Abstract
Homeodomain-interacting protein kinase 2 (HIPK2) is a serine/threonine kinase that phosphorylates and activates the apoptotic program through interaction with diverse downstream targets including tumor suppressor p53. HIPK2 is activated by genotoxic stimuli and modulates cell fate following DNA damage. The DNA damage response (DDR) is triggered by DNA lesions or chromatin alterations. The DDR regulates DNA repair, cell cycle checkpoint activation, and apoptosis to restore genome integrity and cellular homeostasis. Maintenance of the DDR is essential to prevent development of diseases caused by genomic instability, including cancer, defects of development, and neurodegenerative disorders. Recent studies reveal a novel HIPK2-mediated pathway for DDR through interaction with chromatin remodeling factor homeodomain protein 1γ. In this review, we will highlight the molecular mechanisms of HIPK2 and show its functions as a crucial DDR regulator.
Keywords: DNA damage responses, HIPK2, HP1γ, cell cycle, apoptosis
1. Introduction
Cells are constantly subjected to DNA damage events caused by environmental or endogenous genotoxic agents [1,2]. Metabolic by-products, such as reactive oxygen species (ROS), are probably the most frequent intrinsic source of DNA damage. Extrinsic or environmental sources of DNA damage include ultraviolet (UV) light, ionizing radiation (IR), and exposure to numerous chemotherapeutic drugs, such as cisplatin, Adriamycin, and roscovitine [3,4]. Many studies have shown that DNA-damaging agents promote genome instability, which is a main driving force of tumorigenesis [5,6,7]. Eukaryotic cells rely on a strictly coordinated series of events, termed the DNA damage response (DDR), to cope with genotoxic insults to maintain homeostasis. The DDR includes cell-cycle checkpoint activation, regulation of DNA replication, and DNA damage repair [8,9]. Recent studies have shown that the DDR functions as a potent intracellular regulator of transformation and tumorigenesis [5,10,11]. In fact, dysregulation of DDR affects the progression of clinically pertinent diseases, including premature aging, immunological disorders, neurodegenerative diseases, and cancer.
Although molecular mechanisms of DDR are still not fully understood, numerous DDR factors have been identified as tumor suppressors that prevent cancer progression [5,12]. Homeodomain-interacting protein kinase 2 (HIPK2) is a DNA damage-responsive serine/threonine kinase that activates the apoptotic program through interaction with diverse downstream targets, including tumor suppressor p53 [13,14]. In DDR, HIPK2 modulates the activity of several proteins thorough site-specific phosphorylation in response to the DNA damage. To date, epigenetic factors related to chromatin remodeling and histone modification regulate the integrated response of chromatin for promoting DNA damage-triggered signaling and DNA repair [15]. Here, we summarize the molecular mechanisms of the HIPK2-mediated DDR and suggest a possible role of this kinase as an epigenetic regulator linking DNA repair.
2. Signaling Pathways for the DNA Damage Response (DDR)
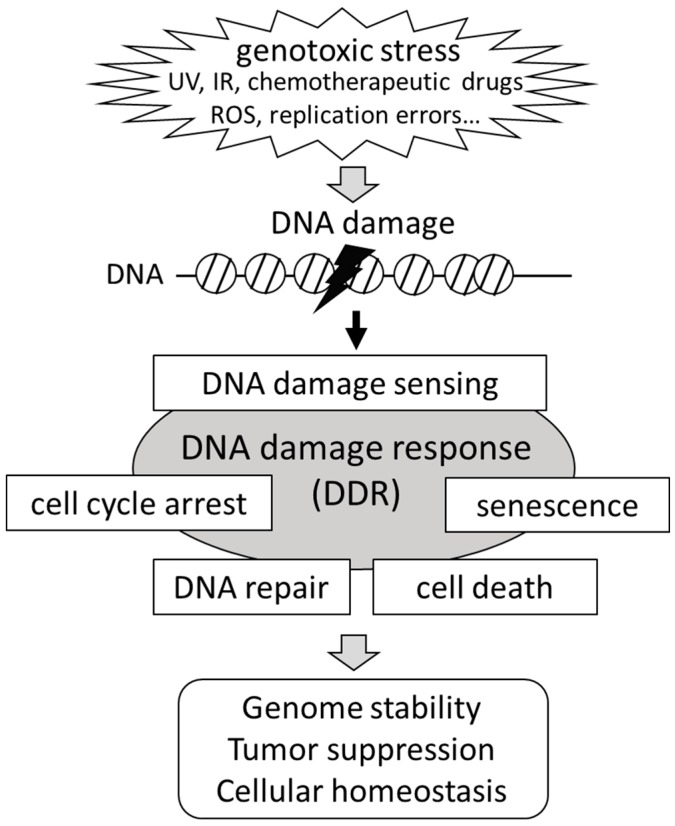
Once cells are exposed to environmental or cell-derived stressors, like UV light or ROS, these insults can cause DNA lesions, such as single-strand breaks (SSBs) or double-strand DNA breaks (DSBs) [5,16]. The DDR employs appropriate cellular machinery to detect DNA damage and maintain genomic integrity. The DDR in damaged cells provokes various cellular events, including cell cycle arrest, DNA repair, cellular senescence, or cell death. While cellular senescence and cell cycle arrest of the damaged cells block proliferation, cell death signaling facilitates removal of the cells that are irreversibly damaged [10] (Figure 1). In general, DDR pathways consist of multi-regulated steps: initial detection of DNA damage, recruitment of DNA repair factors to the damaged lesions and DNA repair.
Figure 1.
Schematic representation of the DNA damage response (DDR). Cells are constantly exposed to various environmental or endogenous genotoxic agents. Through the DDR pathway, the DNA damage is recognized by sensor protein complexes. The accumulation of DNA damage provokes various cellular events, including cell cycle arrest, DNA repair, cellular senescence, or cell death. The DDR plays a key role in the genomic stability that protects cells from tumorgenesis. UV, ultraviolet; IR, ionizing radiation; ROS, reactive oxygen species.
DSBs constitute the most deleterious form of DNA lesions. DSBs are caused spontaneously during DNA replication when arrested replication forks collapse [17,18]. In addition, extrinsic or environmental sources of DNA damage, such as ultraviolet-C (UV-C), IR, and genotoxic drugs, can trigger DSBs [1,2,3,4]. In mammalian cells, DSBs are primarily repaired by two different pathways, homologous recombination (HR) and the non-homologous end-joining (NHEJ) pathway [5]. HR repair is a relatively accurate and efficient pathway, but it depends on the remains of the undamaged sister chromatid DNA, while the NHEJ pathway is not affected by the presence of replicated DNA [19,20,21,22]. The DSB repair pathway is tightly controlled during the cell cycle through DNA damage-induced signaling. If the damage is unrepaired or aberrantly repaired, DSBs latently lead to chromosomal rearrangements and aberrations, resulting in developmental and neurodegenerative disorders, and cancer [2,5]. NHEJ is the crucial mechanism for DNA repair of IR-induced DNA damage. A homeodomain-containing protein, HOXB7, interacts with DNA-dependent protein kinase holoenzymes, including Ku70, Ku80, the catalytic subunit of DNA-PK (DNA-PKcs), and poly (adenosine diphosphate (ADP)) ribose polymerase, which, in turn, enhances NHEJ by stimulating DNA-PK activity and contributes to DNA damage repair in response to IR [23].
