Abstract
Serious mental illness (SMI) is associated with disproportionately high rates of cigarette smoking. The identification of factors that contribute to persistent smoking in people with SMI may lead to the development and adoption of tobacco control policies and treatment approaches that help these smokers quit. This commentary examines factors underlying smoking persistence in people with SMI from the perspective of behavioral economics, a discipline that applies economic principles to understanding drug abuse and dependence. Studies, conducted in the Northeastern US within the past 30 years, that compare the reinforcing effects of nicotine and the costs of smoking in smokers with and without schizophrenia and depression are discussed, and interventions that may reduce the reinforcing efficacy of nicotine and increase the costs of smoking in people with SMI are described.
Keywords: Schizophrenia, Depression, Comorbidity, Cigarette smoking, Tobacco dependence, Tobacco control, Socioeconomic status, Behavioral economics
People with serious mental illness (SMI) are 2–3 times more likely to smoke cigarettes, and smoke more cigarettes per day than those without mental illness (Lasser et al., 2000; McClave et al., 2010). Thus, people with current SMI, who make up approximately 28% of the US population, smoke approximately 44% of cigarettes sold in the US (Lasser et al., 2000). Approximately 50% of deaths in people with SMI are thought to be due to tobacco (Callaghan et al., 2014, Kelly et al., 2011). Pharmacological and behavioral treatments increase cessation among people with SMI (Evins et al., 2015), but, overall, their cessation rates are extremely low (Cook et al., 2014).
Numerous biological and environmental risk factors contribute to smoking among people with SMI, including poverty, unemployment, lack of clinical attention to tobacco use, deficits in working memory and task persistence, a paucity of, or insensitivity to, alternative reinforcers, and high levels of negative affect and low levels of positive affect (anhedonia), along with expectancies that smoking will improve these states (Tidey & Miller, 2015; Ziedonis et al., 2008). These factors can be considered through the lens of behavioral economics, a discipline that applies economic principles when examining why individuals engage in maladaptive behaviors (Bickel et al., 2014). According to this perspective, drug use is a function of both its reinforcing efficacy and its cost, in terms of monetary price, effort, or a relinquished opportunity to obtain an alternative reinforcer. Those who persistently use a drug are thought to overvalue its reinforcing effects and undervalue its costs, due to endogenous factors, such as a pathological reinforcement system, and exogenous factors, such as environmental deprivation. A behavioral economics perspective is conceptually sophisticated because it describes how persistent smoking results from interactions among these endogenous and exogenous costs and benefits, and is useful because it suggests novel approaches for reducing smoking in people with SMI. This commentary discusses results from experimental studies that have compared sensitivities to cigarette reinforcement and cigarette cost factors in smokers with and without SMI from a behavioral economics perspective. It focuses on smokers with schizophrenia (SS) and smokers with depression (SD), because the majority of experimental research on smoking in SMI has examined these populations.
Overvaluation of Cigarette Reinforcement among People with SMI
The prevalence of heavy smoking among SS and SD is higher than that of non-psychiatric smokers (de Leon & Diaz, 2005; Pratt & Brody, 2010). Furthermore, even after controlling for daily smoking rate, SS have higher levels of nicotine intake than non-psychiatric smokers, due to their intense smoking topography characteristics (Tidey et al., 2005; Williams et al., 2011). The functional significance of their elevated nicotine intake is unknown, but is often interpreted as reflecting attempts by SS to stimulate low-affinity alpha-7 nicotinic acetylcholine receptors (nAChRs) to remediate aberrant sensory gating and related cognitive deficits (Brunzell & McIntosh 2012). While this hypothesis is intuitively appealing, the evidence that SS experience stronger pro-cognitive effects of nicotine than other smokers is mixed (Hahn et al., 2013).
