Abstract
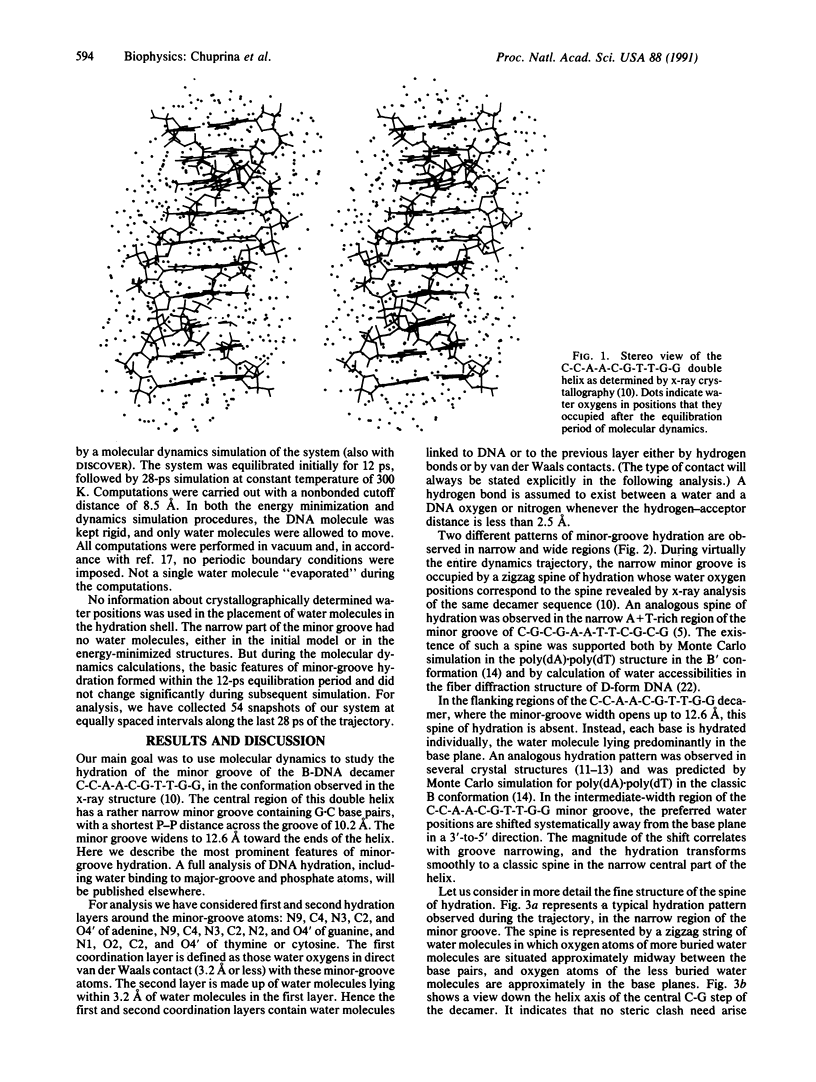
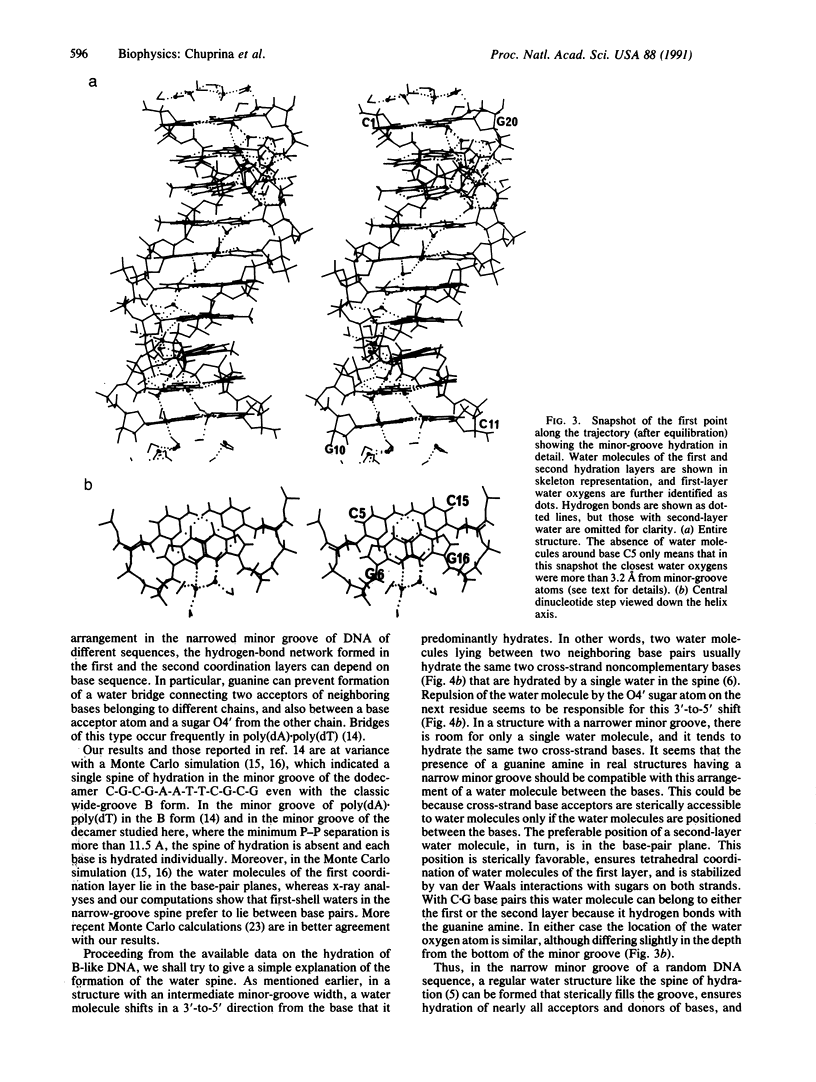
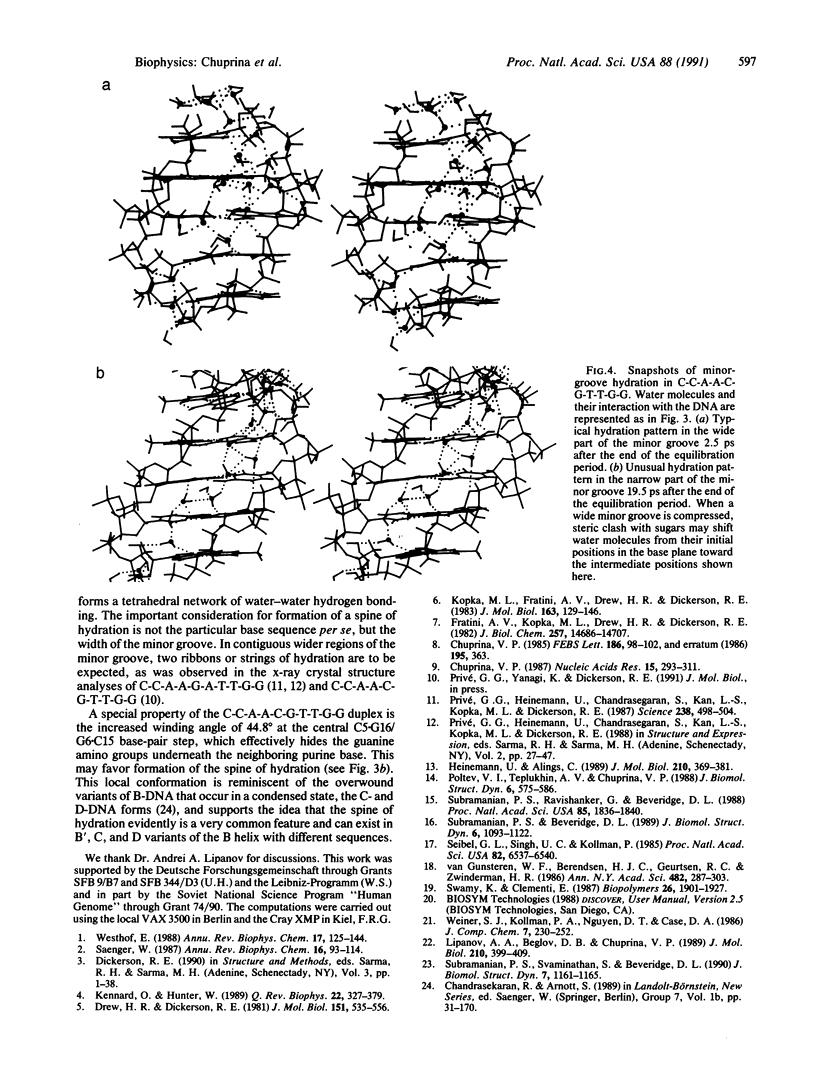
The conformation of the self-complementary B-DNA decamer C-C-A-A-C-G-T-T-G-G is known from a high-resolution x-ray crystal structure analysis. Molecular dynamics simulation of the hydration shell of the decamer has revealed two main types of minor-groove hydration, depending on groove width. The narrow part of the minor groove has a spine of hydration analogous to that described for the A + T-rich center of the minor groove in the dodecamer C-G-C-G-A-A-T-T-C-G-C-G [Drew, H. R. & Dickerson, R. E. (1981) J. Mol. Biol. 151, 535-556], the first hydration layer of which contains one water molecular per base pair. In contrast, in the wide part of the minor groove, each base is hydrated individually, water molecules lying predominantly in the base plane. In intermediate-width regions, preferred water-molecule sites are shifted away from the base plane in a 3'-to-5' direction. This shift becomes more pronounced as the minor groove narrows, until the two water molecules lie approximately midway between base pairs. If the minor groove is narrowed still further, it accommodates only one water molecule, and the hydration transforms to the well-known water spine. The observed pattern agrees with available crystallographic data and with our earlier calculations. The results confirm the assumption that preferred positions of water oxygens in the minor groove depend predominantly on groove width rather than on base sequence. However, the location of water hydrogens, and the network of hydrogen bonding, can depend on base sequence. We suggest a simple explanation of water-spine formation in the narrow minor groove of a random DNA sequence. The spine of hydration may be a property of the minor groove of overwound variants of B-DNA, the C and D forms, for which the middle part of the decamer C-C-A-A-C-G-T-T-G-G can serve as a model.