DNA damage is sensed by specified protein complexes that are directly bound to the site of the DNA lesion. Then, the sensor complexes activate master DDR Ser/Thr kinases. These kinases belong to the family of phosphatidylinositide 3-kinase (PI3K)-like kinases and they constitute the apex of the DNA damage signaling cascade. DSBs are sensed by the heterotrimeric Mre11/Rad50/NBS1 (MRN) complex [24]. The MRN complex is recruited to DSBs and triggers auto-phosphorylation of the DNA damage checkpoint kinase ataxia telangiectasia mutated (ATM) at Ser1981 to switch from inactive dimers to active monomers [25,26]. The Rad9-Hus1-Rad1 (9-1-1) clamp interacts with ATM and Rad3-related (ATR) and promotes its activation in response to collapsed DNA replication forks and replication stress [27,28]. The activation of ATM or ATR subsequently mediates the phosphorylation of downstream substrates that are implicated in DNA repair, cell cycle arrest, and cell death.
3. Homeodomain-Interacting Protein Kinase 2 (HIPK2)
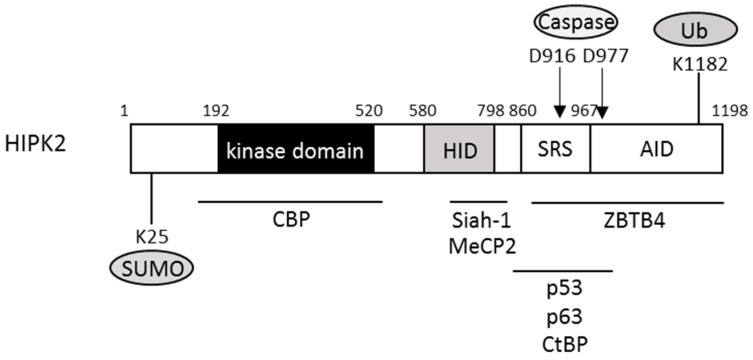
The family of homeodomain-interacting protein kinases (HIPKs) is evolutionally conserved and consists of four related kinases, HIPK1 to HIPK4 [29,30]. HIPK2 was first identified as an interactor with homeodomain transcription factor Nkx1.2 [29]. HIPK2 contains a 330 amino acid-long serine/threonine kinase domain located at the N-terminus of the molecule (Figure 2). The C-terminal part contains an interaction domain for the homeodomain transcription factors (HID), homeobox-interacting domain) and a binding motif for a small ubiquitin-like modifier (SUMO) in the speckle-retention signal (SRS) (Figure 2). The SRS is essential for the subcellular localization of HIPK2 in nuclear bodies. The C-terminal end of HIPK2 contains an interaction domain for the homeodomain transcription factors and an auto-inhibitory domain (AID) [31].
Figure 2.
Schematic summary of homeodomain-interacting protein kinase 2 (HIPK2) structure. The N-terminal domain contains the sumoylation sites (K25). D916 and D977 are caspase-6 cleavage sites. K1182 is the ubiquitylation site. Some HIPK2-interacting proteins are shown. SUMO, sumoylation; Ub, ubiquitylation; HID, homeobox-interacting domain; SRS, speckle-retention signal; AID, auto-inhibitory domain; MeCP, methyl-CpG-binding protein 2; CtBP, C-terminal binding protein; CBP, cAMP-response element-binding protein (CREB)-binding protein; ZBTB4, zinc-finger and BTB domain containing 4.
Studies of Hipk1 and Hipk2 single knockout mice suggest functional redundancy between HIPK1 and HIPK2 [32,33]. However, Hipk1/Hipk2 double knockout mice are embryonically lethal and show defects in neural tube closure, hematopoietic cell differentiation, vasculogenesis, and angiogenesis [34]. HIPK2 has been classically identified as a nuclear kinase that acts as a corepressor for the homeodomain transcription factors [29]. Recently, HIPK2 was recognized as a signaling transducer transductor that is involved in a variety of intracellular signal pathways, including p53, transforming growth factor (TGF)-β, Notch, Wnt, JNK, Hedgehog, and Hippo [35,36,37,38,39,40]. In response to genotoxic stress, HIPK2 phosphorylates downstream substrates to regulate signaling of cellular development, cell cycle, cell proliferation, differentiation, and DDR [30,41,42]. HIPK2 binds and phosphorylates a large number of targets, including signal transducers, transcription factors, epigenetic regulators, and ubiquitin ligases (Table 1). Subsequently, it also associates with neurogenesis, myogenesis, angiogenesis, fat development, and hematopoiesis [37,43,44,45,46].
Table 1.
Phosphorylation targets of Homeodomain-Interacting Protein Kinase 2 (HIPK2) and functional roles.
| Target Proteins | Cellular Function | Reference |
|---|---|---|
| p53 | cell cycle regulation and apoptosis | [13,14,33] |
| ΔNp63α | cell cycle regulation and apoptosis | [47] |
| p27 | cell cycle regulation and cell motility | [48] |
| PML | cell proliferation | [49] |
| H2B | cytokinesis | [50] |
| WIP1 | DNA repair | [51] |
| PDX1 | pancreatic development and mature β-cell function | [52] |
| β-catenin | activation of Wnt/β-catenin signaling | [36] |
| Pc2 | SUMOylation | [53] |
| SIRT1 | acetylation | [54] |
| Siah1, Siah2 | ubiquitination | [55] |
| MDM2 | ubiquitination | [56] |
| C/EBPβ | activation of transcription factor | [57] |
| CtBP | transcription corepression | [35] |
| p300, AML1 | transcription activation; hematopoiesis | [44] |
| Che-1 | transcription activation; cell proliferation | [58] |
| DAXX | transcription repression | [40] |
| MeCP2 | DNA methylation and epigenetic regulation | [59] |
| ZBTB4 | transcription and epigenetic regulation | [60] |
| HMGA1a | transcriptional regulation and chromatin remodeling | [61] |
| HP1γ | epigenetic regulation | [62] |
PML, promyelocytic leukemia protein; WIP1, wild-type p53-inducible phosphatase 1; PDX1, pancreatic and duodenal homeobox 1; Pc2, Proprotein convertase 2; SIRT1, sirtuin 1; Siah-1, seven in absentia-homolog 1; C/EBPβ, CCAAT/enhancer binding protein β; AML1, acute myeloid leukemia 1; DAXX, death domain associated protein; HMGA1a, high mobility group AT-hook 1a; HP1γ, heterochromatin protein 1γ.