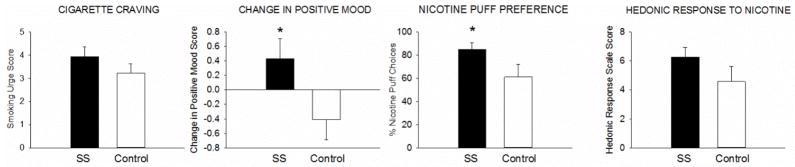
An alternative hypothesis is that the neuropathology underlying schizophrenia and depression confers heightened vulnerability to the reinforcing effects of nicotine (Brody et al., 2009; Chambers et al., 2001). This hypothesis is supported by preclinical research (Berg et al., 2014), but has been understudied in humans. One relevant study found that SS and SD made twice as many hypothetical choices for smoking versus alternative reinforcers (candy, seeing a movie) than the controls, suggesting that SS and SD experience stronger smoking reinforcement (Spring et al., 2003). Also consistent with this hypothesis is a study that found that after 3 days of continuous abstinence, SS reported stronger cigarette craving, a stronger smoking-related increase in positive mood, a stronger nicotine preference, and a greater hedonic response to nicotine than controls (Tidey et al., 2014; Figure 1). Likewise, SD made more responses for cigarette puffs vs. a monetary reinforcer and experienced a larger increase in positive affect after smoking than controls (Audrain-McGovern et al., 2014). Finally, results from a study that used the Cigarette Purchase Task to compare SS and controls on hypothetical cigarette demand as a function of price are consistent with this hypothesis (MacKillop & Tidey, 2011). On this task, participants are asked to report the number of cigarettes that they would consume in one day at various costs. From these values, a demand curve can be constructed that relates price to consumption, and several demand indices can be generated: (a) demand intensity, which is consumption when the price is zero, (b) Omax, which is the maximum amount of money allocated to cigarettes, (c) breakpoint, which is the first price at which participant reports zero consumption, (d) Pmax, which is the price at which Omax occurs, and (e) α, which is the rate of decrease in consumption as a function of price (Hursh et al., 1988). This study found that SS reported a greater demand intensity, which was associated with higher ad libitum smoking rate, consistent with the idea that the incentive value of smoking is stronger among SS (MacKillop & Tidey, 2011).
Figure 1.
Cigarette craving, smoking-related change in positive-mood, percent choices for nicotine puffs, and hedonic response to nicotine puffs, in smokers with schizophrenia (SS) and equally-heavy smokers without psychiatric illness. Bars represent Mean +/− SEM.
* indicates a significant difference between groups (p < .05). Data were collected in Providence, RI, in 2009–2012. From Tidey et al., 2014.
In addition to its primary reinforcing effects, nicotine enhances the reinforcing effects of other stimuli, and these effects may be even more critical to maintaining nicotine self-administration than its primary reinforcing effects (Chaudri et al., 2006; Donny et al. 2003). This raises the question of whether stronger reinforcement-enhancing effects of nicotine in smokers with SMI compared to controls may contribute to smoking persistence in SMI. This hypothesis was recently supported by a study that found that SD, but not controls, reported greater enjoyment of activities in their natural environments while smoking than while abstinent (Audrain-McGovern et al., 2014). Among SS, this hypothesis was recently tested using a preclinical model of schizophrenia that examined whether pretreating rats with phencyclidine, to model the negative symptoms of schizophrenia, altered their responding for a visual stimulus paired with nicotine (Swalve et al., 2015). Although the results of this study were mixed, the hypothesis that SS experience stronger reinforcement-enhancing effects of nicotine than those without SMI merits investigation in human experimental studies.
Undervaluation of Smoking Costs among People with SMI
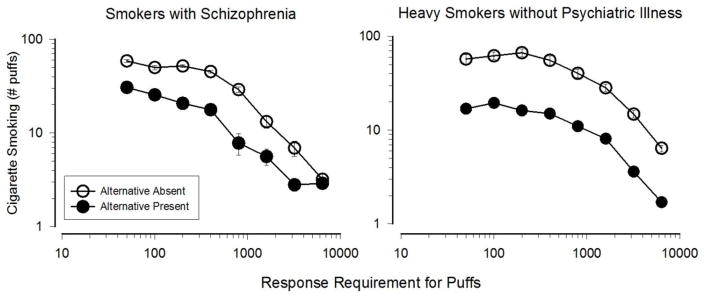
The costs of smoking include the price of cigarettes, the effort required to obtain them, the health consequences of smoking, and the opportunity cost incurred by forgoing an alternative reinforcer in order to smoke (Bickel et al., 2014). From a behavioral economic perspective, individuals who are less sensitive to these costs are more likely to smoke. Although few experimental studies have directly compared sensitivities to the monetary and behavioral costs of smoking in smokers with vs. without SMI, one study examined sensitivity to these costs in SS (Tidey et al., 1999) using the same experimental procedure and equipment as a study that had compared them in heavy smokers without psychiatric illness (Bickel et al., 1995). The effect of response cost on cigarette puff consumption was similar in these groups (Figure 2). A study that used a similar procedure to compare responses for cigarette puffs versus money in SD and controls across a range of response costs found that SD made twice as many responses for puffs than controls, but were not differentially sensitive to increases in response cost (Audrain-McGovern et al., 2014). Finally, a study that compared changes in cigarette demand as a function of price in SS and controls found that the groups were equally sensitive to price increases (MacKillop & Tidey, 2011). Based on this limited evidence, SS and SD do not appear to be less sensitive to the monetary and behavioral costs of smoking than controls.
