Abstract
Objectives
To evaluate the hepatocellular carcinoma (HCC) risk in hepatitis B virus (HBV)-infected Alaska Native (AN) children and young adults.
Study design
Retrospective analysis of a population-based cohort of HBV-infected AN persons followed during 1982–2012. All HBV-infected persons were offered HCC screening regardless of age using alpha-fetoprotein (AFP) every 6 months; persons with an elevated AFP or persons at high-risk for HCC, such as cirrhosis, family history of HCC, were offered ultrasound. We calculated the HCC incidence/1,000 person-years from date of cohort entry until death, diagnosis of HCC, or attaining the age of 40 years (males) or 50 years (females).
Results
We followed 1083 HBV-infected persons (56% male) comprising 5 genotypes (A2 [12.5%], B6 [1.7%], C [5.3%], D [49.7%], F1 [18.6%], unknown [12.4%]) for a median of 23.4 years/person. We observed 22 HCC cases (incidence/1,000 person-years follow-up: 1.0); 19 HCC cases among persons with genotype F1. There was no significant difference in HCC incidence between males (1.4) and females (0.6). The HCC incidence was significantly higher for persons with genotype F1 (4.4) compared with genotype A2 (0.4) and D (0.2) and remained higher among persons with HBV genotype F1 excluding persons with HCC family history/cirrhosis (1.9).
Conclusions
HBV genotype F1-infected AN children and young adults are at high risk for HCC and should receive HCC surveillance. For HBV-infected males and females age <40 and <50 years, respectively, in regions of the world with a high genotype F prevalence, testing/confirming genotype F can identify persons who could benefit from HCC surveillance.
Keywords: cancer screening, diagnosis, epidemiology, cohort study, incidence study
Persons with chronic hepatitis B virus (HBV) infection are at increased risk for death from cirrhosis and hepatocellular carcinoma (HCC) (1). The American Association for the Study of Liver Diseases (AASLD) practice guidelines recommend semiannual HCC surveillance for HBV carriers at high risk (2). The AASLD practice guidelines consider HCC surveillance cost-effective if the HCC incidence exceeds 0.2%/year (incidence >2/1,000 person-years of follow-up) in persons with HBV infection (2). Persons for whom HCC surveillance is indicated include HBV carriers with cirrhosis, a family history of HCC, and Asian males aged ≥40 years and Asian females ≥50 years (2). The prevalence of chronic HBV infection in the United States during 1999–2006 among children aged 6–19 years was 0.05% (29,000) and among adults aged 20–49 years was 0.30% (3). Recommendations for HCC surveillance in HBV-infected males aged <40 years and females aged <50 years are lacking because the benefits of surveillance are uncertain.
We have prospectively followed a population-based cohort of HBV-infected Alaska Native (AN) persons in Alaska since 1982 (4). All cohort persons have been offered HCC surveillance regardless of age or risk factors (5). A previous study using data from this cohort of AN people with chronic HBV infection reported an HCC incidence of 0.19%/year (0.23%/year in men and 0.12%/year in women) (6). The HCC risk varies by HBV genotype with the highest risk among persons with HBV genotypes C and F1(7, 8). Additionally, AN persons with HBV genotype F1 developed HCC at younger age (23 years) compared with those infected by one of the other four genotypes (60 years) found in this population (9); infection with HBV genotype F1 was identified in 68% of HBV-infected AN persons with HCC compared with 18% of persons not developing HCC (9). A recent analysis evaluating the incidence of HCC by genotype among AN persons demonstrated that the regional differences in HCC risk were attributable to the geographic distribution of specific HBV genotypes (8).
The prevalence of chronic HBV infection has declined among the younger age-groups in North America and Western Europe in recent years because of widespread HBV vaccine implementation; however, chronic HBV infection remains endemic in many parts of the world with hepatitis B surface antigen (HBsAg) seroprevalence among persons aged 0–19 years exceeding in 8% in Western sub-Saharan countries and exceeding 3% in South Asia (10). None of the previous studies among HBV-infected AN persons have specifically evaluated the risk for HCC among those not meeting age/sex criteria for HCC surveillance according to AASLD practice guidelines (2). The objective of this study is to evaluate whether certain subgroups of HBV-infected AN children and young adults (males aged <40 years and females aged <50 years) have a high enough HCC incidence (>0.2%/year) to benefit from HCC surveillance.