4. Regulation of HIPK2 Activities
HIPK2 functions are tightly coordinated by its catalytic activity, stability, and subcellular localization, which, in turn, are dynamically regulated by diverse post-translational modifications. In the absence of cellular stress, HIPK2 is generally maintained at low levels by ubiquitination-dependent proteasomal degradation mediated by E3 ubiquitin ligases, seven in absentia-homolog (Siah)-1 and tryptophan–aspartic acid (WD) repeats, and suppressor of cytokine signaling (SOCS) box-containing protein 1 (WSB-1) [51,55]. WSB-1 ubiquitinates the C-terminus of HIPK2 and thereby promotes proteasomal degradation [51]. Once cells are exposed to genotoxic stimuli, HIPK2 undergoes auto-phosphorylation and dissociates from WSB-1, thus protecting HIPK2 against proteasomal degradation [63]. Promyelocytic leukemia protein (PML) is a tumor suppressor that mainly localizes to punctate nuclear structures that are called PML nuclear bodies (PML-NBs). Fbx3 is a component of the Skp1-Cullin 1-F-box (SCF) E3 ligase complex in PML-NBs. HIPK2 is constitutively degraded by Fbx3-mediated polyubiquitination under normal conditions [64]. Increased stability and SUMOylation of PML protein in response to DNA damage mediates HIPK2 activation and induces cell death [65]. Siah-1 is another E3 ligase that mediates HIPK2 degradation in the absence of stress. In response to lethal DNA damage, ATM is activated and phosphorylates Siah-1 at Ser19, resulting in the stabilization of HIPK2 by escape from phosphorylated Siah-1 [55].
Hofmann et al. [65] have demonstrated that SUMO protease SuPr-1 interacts with HIPK2 and deconjugates SUMO from HIPK2, thereby antagonizing the inhibitory effect of SUMO-1 on HIPK2-mediated JNK activation. Thus, HIPK2 function on JNK and its p53-independent growth suppressing activity is modulated by reversible SUMO-1 conjugation and deconjugation. SUMOylation at K25 of HIPK2 is important for low level expression under normal conditions. Attachment of SUMO1 to K25 of HIPK2 is promoted by the SUMO E3 ligase Proprotein convertase 2 (Pc2) [53]. In unstressed cells, SUMOylated HIPK2 recruits histone deacetylase (HDAC) 3 and maintains HIPK2 in a deacetylated state. Exposure to oxidative stress mediates HIPK2 deSUMOylation and acetylation at K10 residues by cAMP-response element-binding protein (CREB)-binding protein (CBP) acetyl-transferase [66]. The changes of HIPK2 modification from SUMOylation to acetylation alters intracellular distribution of HIPK2 from nuclear speckles to the nucleoplasm. This subsequently relieves HIPK2-mediated transcriptional repression of several redox-sensitive genes, such as heme oxygenase 1 and peroxiredoxins [67,68]. Thus, proper acetylation of HIPK2 is essential for tolerance against sublethal DNA damage. Since HIPK2-mediated phosphorylation of Pc2 is required for its activation in response to genotoxic stress, Pc2, in turn, SUMOylates HIPK2, which then participates in the transcriptional repression of proapoptotic genes, such as BAX, to inhibit apoptosis and promote cell survival [53].
5. Roles of HIPK2 in DDR
HIPK2 is a DNA damage-responsive kinase that activates downstream targets, including tumor suppressor p53, which is often mutated or functionally inactivated in various types of cancer. The two master DNA damage checkpoint kinases, ATM and ATR, directly switch on HIPK2 upon different kinds of DNA damage by triggering its stabilization [55]. Exposure to IR is one of the genotoxic stresses which causes various types of complex clustered DNA damage, including DSBs, SSBs, oxidized bases, and nucleic mutations [5,69]. The multi-pathway of DNA repair mechanisms is triggered depending on the doses of IR to determine cell fate. p53 Ser46 phosphorylation plays a crucial role in IR-activated apoptotic programs. Dauth et al. [70] found that accumulation and activation of HIPK2 are significantly correlated with IR-induced phosphorylation of p53 Ser46. In addition, activation of ATM is required for IR-mediated HIPK2 accumulation. Thus, HIPK2 is the IR-triggered p53 Ser46 kinase and its function is regulated through DNA damage checkpoint kinase ATM. In addition, c-Abl, a tyrosine kinase which also activated by ATM, phosphorylates HIPK2 and this leads to HIPK2 accumulation and phosphorylation of p53 in response to γ- and UV-radiation, resulting in promoted apoptosis [71]. HIPK2 is an important modulator that senses the severity of DNA damage. For instance, in unstressed cells, HIPK2 is constantly degraded by the ubiquitin-proteasome system. In response to genotoxic stress, HIPK2 is stabilized by dissociation of E3 ubiquitin ligases. DNA damage also activates HIPK2 through caspase-6-mediated cleavage at Asp916 and Asp977. Caspase-mediated processing and the consequent removal of the C-terminal AID results in an increased activity in p53 Ser46 phosphorylation [49]. Once the damage is irreparable, these cells can ultimately trigger the cell death response to eliminate damaged, potentially threatening cells. Phosphorylation of p53 at Ser46 by HIPK2 in response to lethal DNA damage activates apoptotic target genes, such as PUMA, BAX, NOXA, and BID [72]. MDM2 is one of the potent p53 negative regulators. HIPK2 phosphorylates MDM2 and induces its proteasomal degradation, which leads to restoring p53 apoptotic activity [56]. In addition, HIPK2 can promote p53-independent apoptosis through interaction with C-terminal binding protein (CtBP) and Δp63α. CtBP is an anti-apoptotic transcriptional corepressor that inhibits cell death. UV-triggered CtBP phosphorylation at Ser422 by HIPK2 induces protein degradation in p53-null cells which promotes apoptosis [35]. The anti-apoptotic p63 isoform Δp63α is also phosphorylated by HIPK2 and targeted for degradation in a p53-independent manner [47]. In contrast, when DNA damage is less severe, repair is achieved by the DNA repair system in association with the required cell cycle arrest. In this case, HIPK2 does not affect phosphorylation of p53 at Ser46. Instead, HIPK2 mediates p53 recruitment onto the CDKN1A promoter through acetylation of p53 by p300/CBP-associated factor to induce cell cycle arrest followed by DNA repair [73]. HIPK2 resides in the nucleus where it partially co-localizes with the PML-NB [14]. PML-NB is essential for HIPK2-mediated p53 Ser46 phosphorylation and stabilization for the apoptosis-inducing function of HIPK2 after DNA damage [74]. In addition, the integrity of PML-NB is also regulated by HIPK2-dependent PML phosphorylation. During early stages of DNA damage, HIPK2 phosphorylates PML at Ser8 and Ser38 to increase stability of PML [75]. The deacetylase Sirtuin 1 (SIRT1) suppresses cell death after DNA damage by antagonizing acetylation of p53 [76]. Conrad et al. found that DNA damage initiates interaction between SIRT1 and HIPK2, which phosphorylates SIRT1 at Ser682 in response to lethal damage [54]. Phosphorylation of Ser682 inhibits SIRT1 activity in p53 acetylation and impacts expression of apoptotic p53 target genes and apoptosis. Thus, in response of severe DNA damage, HIPK2 strictly regulates SIRT1 activity through phosphorylation of SIRT1 in the PML-NBs. Thus, HIPK2 functions in the DDR by regulating cell cycle arrest and apoptosis, thereby helping to prevent mutations, genomic instability, and carcinogenesis.