Figure 2.
Effects of varying the effort (response requirement) for cigarette puffs, and the presence or absence of an alternative monetary reinforcer, on smoking in smokers with schizophrenia (left; reprinted from Tidey et al., 1999) and smokers without psychiatric disoders (right; data from Bickel et al., 1995). Data were collected in the 1990s in Burlington. VT. Points represent Mean +/− SEM.
The most significant costs of smoking are its effects on health. SS, SD and controls matched on smoking rate report comparable levels of concern about the health costs of smoking (Tidey & Rohsenow, 2009; Weinberger et al., 2011). Furthermore, among both SS and controls, level of concern about the current and future negative health consequences of smoking is related to intention to quit within 6 months (Tidey & Rohsenow, 2009); however, whether level of health concern predicts cessation outcomes is unknown.
One cost factor on which smokers with and without SMI do differ is access and sensitivity to alternative reinforcers, and this may be an important link in the association between SMI and smoking. For example, depression is associated with a decrease in the frequency and pleasantness of non-smoking-related reinforcers, and this decrease is associated with smoking escalation in young adulthood (Audrain-McGovern et al., 2011). One mechanism underlying this association appears to be anhedonia, defined as diminished anticipation and enjoyment of events that are normally considered pleasurable. The association between anhedonia and smoking has been found in both experimental and longitudinal studies (Audrain-McGovern et al., 2012; Leventhal et al., 2014). Frequently associated with depression, anhedonia is also a common symptom of schizophrenia (Watson & Naragon-Gainey, 2010). The role of anhedonia in the association between schizophrenia and smoking is understudied, but one recent study found that anhedonia was associated with cigarette craving in SS, and a related behavioral measure, reward-based learning, was associated with nicotine dependence severity (AhnAllen et al., 2012). Anhedonia may also partly account for the finding that alternative reinforcers competed relatively poorly for smoking reinforcement among SS and SD (Spring et al., 2003).
Behavioral Economic Approaches to Reducing Smoking in SMI
From a behavioral economic perspective, smoking cessation rates among people with SMI can be increased by reducing the reinforcing efficacy of cigarettes and increasing the costs of smoking. The reinforcing efficacy of cigarettes can be reduced by lowering their nicotine content below an addiction threshold (Benowitz & Henningfield, 2013). Among a general population sample of smokers, use of very low nicotine content (VLNC) cigarettes decreases smoking and cigarette demand, and increases sensitivity to cigarette cost (Donny et al., 2015; Smith et al., 2015). This suggests that an FDA-mandated reduction in the nicotine content of cigarettes, as authorized by the 2009 Tobacco Control Act, may increase the effectiveness of other tobacco control approaches and smoking cessation treatments. Among SS, acute VLNC cigarette use reduces cigarette craving, withdrawal, and smoking (Tidey et al., 2013), and studies of extended VLNC cigarette use in SS and SD are underway (https://clinicaltrials.gov/ct2/show/NCT02019459, https://clinicaltrials.gov/ct2/show/NCT01928758, https://clinicaltrials.gov/ct2/show/NCT02232737). Another method of reducing the reinforcing efficacy of cigarettes is varenicline treatment. As a low-efficacy agonist at alpha4beta2 nAChRs, varenicline both partially substitutes for and blocks the full agonist effects of nicotine (Mihalak et al., 2006), and recent studies have found it to be safe and effective in SS and SD (Evins et al., 2015). Furthermore, varenicline also substitutes for and blocks the reinforcement-enhancing effects of nicotine (Levin et al., 2012).