METHODS
Study cohort of Alaska Native persons with HBV infection
During 1982–1986, approximately 54,000 AN persons (84% of the AN population in Alaska) were screened for HBV infection as part of the comprehensive statewide screening and vaccination program (4). Persons without evidence of immunity to HBV were offered HBV vaccination. Persons identified as having chronic HBV infection (two positive HBsAg results ≥6 months apart) during and subsequent to the vaccination campaign were entered in a HBV clinical registry maintained by the Alaska Tribal Health System. All HBV-infected AN persons were eligible to receive care through the Alaska Tribal Health System. The Liver Disease and Hepatitis Program of the Alaska Native Tribal Health Consortium (ANTHC) uses the HBV clinical registry to track when patients were due for routine appointments to monitor their disease and to send patients reminders every 6 months to follow-up with a provider for clinical evaluation and laboratory testing. Patients in the HBV registry providing consent were enrolled into a long-term prospective study cohort. For this present analysis, we selected study cohort participants if they were a male aged <40 years or a female aged <50 years at any point during 1982–2012 (the study period). Because our study population represents an observational cohort, information on other risk factors for cirrhosis and liver cancer, such as alcohol use, fatty liver disease, human immunodeficiency virus (HIV) infection and hepatitis C virus (HCV) infection, was not available for all study participants. However, persons with known HIV or HCV infection were excluded from analysis.
Laboratory testing
All AN persons with chronic HBV infection have been reminded by mail to have their blood drawn semiannually for serologic tests since 1982. The sera were sent to the Alaska Native Medical Center in Anchorage, Alaska for testing that included alpha-fetoprotein (AFP) and HBsAg (6). Beginning in 2001, sera were also tested for HBV DNA and aspartate/alanine aminotransferase (AST/ALT) levels; additionally, the HBV genotype/subgenotype was determined by sequencing the S gene of the HBV genome (9). A complete blood count was not routinely requested because of specimen instability during transport from rural Alaska to the Alaska Native Medical Center.
Identification of HBV-infected AN persons with HCC
The ANTHC Liver Disease and Hepatitis Program has used AFP as the initial screening tool for HCC surveillance because many HBV-infected persons lived in remote villages where ultrasound was unavailable; persons with an elevated AFP were referred for an ultrasound or computed tomography of the liver (5). The AFP cut-off threshold triggering imaging referral was >25 ng/mL during 1982–1992, >15 ng/mL for 1993–1999, and >10 ng/ml after 2000. In addition, semiannual ultrasound examination of the liver was offered to persons living in areas where ultrasound was readily available and to all HBV infected persons with a family history of HCC or cirrhosis. Patients with a suspicious lesion on radiologic imaging were recommended to receive a biopsy or surgical resection of the lesion at the Alaska Native Medical Center (6).
Cohort persons with HCC were primarily identified by pathologic confirmation if biopsy/surgical specimen was available for examination (9). For persons who declined further evaluation and management, including biopsy, we relied on the treating hepatologist’s clinical diagnosis of HCC based on the patient’s clinical characteristics and tumor features on imaging. Additionally, to identify persons with HCC who might have received care outside the Alaska Tribal Health System, we cross-referenced the names of HBV-infected AN persons with the Alaska Native Tumor Registry and with death certificate data from the Alaska Department of Health and Social Services’ Bureau of Vital Statistics (11). As part of the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, the Alaska Native Tumor Registry follows the Program’s guidance for identifying and reporting of HCC cases (12). For patients with available liver histology, we defined cirrhosis as an Ishak fibrosis score 5 or 6.
Statistical analysis
We calculated the HCC incidence/1,000 person-years of follow-up. Person-years of follow-up began on January 1, 1982 for persons with a known HBsAg positive result prior to that date or on the date of first positive HBsAg result occurring after 1982. Person-years of follow-up ended on the date of death, date of HCC diagnosis, the date males attained age 40 years and females attained age 50 years, or at the conclusion of the study (December 31, 2012). An HCC case was defined as an HCC tumor identified in an HBV-infected AN person consenting to participate in our study. We categorized HCC tumors as early-stage (single tumor ≤5 cm in diameter or ≤3 tumors each ≤3 cm in diameter) or late-stage based on Milan criteria for liver transplantation (13). Survival >5 years after HCC diagnosis (i.e., 5-year survival) was calculated from the date of HCC diagnosis. Persons diagnosed with HCC and alive on December 31, 2012 were excluded from 5-year survival analysis if they were followed for <5 years after HCC diagnosis. We compared median survival between groups by equality of medians test and log rank test, and the 5-year survival by Fisher’s exact test. Poisson regression was used to evaluate whether HCC incidence increased with duration of infection as measured by years of follow-up (<5, 5–9, 10–14, 15–19, 20–24, 25–29, and ≥30 years). Analysis was conducted using Stata 10.