6. HIPK2 as an Epigenetic Regulator
Several studies have suggested that HIPK2 has a novel function as an epigenetic regulator of chromatin structure (Figure 3). For example, HIPK2 contributes to cell proliferation during cytokinesis through the phosphorylation of histone H2B at Ser14 [50]. A loss of H2B phosphorylation at the midbody caused by HIPK2 depletion prevents cell cleavage and tetra- and polyploidization. In addition, HIPK2 associates with chromatin modification factors, including methyl-CpG-binding protein 2 (MeCP2), methyl-binding transcription factor Zinc finger, and BTB domain-containing 4 (ZBTB4), transcriptional corepressor CtBP, and polycomb protein Pc2 [35,53,59,60]. Chromatin components and epigenetic factors promote DNA damage signaling and repair by regulating the integrated response of chromatin remodeling. MeCP2 represses transcription by its association with methylated DNA and recruitment of co-repressor proteins [77]. Phosphorylation of MeCP2 at Ser80 mediated by HIPK2 is required for DNA binding activity [59]. ZBTB4 also binds methylated DNA in vitro and in vivo and represses methylated sequences [78]. In response to DNA damage, HIPK2 phosphorylates threonine residues of ZBTB4 and accelerates its degradation [60].
Figure 3.
HIPK2 targets in epigenetic regulation. HIPK2 phosphorylates and activates epigenetic factors which are involved in DNA methylation, histone modification, chromatin remodeling, and transcription. Phosphorylated sites (P) by HIPK2 are shown.
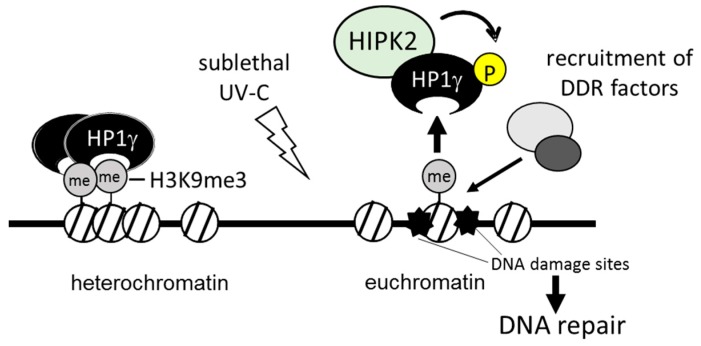
Heterochromatin protein 1γ (HP1γ) consists of three orthologs, HP1α, HP1β, and HP1γ [79]. HP1 proteins are heterochromatin components that are associated with histone H3 di- or trimethylated at Lys9 (histone H3K9me2 and histone H3K9me3, respectively) through their N-terminal chromo domain [80]. HP1 proteins dimerize through their C-terminal chromo-shadow domain to promote heterochromatin formation, which induces gene silencing [79]. In addition, HP1 proteins associate with a number of chromatin modification proteins, including transcription regulators, DNA replication and repair-related proteins, via the chromo-shadow domain [80]. In response to genotoxic stress, chromatin structure is remodeled to increase the accessibility of the DDR machinery to the DNA damage sites. Several studies have demonstrated that HP1 proteins are released from histone H3K9me3 and facilitate recruitment of DDR factors to damaged sites, resulting in progression of DNA repair [81]. HP1 proteins are recruited again to DNA damage sites after they are released from chromatin [82]. These results suggest that the HP1 family may be involved in regulation of the chromatin structure by changing their binding affinity to chromatin in response to DNA damage and mediate recruitment of DNA repair factors to the damaged lesions. HIPK2 is specifically bound to HP1γ through its conserved pentapeptide motifs (883-PTVSV-887) called HP1box [62]. This association mediated phosphorylation of HP1γ in response to sublethal UV-C irradiation that resulted in facilitating the dissociation of HP1γ from histone H3K9me3 (Figure 4). UV-C induces pyrimidine–pyrimidone (6–4) photoproducts (6–4PPs), a major class of DNA lesions, which are removed by the nucleotide excision repair system [83]. The remaining photoproducts induces DSBs and then the surrounding histone H2A.X is phosphorylated at Ser139 (γ-H2AX) to initiate recruitment of DNA repair factors to DNA damaged sites. Knockdown of HIPK2 impaired the removal of 6–4PPs and enhanced accumulation of γ-H2AX after UV-C irradiation [62]. This suggests HIPK2-dependent phosphorylation of HP1γ may regulate the dynamic interaction between HP1γ and chromatin for DNA damage repair. These data show a novel HIPK2-mediated pathway for DDR through the phosphorylation of chromatin remodeling factors.
Figure 4.
Schematic summary of HIPK2-mediated chromatin modification. Heterochromatin protein 1γ (HP1γ) binds to the methylated histone H3 and forms heterochromatin. In response to sublethal stress, HIPK2 phosphorylates HP1γ and facilitates its release from histone and promotes recruitment of DDR factors to the damaged sites for DNA repair. UV-C, ultraviolet-C.
7. A Role for HIPK2 in Notch1-Associated Tumorigenesis
After HIPK2 was first reported to induce p53 apoptotic activity through Ser46 phosphorylation, a number of studies uncovered additional molecular mechanisms for HIPK2-mediated p53 regulation and other downstream transcription factors that participate in cancer progression [84]. HIPK2 is now known to be a crucial regulator of DNA damage signaling and tumor suppression. A recent study identified Notch1 as a crucial downstream target of HIPK2. HIPK2 facilitates Fbw7-dependent proteasomal degradation of Notch1 by phosphorylating its intracellular domain (Notch1-IC) within the Cdc4 phosphodegron (CPD) motif. Under genotoxic stress, HIPK2 phosphorylates the residue T2512 in Notch1-IC and keeps it at a low level through proteasomal degradation [85]. Notch1 signaling is aberrantly activated in breast cancer. Moreover, increased expression of Notch1 intracellular domain (Notch1-IC) is associated with poor survival in patients with breast cancer and other tumors. Breast cancer tissues have increased Notch1-IC levels and show decreased levels of phosphorylated Notch1-IC T2512, HIPK2, and Fbw7. However, these changes are absent in cells expressing mutated Notch1-IC (T2512A), as well as the somatic mutants Notch1-IC P2513L and Notch1-IC P2515 frameshift. This is because these mutations block the HIPK2-induced phosphorylation of Notch1-IC and increase Notch1 stability. These studies have uncovered a novel mechanism for Notch1-dependent cancer progression and suggested an important role of HIPK2-induced phosphorylation of Notch1-IC at the T2512 residue in cancer prevention.