Several potential methods of increasing the costs of smoking in SMI should be explored. Increasing cigarette excise taxes and reducing cigarette outlets in socioeconomically-deprived areas should help to reduce smoking persistence in SMI (Institute of Medicine, 2007), particularly if low-cost treatments are also made available to these smokers. Providing smokers with personalized risk profiles, such as their lung age or heart age (Morris & Temple, 1985; Davies et al., 2013), and training them to imagine positive future events (e.g., Daniel et al., 2013) may help motivate smokers with SMI to seek treatment by increasing the immediacy and salience of the health costs of smoking and the benefits of abstinence. Helping them to identify and engage in substitute reinforcing activities may reduce the likelihood of relapse during cessation treatment by increasing the opportunity cost of smoking (Goelz et al., 2014).
Finally, contingency management (CM) interventions directly increase the opportunity cost of smoking by delivering tangible incentives upon objective evidence of abstinence (Higgins et al., 2002). CM interventions are highly effective in people with SMI (Tidey, 2012). For example, among SS, a 3-week CM intervention decreased nicotine intake by 34%, compared to a 4% decrease among those randomized to the control condition (Tidey et al., 2011). Among depression-prone pregnant and post-partum women, CM reduced smoking and depression severity (Lopez et al., 2015). These promising findings warrant the development of longer CM smoking cessation interventions for people with SMI, including internet- and smart phone-based CM approaches (e.g., Dallery et al., 2013). Considering the high rates of smoking persistence among people with SMI, combined treatments that both reduce the reinforcing value of smoking and increase the cost of smoking are also warranted. For example, the effectiveness of CM interventions for smoking would likely be enhanced if combined with an approach that reduces the reinforcing efficacy of cigarettes, such as varenicline or VLNC cigarettes.
Conclusions
People with SMI are not benefiting from current tobacco control policies and treatments. From a behavioral economic perspective, improving their cessation rates will require changing the benefit-cost ratio of smoking. As reviewed in this commentary, policies and treatment approaches that can increase the costs and reduce the reinforcing efficacy of cigarettes in people with SMI include reducing the nicotine content of cigarettes to a minimally-addictive level, reducing cigarette availability, and facilitating access to varenicline and contingency management treatments. In addition, research is warranted to examine whether combinations of these approaches, such as varenicline combined with contingency management or very low nicotine content cigarettes, will help people with SMI achieve and maintain smoking abstinence.
Highlights.
People with serious mental illness have very low smoking cessation rates.
Reducing smoking requires decreasing cigarette reinforcement and increasing its cost.
Very low nicotine cigarettes and varenicline increase reduce smoking reinforcement.
Contingency management interventions increase the opportunity cost of smoking.
Combining these interventions may have additive effects.
Acknowledgments
This manuscript is based on symposium papers presented at the 2015 meetings of the College on Problems of Drug Dependence and the Vermont Center on Behavior and Health. I am grateful to Adam M. Leventhal, Ph.D., and Stephen T. Higgins, Ph.D., for inviting me to participate in those symposia, and to Warren K. Bickel, Ph.D., whose insights spurred my interest in behavioral economics. The preparation of this manuscript was supported by grants P50DA036114 and U54DA031659 from National Institute on Drug Abuse and the Food and Drug Administration’s Center for Tobacco Products.