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuprina V. P. Anomalous structure and properties of poly (dA).poly(dT). Computer simulation of the polynucleotide structure with the spine of hydration in the minor groove. Nucleic Acids Res. 1987 Jan 12;15(1):293–311. doi: 10.1093/nar/15.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuprina V. P. Regularities in formation of the spine of hydration in the DNA minor groove and its influence on the DNA structure. FEBS Lett. 1985 Jul 1;186(1):98–102. doi: 10.1016/0014-5793(85)81347-8. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Fratini A. V., Kopka M. L., Drew H. R., Dickerson R. E. Reversible bending and helix geometry in a B-DNA dodecamer: CGCGAATTBrCGCG. J Biol Chem. 1982 Dec 25;257(24):14686–14707. [PubMed] [Google Scholar]
- Heinemann U., Alings C. Crystallographic study of one turn of G/C-rich B-DNA. J Mol Biol. 1989 Nov 20;210(2):369–381. doi: 10.1016/0022-2836(89)90337-9. [DOI] [PubMed] [Google Scholar]
- Kennard O., Hunter W. N. Oligonucleotide structure: a decade of results from single crystal X-ray diffraction studies. Q Rev Biophys. 1989 Aug;22(3):327–379. doi: 10.1017/s0033583500002997. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Fratini A. V., Drew H. R., Dickerson R. E. Ordered water structure around a B-DNA dodecamer. A quantitative study. J Mol Biol. 1983 Jan 5;163(1):129–146. doi: 10.1016/0022-2836(83)90033-5. [DOI] [PubMed] [Google Scholar]
- Lipanov A. A., Beglov D. B., Chuprina V. P. DNA B to D transition can be explained in terms of hydration economy of the minor groove atoms. J Mol Biol. 1989 Nov 20;210(2):399–409. doi: 10.1016/0022-2836(89)90339-2. [DOI] [PubMed] [Google Scholar]
- Poltev V. I., Teplukhin A. V., Chuprina V. P. Monte-Carlo simulation of DNA duplex hydration. B and B' conformations of poly(dA).poly(dT) have different hydration shells. J Biomol Struct Dyn. 1988 Dec;6(3):575–586. doi: 10.1080/07391102.1988.10506508. [DOI] [PubMed] [Google Scholar]
- Privé G. G., Heinemann U., Chandrasegaran S., Kan L. S., Kopka M. L., Dickerson R. E. Helix geometry, hydration, and G.A mismatch in a B-DNA decamer. Science. 1987 Oct 23;238(4826):498–504. doi: 10.1126/science.3310237. [DOI] [PubMed] [Google Scholar]