8. Pathophysiological Functions of HIPK2
Cellular responses to DNA damage are crucial to protect cells from genomic instability. Recent studies reveal that the DDR is correlated with immune response signaling networks and works together to maintain the harmonized multi-cellular function [86]. For instance, it is well established that viral genetic material triggers immunity of host cells by directly inducing cytokines, especially interferons (IFNs). Recent studies have documented that viral infection induces systemic DNA genetic and epigenetic alterations, including an increased frequency of homologous recombination (HR) [86]. There is increasing evidence that the DDR pathway is activated by microbial infection in humans. IFNα/β promotes p53 in turn evoking cell death that is crucial for antiviral immunity, thus, suggesting a novel relationship between IFNs and p53 signaling pathway [87]. Moreover, recent studies demonstrate that ATM modulates nuclear factor (NF)-κB activity through the activation of IκB kinases [88] and the phosphorylation of NF-κB at Ser547 [89]. Thus, ATM plays a key role as a hub of the DDR and immune response. In HIV-infected renal tubular epithelial cells, HIPK2 induced expression of pro-fibrosis markers via activating p53, TGF-β, and Wnt/Notch pathways [84]. Downregulation of HIPK2 leads to upregulated C-cadherin and decreases epithelial to mesenchymal transition [90]. These results suggest that HIPK2 is one of crucial regulators of kidney fibrosis by activating the upstream fibrosis signaling pathway. The tumor-suppressing functions of HIPK2 have been investigated to reside in its p53-dependent and p53-independent proapoptotic signaling transduction. In addition, HIPK2 is implicated in cytokinesis and prevents tetraploidization by phosphorylating histone H2B [50]. Failure in cytokinesis, the final step in cell division, causes chromosomal instability, a hallmark of cancer. Hence, HIPK2 contributes to cell proliferation and protects cells from tumorgenesis by controlling cytokinesis.
9. Conclusions
Although HIPK2 was originally thought to be a corepressor of a transcription factor, a large amount of data has shown that it also participates in diverse cellular activities, including a pro-apoptotic response to genetic damage by its serine/threonine kinase activity. Cellular responses to DNA damage are important to protect cells against genomic instability. The fundamental role of HIPK2 is acting as an integrating sensor for diverse cellular signaling. HIPK2 mediates different signaling pathways depending on dosages or types of the stimuli through its post-transcriptional modifications, subcellular localization, kinase activity, and substrate specificity results in the determination of cell fates. In recent years, increasing evidence reveals that the coordinated maintenance of the genome and epigenome in response to DNA damage is regulated by interactions between DDR factors and chromatin modifiers. Future studies could clarify the functions of HIPK2 in epigenetic regulation to promote DNA repair and genome stability. Finally, the contributions of HIPK2 to tumor regression and the response to anticancer drugs suggest that HIPK2 might be a diagnostic marker and a therapeutic target.
Acknowledgments
A part of this work was funded by the Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (No. 26293169 to Kazuhito Rokutan, 15K15294 and 26713027 to Yuki Kuwano).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeijmakers J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein M., Kastan M.B. The DNA damage response: Implications for tumor responses to radiation and chemotherapy. Annu. Rev. Med. 2015;66:129–143. doi: 10.1146/annurev-med-081313-121208. [DOI] [PubMed] [Google Scholar]
- 4.Ciccia A., Elledge S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor M.J. Targeting the DNA damage response in cancer. Mol. Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Corcoran N.M., Clarkson M.J., Stuchbery R., Hovens C.M. Molecular pathways: Targeting DNA repair pathway defects enriched in metastasis. Clin. Cancer Res. 2016;22:3132–3137. doi: 10.1158/1078-0432.CCR-15-1050. [DOI] [PubMed] [Google Scholar]
- 7.Hoeijmakers J.H. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 8.Polo S.E., Almouzni G. Chromatin dynamics after DNA damage: The legacy of the access-repair-restore model. DNA Repair. 2015;36:114–121. doi: 10.1016/j.dnarep.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y., Li Y., Lu X. Regulators in the DNA damage response. Arch. Biochem. Biophys. 2016;594:18–25. doi: 10.1016/j.abb.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Matt S., Hofmann T.G. The DNA damage-induced cell death response: A roadmap to kill cancer cells. Cell. Mol. Life Sci. 2016;73:2829–2850. doi: 10.1007/s00018-016-2130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolai S., Rossi A., di Daniele N., Melino G., Annicchiarico-Petruzzelli M., Raschellà G. DNA repair and aging: The impact of the p53 family. Aging. 2015;7:1050–1065. doi: 10.18632/aging.100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulli G., Di Micco R., d’Adda di Fagagna F. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat. Rev. Cancer. 2012;12:709–720. doi: 10.1038/nrc3344. [DOI] [PubMed] [Google Scholar]
- 13.D’Orazi G., Cecchinelli B., Bruno T., Manni I., Higashimoto Y., Saito S., Gostissa M., Coen S., Marchetti A., del Sal G., et al. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat. Cell Biol. 2002;4:11–19. doi: 10.1038/ncb714. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann T.G., Möller A., Sirma H., Zentgraf H., Taya Y., Dröge W., Will H., Schmitz M.L. Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat. Cell Biol. 2002;4:1–10. doi: 10.1038/ncb715. [DOI] [PubMed] [Google Scholar]
- 15.Papamichos-Chronakis M., Peterson C.L. Chromatin and the genome integrity network. Nat. Rev. Genet. 2013;14:62–75. doi: 10.1038/nrg3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen D.H., Stucki M. Nucleolar responses to DNA double-strand breaks. Nucleic Acids Res. 2016;44:538–544. doi: 10.1093/nar/gkv1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilgner K., Neganova I., Moreno-Gimeno I., Al-Aama J.Y., Burks D., Yung S., Singhapol C., Saretzki G., Evans J., Gorbunova V., et al. A human iPSC model of ligase IV deficiency reveals an important role for NHEJ-mediated-DSB repair in the survival and genomic stability of induced pluripotent stem cells and emerging haematopoietic progenitors. Cell Death Differ. 2013;20:1089–1100. doi: 10.1038/cdd.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branzei D., Foiani M. The DNA damage response during DNA replication. Curr. Opin. Cell Biol. 2005;17:568–575. doi: 10.1016/j.ceb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Gaillard H., García-Muse T., Aguilera A. Replication stress and cancer. Nat. Rev. Cancer. 2015;15:276–289. doi: 10.1038/nrc3916. [DOI] [PubMed] [Google Scholar]
