Vinorelbine was decreased and administered intravenously as 20 mg/m2 on day 1 and 8 with diluents as described above. On day 5 ½ normal saline was delivered over IVP. The doxorubicin was given as 50 mg/m2 on day 1 only and it was administered slowly into tubing of freely running infusion of 0.9% Sodium Chloride or 5% Glucose as described previously. [6] While, the Cisplatin was administered intravenously with a dose of 40mg/m2 on day 1 and with the diluents (normal saline) on day 5 ½ and was delivered over IVP. [7]
Sample Collection and Monocyte Count
The 3 ml of blood samples were drawn from brachial veins in 5 cc disposal syringes and transferred to appropriately labeled (complete blood count (C.B.C) vials containing 2.0 % w/v of EDTA. The monocyte count was performed using a computerized auto-analyzer (Technicon 113, Bayer Laboratories, USA) at the Pathology laboratory, SKMCH&RC.
Data Analysis
The means of two groups were compared by student t-test to avoid the consistent deviation of analytical results or systematic errors in the procedure. ANOVA used to identify any factor influencing the test results.
Results
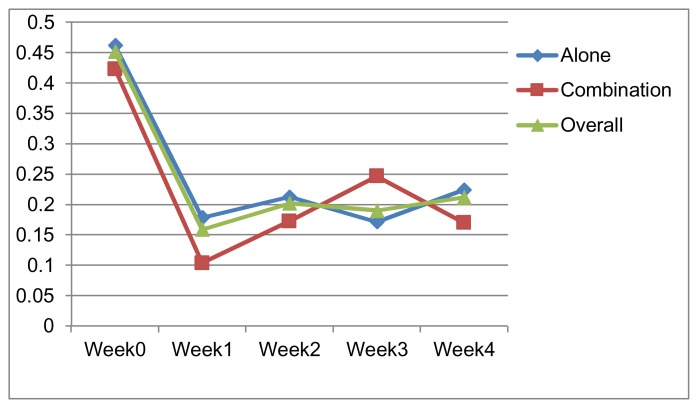
The effect of different treatments on the monocyte count is given in Table 2. On week 1, 2 and 4 of the treatment no significant difference in monocytopenia was observed (Figure 1). However, as shown by the monocyte count on week 3, significant potential for monocytopenia was noticed. When the mean monocytic counts before therapy (Week 0) were compared with that of after therapy (week 4), there a significant decrease was noted in the patients on treatment protocol of vinorelbine alone only.
Table 2.
The mean ± SEM Monocyte count (×103) per μl, Pre and post chemotherapy of cancer patients on the treatment protocol of vinorelbine (Group I), vinorelbine based combinations(Group II) and overall total (60) patients.
| Time (week) | Monocyte count (×103) per μl in Group-I | Monocyte count (×103) per μl Group-II | Overall Monocyte count (×103) per μl | P value2 | |||
|---|---|---|---|---|---|---|---|
| Mean | ± SEM | Mean | ± SEM | Mean | ± SEM | ||
| Week 0 | 0.4621 | 0.0343490 | 0.42330 | 0.0495568 | 0.4519 | 0.0283423 | 0.551 |
| Week 1 | 0.1786 | 0.0197340 | 0.10400 | 0.0544390 | 0.1593 | 0.0204869 | 0.112 |
| Week 2 | 0.2129 | 0.0346626 | 0.17266 | 0.0621555 | 0.2021 | 0.0301487 | 0.559 |
| Week 3 | 0.1716 | 0.0247280 | 0.24666 | 0.0774140 | 0.1900 | 0.0265393 | 0.372 |
| Week 4 | 0.2246 | 0.0380977 | 0.17000 | 0.0474040 | 0.2120 | 0.0312281 | 0.468 |
| P value1 | <0.001 | 0.08 | <0.001 | ||||
| P value3 | <0.001 | 0.053 | |||||
P value1 -represent an overall comparison of mean values over time,
P value2 -represent the independent comparison of mean values for two groups at every week
P value3 -represent a comparison of mean values observed before therapy with that of week 4 (after therapy)
p< 0.001 considered extremely significant and p < 0.05 considered significant
All values are expressed in Mean ± SEM, n = 60
Figure 1.
Mean Monocyte Count (x 103/ ml) Vs time (in weeks).
Discussion
The finding under discussion are in line with Cao et al, [8] who reported similar incidences of leucopenia with different response rate of two vinorelbine combinations; Vinorelbine+cisplatin+rh-endostatin and Vinorelbine+cisplatin. The advanced treatment of NSCLC with platinum-based combination improves the response rate without potential hematological toxicity. However, the response rates have a similar outcome.
The results of our study are in line with Kondo et al., [9] reported the correlation of monocytopenia with cisplatin. The patients of advanced lung cancer taking chemotherapy of cisplatin based combinations at 3 – 4 week intervals were analyzed retrospectively. The mean monocyte count among 80% of the patients was significantly lower. Moreover, all 75% of the patients have a monocyte count of less than 150/microlitre despite of the absence of a correlation between the leukocyte count on days 6 to 8.
Our finding substantiated by Ciobotaro et al., [10] reported the leukocytopenia induced by doxorubicin. They described the non-significant change in cell surface level of CD63, CD49d and CD11a–c after chemotherapy. However, a significant reduction in the membrane level of CD18 was demonstrated in polymorphonuclear cells after doxorubicin (p<0.005), suggesting a direct effect of doxorubicin rather than an apoptosis-associated phenomenon. Furthermore an expected leukocytopenia 10 days after doxorubicin administration with no correlation to apoptosis, suggesting that leukocytopenia by doxorubicin is largely attributed to toxicity of blood progenitors.