- Seibel G. L., Singh U. C., Kollman P. A. A molecular dynamics simulation of double-helical B-DNA including counterions and water. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6537–6540. doi: 10.1073/pnas.82.19.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian P. S., Beveridge D. L. A theoretical study of the aqueous hydration of canonical B d(CGCGAATTCGCG): Monte Carlo simulation and comparison with crystallographic ordered water sites. J Biomol Struct Dyn. 1989 Jun;6(6):1093–1122. doi: 10.1080/07391102.1989.10506539. [DOI] [PubMed] [Google Scholar]
- Subramanian P. S., Ravishanker G., Beveridge D. L. Theoretical considerations on the "spine of hydration" in the minor groove of d(CGCGAATTCGCG).d(GCGCTTAAGCGC): Monte Carlo computer simulation. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1836–1840. doi: 10.1073/pnas.85.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian P. S., Swaminathan S., Beveridge D. L. Theoretical account of the 'spine of hydration' in the minor groove of duplex d(CGCGAATTCGCG). J Biomol Struct Dyn. 1990 Apr;7(5):1161–1165. doi: 10.1080/07391102.1990.10508553. [DOI] [PubMed] [Google Scholar]
- Swamy K. N., Clementi E. Hydration structure and dynamics of B- and Z-DNA in the presence of counterions via molecular dynamics simulations. Biopolymers. 1987 Nov;26(11):1901–1927. doi: 10.1002/bip.360261106. [DOI] [PubMed] [Google Scholar]
- Van Gunsteren W. F., Berendsen H. J., Geurtsen R. G., Zwinderman H. R. A molecular dynamics computer simulation of an eight-base-pair DNA fragment in aqueous solution: comparison with experimental two-dimensional NMR data. Ann N Y Acad Sci. 1986;482:287–303. doi: 10.1111/j.1749-6632.1986.tb20962.x. [DOI] [PubMed] [Google Scholar]
- Westhof E. Water: an integral part of nucleic acid structure. Annu Rev Biophys Biophys Chem. 1988;17:125–144. doi: 10.1146/annurev.bb.17.060188.001013. [DOI] [PubMed] [Google Scholar]