- 20.Moynahan M.E., Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat. Rev. Mol. Cell Biol. 2010;11:196–207. doi: 10.1038/nrm2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceccaldi R., Liu J.C., Amunugama R., Hajdu I., Primack B., Petalcorin M.I., O’Connor K.W., Konstantinopoulos P.A., Elledge S.J., Boulton S.J., et al. Homologous-recombination-deficient tumours are dependent on polθ-mediated repair. Nature. 2015;518:258–262. doi: 10.1038/nature14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mateos-Gomez P.A., Gong F., Nair N., Miller K.M., Lazzerini-Denchi E., Sfeir A. Mammalian polymerase θ promotes alternative NHEJ and suppresses recombination. Nature. 2015;518:254–257. doi: 10.1038/nature14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin E., Wu X., Zhu T., Cheung J.C., Chen H., Lorincz A., Pandita R.K., Sharma G.G., Ha H.C., Gasson J., et al. A role for the HOXB7 homeodomain protein in DNA repair. Cancer Res. 2007;67:1527–1535. doi: 10.1158/0008-5472.CAN-06-4283. [DOI] [PubMed] [Google Scholar]
- 24.Radhakrishnan S.K., Jette N., Lees-Miller S.P. Non-homologous end joining: Emerging themes and unanswered questions. DNA Repair. 2014;17:2–8. doi: 10.1016/j.dnarep.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carney J.P., Maser R.S., Olivares H., Davis E.M., Le Beau M., Yates J.R., Hays L., Morgan W.F., Petrini J.H. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: Linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/S0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 26.Shiloh Y. ATM and related protein kinases: Safeguarding genome integrity. Nat. Rev. Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 27.Kozlov S.V., Graham M.E., Jakob B., Tobias F., Kijas A.W., Tanuji M., Chen P., Robinson P.J., Taucher-Scholz G., Suzuki K., et al. Autophosphorylation and ATM activation: Additional sites add to the complexity. J. Biol. Chem. 2011;286:9107–9119. doi: 10.1074/jbc.M110.204065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delacroix S., Wagner J.M., Kobayashi M., Yamamoto K., Karnitz L.M. The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev. 2007;21:1472–1477. doi: 10.1101/gad.1547007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y.H., Choi C.Y., Lee S.J., Conti M.A., Kim Y. Homeodomain-interacting protein kinases, a novel family of co-repressors for homeodomain transcription factors. J. Biol. Chem. 1998;273:25875–25879. doi: 10.1074/jbc.273.40.25875. [DOI] [PubMed] [Google Scholar]
- 30.Rinaldo C., Siepi F., Prodosmo A., Soddu S. HIPKs: Jack of all trades in basic nuclear activities. Biochim. Biophys. Acta. 2008;1783:2124–2129. doi: 10.1016/j.bbamcr.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Saul V.V., de la Vega L., Milanovic M., Krüger M., Braun T., Fritz-Wolf K., Becker K., Schmitz M.L. HIPK2 kinase activity depends on cis-autophosphorylation of its activation loop. J. Mol. Cell Biol. 2013;5:27–38. doi: 10.1093/jmcb/mjs053. [DOI] [PubMed] [Google Scholar]
- 32.Wiggins A.K., Wei G., Doxakis E., Wong C., Tang A.A., Zang K., Luo E.J., Neve R.L., Reichardt L.F., Huang E.J. Interaction of Brn3a and HIPK2 mediates transcriptional repression of sensory neuron survival. J. Cell Biol. 2004;167:257–267. doi: 10.1083/jcb.200406131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo S., Lu Y., Debbas M., Lin A.W., Sarosi I., Itie A., Wakeham A., Tuan J., Saris C., Elliott G., et al. Characterization of cells and gene-targeted mice deficient for the p53-binding kinase homeodomain-interacting protein kinase 1 (HIPK1) Proc. Natl. Acad. Sci. USA. 2003;100:5431–5436. doi: 10.1073/pnas.0530308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isono K., Nemoto K., Li Y., Takada Y., Suzuki R., Katsuki M., Nakagawara A., Koseki H. Overlapping roles for homeodomain-interacting protein kinases HIPK1 and HIPK2 in the mediation of cell growth in response to morphogenetic and genotoxic signals. Mol. Cell. Biol. 2006;26:2758–2771. doi: 10.1128/MCB.26.7.2758-2771.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q., Yoshimatsu Y., Hildebrand J., Frisch S.M., Goodman R.H. Homeodomain interacting protein kinase 2 promotes apoptosis by downregulating the transcriptional corepressor CtBP. Cell. 2003;115:177–186. doi: 10.1016/S0092-8674(03)00802-X. [DOI] [PubMed] [Google Scholar]
- 36.Nardinocchi L., Puca R., Givol D., D’Orazi G. HIPK2—A therapeutical target to be (re)activated for tumor suppression: Role in p53 activation and HIF-1α inhibition. Cell Cycle. 2010;9:1270–1275. doi: 10.4161/cc.9.7.11125. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Pho V., Bonasera S.J., Holtzman J., Tang A.T., Hellmuth J., Tang S., Janak P.H., Tecott L.H., Huang E.J. Essential function of HIPK2 in TGFβ-dependent survival of midbrain dopamine neurons. Nat. Neurosci. 2007;10:77–86. doi: 10.1038/nn1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei G., Ku S., Ma G.K., Saito S., Tang A.A., Zhang J., Mao J.H., Appella E., Balmain A., Huang E.J. HIPK2 represses β-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc. Natl. Acad. Sci. USA. 2007;104:13040–13045. doi: 10.1073/pnas.0703213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee W., Andrews B.C., Faust M., Walldorf U., Verheyen E.M. HIPK is an essential protein that promotes notch signal transduction in the drosophila eye by inhibition of the global co-repressor groucho. Dev. Biol. 2009;325:263–272. doi: 10.1016/j.ydbio.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann T.G., Stollberg N., Schmitz M.L., Will H. HIPK2 regulates transforming growth factor-β-induced c-Jun NH2-terminal kinase activation and apoptosis in human hepatoma cells. Cancer Res. 2003;63:8271–8277. [PubMed] [Google Scholar]
- 41.Calzado M.A., Renner F., Roscic A., Schmitz M.L. HIPK2: A versatile switchboard regulating the transcription machinery and cell death. Cell Cycle. 2007;6:139–143. doi: 10.4161/cc.6.2.3788. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann T.G., Glas C., Bitomsky N. HIPK2: A tumour suppressor that controls DNA damage-induced cell fate and cytokinesis. Bioessays. 2013;35:55–64. doi: 10.1002/bies.201200060. [DOI] [PubMed] [Google Scholar]