Footnotes
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Conflict of Interest
The author reports no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn WY, Rass O, Fridberg DJ, Bishara AJ, Forsyth JK, Breier A, Busemeyer JR, Hetrick WP, Bolbecker AR, O’Donnell BF. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. J Abnorm Psychol. 2011 Nov;120(4):911–21. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnallen CG, Liverant GI, Gregor KL, Kamholz BW, Levitt JJ, Gulliver SB, Pizzagalli DA, Koneru VK, Kaplan GB. The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry Res. 2012 Mar 30;196(1):9–14. doi: 10.1016/j.psychres.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Leventhal AM, Cuevas J, Rodgers K, Sass J. Where is the pleasure in that? Low hedonic capacity predicts smoking onset and escalation. Nicotine Tob Res. 2012 Oct;14(10):1187–96. doi: 10.1093/ntr/nts017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Rodgers K, Cuevas J. Declining alternative reinforcers link depression to young adult smoking. Addiction. 2011 Jan;106(1):178–87. doi: 10.1111/j.1360-0443.2010.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013 May;22( Suppl 1):i14–7. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg SA, Sentir AM, Cooley BS, Engleman EA, Chambers RA. Nicotine is more addictive, not more cognitively therapeutic in a neurodevelopmental model of schizophrenia produced by neonatal ventral hippocampal lesions. Addict Biol. 2014 Nov;19(6):1020–31. doi: 10.1111/adb.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR, Badger GJ. Effects of simulated employment and recreation on drug taking: A behavioral economic analysis. Exper Clin Psychopharmacol. 1999 Nov;3(4):467–476. http://dx.doi.org/10.1037/1064-1297.3.4.467. [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, Murphy JG. The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol. 2014;10:641–77. doi: 10.1146/annurev-clinpsy-032813-153724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Brody AL, Olmstead RE, Abrams AL, Costello MR, Khan A, Kozman D, Saxena S, Farahi J, London ED, Mandelkern MA. Effect of a history of major depressive disorder on smoking-induced dopamine release. Biol Psychiatry. 2009 Nov 1;66(9):898–901. doi: 10.1016/j.biopsych.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012 Apr;37(5):1134–43. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan RC, Veldhuizen S, Jeysingh T, Orlan C, Graham C, Kakouris G, Remington G, Gatley J. Patterns of tobacco-related mortality among individuals diagnosed with schizophrenia, bipolar disorder, or depression. J Psychiatr Res. 2014 Jan;48(1):102–10. doi: 10.1016/j.jpsychires.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001 Jul 15;50(2):71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006 Mar;184(3–4):353–66. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA. 2014 Jan 8;311(2):172–82. doi: 10.1001/jama.2013.284985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Grabinski MJ. Internet-based contingency management to promote smoking cessation: a randomized controlled study. J Appl Behav Anal. 2013 Dec;46(4):750–64. doi: 10.1002/jaba.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, Epstein LH. The future is now: reducing impulsivity and energy intake using episodic future thinking. Psychol Sci. 2013 Nov 1;24(11):2339–42. doi: 10.1177/0956797613488780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TL, Gompels M, Johnston S, Bovill B, May MT. Mind the gap: difference between Framingham heart age and real age increases with age in HIV-positive individuals-a clinical cohort study. BMJ Open. 2013 Oct 25;3(10):e003245. doi: 10.1136/bmjopen-2013-003245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005 Jul 15;76(2–3):135–57. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003 Aug;169(1):68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, al’Absi M, Carmella SG, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, Le CT, McClernon FJ, Montoya ID, Murphy SE, Robinson JD, Stitzer ML, Strasser AA, Tindle H, Hatsukami DK. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015 Oct;373(14):1340–9. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins AE, Cather C, Laffer A. Treatment of tobacco use disorders in smokers with serious mental illness: toward clinical best practices. Harv Rev Psychiatry. 2015 Mar-Apr;23(2):90–8. doi: 10.1097/HRP.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz PM, Audrain-McGovern JE, Hitsman B, Leone FT, Veluz-Wilkins A, Jepson C, Wileyto EP, D’Avanzo PA, Rivera JG, Schnoll RA. The association between changes in alternative reinforcers and short-term smoking cessation. Drug Alcohol Depend. 2014 May 1;138:67–74. doi: 10.1016/j.drugalcdep.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Holcomb HH, Gold JM. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biol Psychiatry. 2013 Sep 15;74(6):436–43. doi: 10.1016/j.biopsych.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Alessi SM, Dantona RL. Voucher-based incentives. A substance abuse treatment innovation. Addict Behav. 2002 Nov-Dec;27(6):887–910. doi: 10.1016/s0306-4603(02)00297-6. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Raslear TG, Shurtleff D, Bauman R, Simmons L. A cost-benefit analysis of demand for food. J Exper Anal Behav. 