Tubiana et al., [11] reported combination of vinorelbine as first-line regimen for metastatic breast cancer. The possibility of a longer duration of chemotherapy and prolonged infusion free survival has made it an attractive regimen in first line for Human Epidermal growth factor Receptor 2 (HER2) negative metastatic breast cancer patients. While Marty et al., [21] reported leucopenia as noncumulative side effect of short duration (<7 days). Shamseddine et al, [12] reported the acceptable hematological toxicities induced by cisplatin and Vinorelbine combination.
Oostendorp et al., [13] reported the therapeutical potential of vinorelbine combination with doxorubicin in clinical practice. They selected the breast cancer patient because of its wide range anticancer benefits. The studies randomly compared the drugs in combination with targeted agents to provide reasonable scientific evidences for therapeutical usage in advanced breast cancer patients.
Conclusion
In conclusion, the monocytopenia induced in both of the chemotherapy protocols allows the clinical oncologists and consultant physicians to select either of the chemotherapy protocol. The therapeutic efficacy should constitute the intervening consideration to treat the breast, cervix and NSCL cancers.
Abbreviations
- CBC
Complete Blood Count
- CDDP
Cis-Diamminedichloroplatinum (II)
- CD14++
Cluster of differentiation 14 monocyte
- DLT
Dose-Limiting Toxicity
- EDTA
Ethylenediaminetetraacetic Acid
- OPD
Outpatient Department
- NSCLC
Non-Small Cell Lung Cancer
- SKMCH&RC
Shaukat Khanum Memorial Cancer Hospital & Research Center
References
- 1.Forget MA, Voorhees JL, Cole SL, Dakhlallah D, Patterson IL, Gross AC, Moldovan L, Mo X, Evans R, Marsh CB, Eubank TD. Macrophage colony-stimulating factor augments Tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer. PLoS One. 2014 Jun 3;9(6):e98623. doi: 10.1371/journal.pone.0098623. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Ziegler-Heitbrock L. The CD14+ CD16+ Blood Monocytes: their Role in Infection and Inflammation, Review. J Leukocyte Biology. 2007;81:584. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 3.Oguz A, Karadeniz C, Ckitak EC, Cil V. Which one is a risk factor for chemotherapy-induced febrile neutropenia in childhood solid tumors: Early lymphopenia or monocytopenia. Pediatr Hematol Oncol. 2006;23(2):143–51. doi: 10.1080/08880010500457673. [DOI] [PubMed] [Google Scholar]
- 4.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A. Identification of Splenic Reservoir Monocytes and Their Deployment to Inflammatory Sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nazir T, Islam A, Omer MO, Mustafa M. Lymphocytopenia; induced by vinorelbine, doxorubicin and cisplatin in human cancer patients. Breast Dis. 2015;35(1):1–4. doi: 10.3233/BD-140386. [DOI] [PubMed] [Google Scholar]
- 6.Kondo M, Oshita F, Kato Y, Yamada K, Nomura I, Noda K. Early monocytopenia after chemotherapy as a risk factor for neutropenia. Am J Clin Oncol. 1999;22:103–5. doi: 10.1097/00000421-199902000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Hirai F, Seto T, Shimokawa M, Inamasu E, Toyozawa R, Toyokawa G, Yoshida T, Shiraishi Y, Takenaka T, Yamaguchi M, Takenoyama M, Ichinose Y. Split-dose cisplatin and vinorelbine as adjuvant chemotherapy for completely resected non-small cell lung cancer. Anticancer Res. 2014 Feb;34(2):927–31. [PubMed] [Google Scholar]
- 8.Cao D, Ge W, Wang H, Zhang L, Zheng Y, Zhang J. Efficacy and safety of rh-endostatin combined with chemotherapy versus chemotherapy alone for advanced NSCLC: A meta-analysis review. Zhongguo Fei Ai Za Zhi. 2011 May 20;14(5):404–13. doi: 10.3779/j.issn.1009-3419.2011.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo M, Oshita F, Kato Y, Yamada K, Nomura I, Noda K. Early monocytopenia after chemotherapy as a risk factor for neutropenia. Am J Clin Oncol. 1999;22(1):103–5. doi: 10.1097/00000421-199902000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Ciobotaro P, Drucker L, Neumann A, Shapiro H, Shapira J, Radnay J, Lishner M. The effects of doxorubicin on apoptosis and adhesion molecules of normal peripheral blood leukocytes-an ex vivo study. Anticancer Drugs. 2003 Jun;14(5):383–9. doi: 10.1097/00001813-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Tubiana-Mathieu N, Bougnoux P, Becquart D, Chan A, Conte PF, Majois F, Espie M, Morand M, Vaissiere N, Villanova G. All-oral combination of oral vinorelbine and capecitabine as first-line chemotherapy in HER2-negative metastatic breast cancer: An International Phase II Trial. British Journal of Cancer. 2009;101:232–237. doi: 10.1038/sj.bjc.6605156. www.bjcancer.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamseddin AI, Taher A, Dabaja B, Dandashi A, Salem Z, Saghir EL. Combination Cisplatine Vinorelbine for relapse and chemotherapy – Pretreatment metastatic breast cancer. AMI Clin Oncol. 1999;22(3):298–302. doi: 10.1097/00000421-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Oostendorp LJ, Stalmeier PF, Donders AR, van der Graaf WT, Ottevanger PB. Efficacy and safety of palliative chemotherapy for patients with advanced breast cancer pretreated with anthracyclines and taxanes: a systematic review. Lancet Oncol. 2011 Oct;12(11):1053–61. doi: 10.1016/S1470-2045(11)70045-6. [DOI] [PubMed] [Google Scholar]