- 43.De la Vega L., Hornung J., Kremmer E., Milanovic M., Schmitz M.L. Homeodomain-interacting protein kinase 2-dependent repression of myogenic differentiation is relieved by its caspase-mediated cleavage. Nucleic Acids Res. 2013;41:5731–5745. doi: 10.1093/nar/gkt262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aikawa Y., Nguyen L.A., Isono K., Takakura N., Tagata Y., Schmitz M.L., Koseki H., Kitabayashi I. Roles of HIPK1 and HIPK2 in AML1- and p300-dependent transcription, hematopoiesis and blood vessel formation. EMBO J. 2006;25:3955–3965. doi: 10.1038/sj.emboj.7601273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shang Y., Doan C.N., Arnold T.D., Lee S., Tang A.A., Reichardt L.F., Huang E.J. Transcriptional corepressors HIPK1 and HIPK2 control angiogenesis via TGF-β–TAK1-dependent mechanism. PLoS Biol. 2013;11:1638. doi: 10.1371/journal.pbio.1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sjölund J., Pelorosso F.G., Quigley D.A., DelRosario R., Balmain A. Identification of HIPK2 as an essential regulator of white fat development. Proc. Natl. Acad. Sci. USA. 2014;111:7373–7378. doi: 10.1073/pnas.1322275111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazzari C., Prodosmo A., Siepi F., Rinaldo C., Galli F., Gentileschi M., Bartolazzi A., Costanzo A., Sacchi A., Guerrini L., et al. HIPK2 phosphorylates ΔNp63α and promotes its degradation in response to DNA damage. Oncogene. 2011;30:4802–4813. doi: 10.1038/onc.2011.182. [DOI] [PubMed] [Google Scholar]
- 48.Pierantoni G.M., Esposito F., Tornincasa M., Rinaldo C., Viglietto G., Soddu S., Fusco A. Homeodomain-interacting protein kinase-2 stabilizes p27kip1 by its phosphorylation at serine 10 and contributes to cell motility. J. Biol. Chem. 2011;286:29005–29013. doi: 10.1074/jbc.M111.230854. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Gresko E., Roscic A., Ritterhoff S., Vichalkovski A., del Sal G., Schmitz M.L. Autoregulatory control of the p53 response by caspase-mediated processing of HIPK2. EMBO J. 2006;25:1883–1894. doi: 10.1038/sj.emboj.7601077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinaldo C., Moncada A., Gradi A., Ciuffini L., D’Eliseo D., Siepi F., Prodosmo A., Giorgi A., Pierantoni G.M., Trapasso F., et al. HIPK2 controls cytokinesis and prevents tetraploidization by phosphorylating histone H2B at the midbody. Mol. Cell. 2012;47:87–98. doi: 10.1016/j.molcel.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 51.Choi D.W., Seo Y.M., Kim E.A., Sung K.S., Ahn J.W., Park S.J., Lee S.R., Choi C.Y. Ubiquitination and degradation of homeodomain-interacting protein kinase 2 by WD40 repeat/SOCS box protein Wsb-1. J. Biol. Chem. 2008;283:4682–4689. doi: 10.1074/jbc.M708873200. [DOI] [PubMed] [Google Scholar]
- 52.Boucher M.J., Simoneau M., Edlund H. The homeodomain-interacting protein kinase 2 regulates insulin promoter factor-1/pancreatic duodenal homeobox-1 transcriptional activity. Endocrinology. 2009;150:87–97. doi: 10.1210/en.2007-0865. [DOI] [PubMed] [Google Scholar]
- 53.Roscic A., Möller A., Calzado M.A., Renner F., Wimmer V.C., Gresko E., Lüdi K.S., Schmitz M.L. Phosphorylation-dependent control of Pc2 SUMO E3 ligase activity by its substrate protein HIPK2. Mol. Cell. 2006;24:77–89. doi: 10.1016/j.molcel.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Conrad E., Polonio-Vallon T., Meister M., Matt S., Bitomsky N., Herbel C., Liebl M., Greiner V., Kriznik B., Schumacher S., et al. HIPK2 restricts Sirt1 activity upon severe DNA damage by a phosphorylation-controlled mechanism. Cell Death Differ. 2016;23:110–122. doi: 10.1038/cdd.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winter M., Sombroek D., Dauth I., Moehlenbrink J., Scheuermann K., Crone J., Hofmann T.G. Control of HIPK2 stability by ubiquitin ligase Siah-1 and checkpoint kinases ATM and ATR. Nat. Cell Biol. 2008;10:812–824. doi: 10.1038/ncb1743. [DOI] [PubMed] [Google Scholar]
- 56.Di Stefano V., Blandino G., Sacchi A., Soddu S., D’Orazi G. HIPK2 neutralizes MDM2 inhibition rescuing p53 transcriptional activity and apoptotic function. Oncogene. 2004;23:5185–5192. doi: 10.1038/sj.onc.1207656. [DOI] [PubMed] [Google Scholar]
- 57.Steinmann S., Coulibaly A., Ohnheiser J., Jakobs A., Klempnauer K.H. Interaction and cooperation of the CCAAT-box enhancer-binding protein β (C/EBPβ) with the homeodomain-interacting protein kinase 2 (HIPK2) J. Biol. Chem. 2013;288:22257–22269. doi: 10.1074/jbc.M113.487769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Nicola F., Catena V., Rinaldo C., Bruno T., Iezzi S., Sorino C., Desantis A., Camerini S., Crescenzi M., Floridi A., et al. HIPK2 sustains apoptotic response by phosphorylating Che-1/AATF and promoting its degradation. Cell Death Dis. 2014;5:e1414. doi: 10.1038/cddis.2014.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bracaglia G., Conca B., Bergo A., Rusconi L., Zhou Z., Greenberg M.E., Landsberger N., Soddu S., Kilstrup-Nielsen C. Methyl-CpG-binding protein 2 is phosphorylated by homeodomain-interacting protein kinase 2 and contributes to apoptosis. EMBO Rep. 2009;10:1327–1333. doi: 10.1038/embor.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamada D., Pérez-Torrado R., Filion G., Caly M., Jammart B., Devignot V., Sasai N., Ravassard P., Mallet J., Sastre-Garau X., et al. The human protein kinase HIPK2 phosphorylates and downregulates the methyl-binding transcription factor ZBTB4. Oncogene. 2009;28:2535–2544. doi: 10.1038/onc.2009.109. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Q., Wang Y. Homeodomain-interacting protein kinase-2 (HIPK2) phosphorylates HMGA1a at Ser-35, Thr-52, and Thr-77 and modulates its DNA binding affinity. J. Proteome Res. 2007;6:4711–4719. doi: 10.1021/pr700571d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akaike Y., Kuwano Y., Nishida K., Kurokawa K., Kajita K., Kano S., Masuda K., Rokutan K. Homeodomain-interacting protein kinase 2 regulates DNA damage response through interacting with heterochromatin protein 1γ. Oncogene. 2015;34:3463–3473. doi: 10.1038/onc.2014.278. [DOI] [PubMed] [Google Scholar]
- 63.Bitomsky N., Conrad E., Moritz C., Polonio-Vallon T., Sombroek D., Schultheiss K., Glas C., Greiner V., Herbel C., Mantovani F., et al. Autophosphorylation and Pin1 binding coordinate DNA damage-induced HIPK2 activation and cell death. Proc. Natl. Acad. Sci. USA. 2013;110:E4203–E4212. doi: 10.1073/pnas.1310001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shima Y., Shima T., Chiba T., Irimura T., Pandolfi P.P., Kitabayashi I. PML activates transcription by protecting HIPK2 and p300 from SCFFbx3-mediated degradation. Mol. Cell. Biol. 2008;28:7126–7138. doi: 10.1128/MCB.00897-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hofmann T.G., Jaffray E., Stollberg N., Hay R.T., Will H. Regulation of homeodomain-interacting protein kinase 2 (HIPK2) effector function through dynamic small ubiquitin-related modifier-1 (SUMO-1) modification. J. Biol. Chem. 2005;280:29224–29232. doi: 10.1074/jbc.M503921200. [DOI] [PubMed] [Google Scholar]