1988;50:419–40. doi: 10.1901/jeab.1988.50-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Ending the tobacco problem: a blueprint for the nation. Washington D.C: Institute of Medicine of the National Academies; 2007. [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000 Nov;284(20):2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Trujillo M, Ameringer KJ, Tidey JW, Sussman S, Kahler CW. Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. J Abnorm Psychol. 2014 May;123(2):375–86. doi: 10.1037/a0036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Sved AF, Donny EC. Varenicline dose dependently enhances responding for nonpharmacological reinforcers and attenuates the reinforcement-enhancing effects of nicotine. Nicotine Tob Res. 2012 Mar;14(3):299–305. doi: 10.1093/ntr/ntr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AA, Skelly JM, Higgins ST. Financial incentives for smoking cessation among depression-prone pregnant and newly postpartum women: effects on smoking abstinence and depression ratings. Nicotine Tob Res. 2015 Apr;17(4):455–62. doi: 10.1093/ntr/ntu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Murphy CM, Martin RA, Stojek M, Tidey JW, Colby SM, Rohsenow DJ. Predictive validity of a cigarette purchase task in a randomized controlled trial of contingent vouchers for smoking in individuals with substance use disorders. Nicotine Tob Res. 2015 Oct 24; doi: 10.1093/ntr/ntv233. pii: ntv233. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology (Berl) 2011 Jul;216(1):91–9. doi: 10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010 Dec;100(12):2464–72. doi: 10.2105/AJPH.2009.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006 Sep;70(3):801–5. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Morris JF, Temple W. Spirometric “lung age” estimation for motivating smoking cessation. Prev Med. 1985 Sep;14(5):655–62. doi: 10.1016/0091-7435(85)90085-4. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Brody DJ. NCHS data brief. 34. Hyattsville, MD: National Center for Health Statistics; 2010. Depression and smoking in the U.S. household population aged 20 and over, 2005–2008. [PubMed] [Google Scholar]
- Pulcu E, Trotter PD, Thomas EJ, McFarquhar M, Juhasz G, Sahakian BJ, Deakin JF, Zahn R, Anderson IM, Elliott R. Temporal discounting in major depressive disorder. Psychol Med. 2014 Jul;44(9):1825–34. doi: 10.1017/S0033291713002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Cassidy RN, Tidey JW, Luo X, Le CT, Hatsukami DK, Donny EC. Impact of smoking reduced nicotine content cigarettes on sensitivity to cigarette price: Results from a multi-site clinical trial. Presented at the annual NIH Tobacco Regulatory Science conference; Bethesda, MD. 2015. Oct, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swalve N, Pittenger ST, Bevins RA, Li M. Behavioral effects of phencyclidine on nicotine self-administration and reinstatement in the presence or absence of a visual stimulus in rats. Psychopharmacology (Berl) 2015 Aug;232(16):2877–87. doi: 10.1007/s00213-015-3923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW. Using incentives to reduce substance use and other health risk behaviors among people with serious mental illness. Prev Med. 2012 Nov;55( Suppl):S54–60. doi: 10.1016/j.ypmed.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Colby SM, Xavier EM. Effects of smoking abstinence on cigarette craving, nicotine withdrawal, and nicotine reinforcement in smokers with and without schizophrenia. Nicotine Tob Res. 2014 Mar;16(3):326–34. doi: 10.1093/ntr/ntt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Higgins ST, Bickel WK, Steingard S. Effects of response requirement and the availability of an alternative reinforcer on cigarette smoking by schizophrenics. Psychopharmacology (Berl) 1999 Jul;145(1):52–60. doi: 10.1007/s002130051031. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miller ME. Smoking cessation and reduction in people with chronic mental illness. BMJ. 2015 Sep 21;351:h4065. doi: 10.1136/bmj.h4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Ahnallen CG. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res. 2013 Jan;15(1):121–9. doi: 10.1093/ntr/nts098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology (Berl) 2011 Sep;217(2):279–87. doi: 10.1007/s00213-011-2282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM. Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depend. 2005;80(2):259–65. doi: 10.1016/j.drugalcdep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ. Smoking expectancies and intention to quit in smokers with schizophrenia, schizoaffective disorder and non-psychiatric controls. Schizophr Res. 2009 Dec;115(2–3):310–6. doi: 10.1016/j.schres.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clin Psychol Rev. 2010 Nov;30(7):839–48. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, George TP, McKee SA. Differences in smoking expectancies in smokers with and without a history of major depression. Addict Behav. 2011;36(4):434–7. doi: 10.1016/j.addbeh.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Lu SE, Kumar S, Steinberg ML, Cottler B, et al. Shorter interpuff interval is associated with higher nicotine intake in smokers with schizophrenia. Drug Alcohol Depend. 2011;118(2–3):313–9. doi: 10.1016/j.drugalcdep.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Moss TG, Rabin RA, George TP. Effects of cigarette smoking status on delay discounting in schizophrenia and healthy controls. Addict Behav. 2012 Jan;37(1):67–72. doi: 10.1016/j.addbeh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10(12):1691–715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]