- 66.De la Vega L., Grishina I., Moreno R., Krüger M., Braun T., Schmitz M.L. A redox-regulated SUMO/acetylation switch of HIPK2 controls the survival threshold to oxidative stress. Mol. Cell. 2012;46:472–483. doi: 10.1016/j.molcel.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Cano C.E., Gommeaux J., Pietri S., Culcasi M., Garcia S., Seux M., Barelier S., Vasseur S., Spoto R.P., Pébusque M.J., et al. Tumor protein 53-induced nuclear protein 1 is a major mediator of p53 antioxidant function. Cancer Res. 2009;69:219–226. doi: 10.1158/0008-5472.CAN-08-2320. [DOI] [PubMed] [Google Scholar]
- 68.Hailemariam K., Iwasaki K., Huang B.W., Sakamoto K., Tsuji Y. Transcriptional regulation of ferritin and antioxidant genes by HIPK2 under genotoxic stress. J. Cell Sci. 2010;123:3863–3871. doi: 10.1242/jcs.073627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nikitaki Z., Mavragani I.V., Laskaratou D.A., Gika V., Moskvin V.P., Theofilatos K., Vougas K., Stewart R.D., Georgakilas A.G. Systemic mechanisms and effects of ionizing radiation: A new ”old” paradigm of how the bystanders and distant can become the players. Semin. Cancer Biol. 2016;37:77–95. doi: 10.1016/j.semcancer.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Dauth I., Krüger J., Hofmann T.G. Homeodomain-interacting protein kinase 2 is the ionizing radiation-activated p53 serine 46 kinase and is regulated by ATM. Cancer Res. 2007;67:2274–2279. doi: 10.1158/0008-5472.CAN-06-2884. [DOI] [PubMed] [Google Scholar]
- 71.Reuven N., Adler J., Porat Z., Polonio-Vallon T., Hofmann T.G., Shaul Y. The tyrosine kinase c-Abl promotes homeodomain-interacting protein kinase 2 (HIPK2) accumulation and activation in response to DNA damage. J. Biol. Chem. 2015;290:16478–16488. doi: 10.1074/jbc.M114.628982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Youle R.J., Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 73.Di Stefano V., Soddu S., Sacchi A., D’Orazi G. HIPK2 contributes to PCAF-mediated p53 acetylation and selective transactivation of p21Waf1 after nonapoptotic DNA damage. Oncogene. 2005;24:5431–5442. doi: 10.1038/sj.onc.1208717. [DOI] [PubMed] [Google Scholar]
- 74.Möller A., Sirma H., Hofmann T.G., Rueffer S., Klimczak E., Dröge W., Will H., Schmitz M.L. PML is required for homeodomain-interacting protein kinase 2 (HIPK2)-mediated p53 phosphorylation and cell cycle arrest but is dispensable for the formation of HIPK domains. Cancer Res. 2003;63:4310–4314. [PubMed] [Google Scholar]
- 75.Gresko E., Ritterhoff S., Sevilla-Perez J., Roscic A., Fröbius K., Kotevic I., Vichalkovski A., Hess D., Hemmings B.A., Schmitz M.L. PML tumor suppressor is regulated by HIPK2-mediated phosphorylation in response to DNA damage. Oncogene. 2009;28:698–708. doi: 10.1038/onc.2008.420. [DOI] [PubMed] [Google Scholar]
- 76.Vaziri H., Dessain S.K., Ng Eaton E., Imai S.I., Frye R.A., Pandita T.K., Guarente L., Weinberg R.A. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/S0092-8674(01)00527-X. [DOI] [PubMed] [Google Scholar]
- 77.Bestor T.H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 2000;9:2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 78.Filion G.J., Zhenilo S., Salozhin S., Yamada D., Prokhortchouk E., Defossez P.A. A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol. Cell. Biol. 2006;26:169–181. doi: 10.1128/MCB.26.1.169-181.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nielsen A.L., Oulad-Abdelghani M., Ortiz J.A., Remboutsika E., Chambon P., Losson R. Heterochromatin formation in mammalian cells: Interaction between histones and HP1 proteins. Mol. Cell. 2001;7:729–739. doi: 10.1016/S1097-2765(01)00218-0. [DOI] [PubMed] [Google Scholar]
- 80.Canzio D., Larson A., Narlikar G.J. Mechanisms of functional promiscuity by HP1 proteins. Trends Cell Biol. 2014;24:377–386. doi: 10.1016/j.tcb.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ayoub N., Jeyasekharan A.D., Bernal J.A., Venkitaraman A.R. HP1β mobilization promotes chromatin changes that initiate the DNA damage response. Nature. 2008;453:682–686. doi: 10.1038/nature06875. [DOI] [PubMed] [Google Scholar]
- 82.Luijsterburg M.S., Dinant C., Lans H., Stap J., Wiernasz E., Lagerwerf S., Warmerdam D.O., Lindh M., Brink M.C., Dobrucki J.W., et al. Heterochromatin protein 1 is recruited to various types of DNA damage. J. Cell Biol. 2009;185:577–586. doi: 10.1083/jcb.200810035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rastogi R.P., Richa, Kumar A., Tyagi M.B., Sinha R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids. 2010;2010:592980. doi: 10.4061/2010/592980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puca R., Nardinocchi L., Givol D., D’Orazi G. Regulation of p53 activity by HIPK2: Molecular mechanisms and therapeutical implications in human cancer cells. Oncogene. 2010;29:4378–4387. doi: 10.1038/onc.2010.183. [DOI] [PubMed] [Google Scholar]
- 85.Ann E.J., Kim M.Y., Yoon J.H., Ahn J.S., Jo E.H., Lee H.J., Lee H.W., Kang H.G., Choi D.W., Chun K.H., et al. Tumor suppressor HIPK2 regulates malignant growth via phosphorylation of Notch1. Cancer Res. 2016;76:4728–4740. doi: 10.1158/0008-5472.CAN-15-3310. [DOI] [PubMed] [Google Scholar]
- 86.Pateras I.S., Havaki S., Nikitopoulou X., Vougas K., Townsend P.A., Panayiotidis M.I., Georgakilas A.G., Gorgoulis V.G. The DNA damage response and immune signaling alliance: Is it good or bad? Nature decides when and where. Pharmacol. Ther. 2015;154:36–56. doi: 10.1016/j.pharmthera.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 87.Moiseeva O., Mallette F.A., Mukhopadhyay U.K., Moores A., Ferbeyre G. DNA damage signaling and p53-dependent senescence after prolonged β-interferon stimulation. Mol. Biol. Cell. 2006;17:1583–1592. doi: 10.1091/mbc.E05-09-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miyamoto S. Nuclear initiated NF-κB signaling: NEMO and ATM take center stage. Cell Res. 2011;21:116–130. doi: 10.1038/cr.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sabatel H., di Valentin E., Gloire G., Dequiedt F., Piette J., Habraken Y. Phosphorylation of p65(RelA) on Ser547 by atm represses NF-κB-dependent transcription of specific genes after genotoxic stress. PLoS ONE. 2012;7:1638. doi: 10.1371/journal.pone.0038246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang Y., Tong J., He F., Yu X., Fan L., Hu J., Tan J., Chen Z. miR-141 regulates TGF-β1-induced epithelial-mesenchymal transition through repression of HIPK2 expression in renal tubular epithelial cells. Int. J. Mol. Med. 2015;35:311–318. doi: 10.3892/ijmm.2014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]