Human subjects review
The study was approved by the Alaska Area Institutional Review Board and the Centers for Disease Control and Prevention Institutional Review Board. Informed consent was obtained from all living participants. Information from deceased patients was used with Institutional Review Board permission.
RESULTS
Description of study cohort
Of the 1,535 AN persons with chronic HBV infection entered into the HBV registry during and after vaccination campaign, 1,268 persons provided consent to participate in this study and 1,083 persons met our cohort inclusion criteria (Table 1). We identified 5 HBV genotype/subgenotypes among persons in our cohort: 135 (12.5%) with A2, 18 (1.7%) with B6, 57 (5.3%) with C2, 538 (49.7%) with D, and 201 (18.6%) with F1; genotype was unknown for 134 (12.4%) persons. The median age of our cohort at first positive HBsAg result was 15.6 years (range by HBV genotype: 9 years [F1] to 25 years [B6]). The median age at cohort entry was 18.0 years (range by HBV genotype: 15 years [F1] to 31 years [B6]). All-cause mortality during the study period in the overall cohort was 13% and there was no significant difference in mortality by HBV genotype. Median person-years of follow-up in our cohort before censoring or end of study period was 23.4 years (range by HBV genotype: 12 years [B6] to 26 years [D]). Persons with HBV genotype D contributed the greatest number of person-years of follow-up to the cohort (11,604) and persons with genotypes B6 and C2 contributed the least (253 and 1,142, respectively).
Table 1.
Demographic and Clinical Characteristics of a Population-Based Cohort of Hepatitis B Virus (HBV) Infected Alaska Native Males Aged <40 Years and Females Aged <50 Years By HBV Genotype/Subgenotype — Alaska, 1982–2012.a
| Characteristic | HBV Genotype/Subgenotype | Total | P-valueb | |||||
|---|---|---|---|---|---|---|---|---|
| A2 (n=135) | B6 (n=18) | C2 (n=57) | D (n=538) | F1 (n=201) | Unknown (n=134) | (n=1083) | ||
| % Male | 58% | 56% | 42% | 56% | 58% | 57% | 56% | 0.31 |
| Median age (range) at first +HBsAg, years | 19.8 (2.4–49.1) | 25.2 (8.1–47.6) | 17.6 (1.0–45.2) | 14.2 (0–49.2) | 8.7 (0–49.0) | 21.9 (1.6–48.1) | 15.6 (0–49.2) | <0.01 |
| Median age (range) at cohort entry, years | 21.0 (2.4–49.2) | 30.9 (13.0–47.6) | 19.1 (1.0–46.5) | 15.6 (0–49.2) | 14.7 (1.7–49.6) | 23.4 (1.6–49.6) | 18.0 (0–49.6) | <0.01 |
| % died during study period | 10% | 11% | 11% | 12% | 17% | 13% | 13% | 0.31 |
| % aged out of cohortc | 65% | 83% | 61% | 48% | 47% | 63% | 53% | |
| % currently eligibled | 25% | 6% | 28% | 40% | 36% | 24% | 34% | <0.01 |
| Median age (range) of currently eligible, years | 42.3 (31.6–49.7) | 46.4 | 35.3 (22.4–47.9) | 35.2 (20.4–49.6) | 38.0 (30.9–49.4) | 39.8 (31.1–49.6) | 36.6 (20.4–49.7) | <0.01 |
| % developing HCC | 0.7% | 0 | 0 | 0.4% | 9.5% | 0 | 2.0% | <0.01 |
| Median age (range) at HCC diagnosis, years | 32.8 | -- | -- | 23.6 (17.0–30.1) | 19.9 (8.6–40.2) | -- | 20.8 (8.6–40.2) | 1.0 |
| Total person-years of follow-upe | 2605 | 253 | 1142 | 11604 | 4341 | 2354 | 22300 | |
| Median (range) person-years of follow-up | 20.4 (0.8–31.0) | 12.1 (2.0–31) | 21.1 (2.1–31) | 25.9 (<0.1–31) | 24.1 (0.1–31) | 18.2 (<0.1–31) | 23.4 (<0.1–31) | |
Abbreviations: HBsAg, positive hepatitis B surface antigen; HCC, hepatocellular carcinoma
Analysis restricted to men aged <40 years and women aged <50 years at cohort entry
Equality of medians test comparing medians, Chi-squared or randomization test for proportions (significant results indicated in bold).
Males and females attaining age 40 and 50 years, respectively, before end of study period
Alive at end of study period and not aged out of cohort
Person-years of follow-up calculated from cohort entry until death, HCC diagnosis, aged out of cohort, or end of study period
Characteristics of persons with HCC
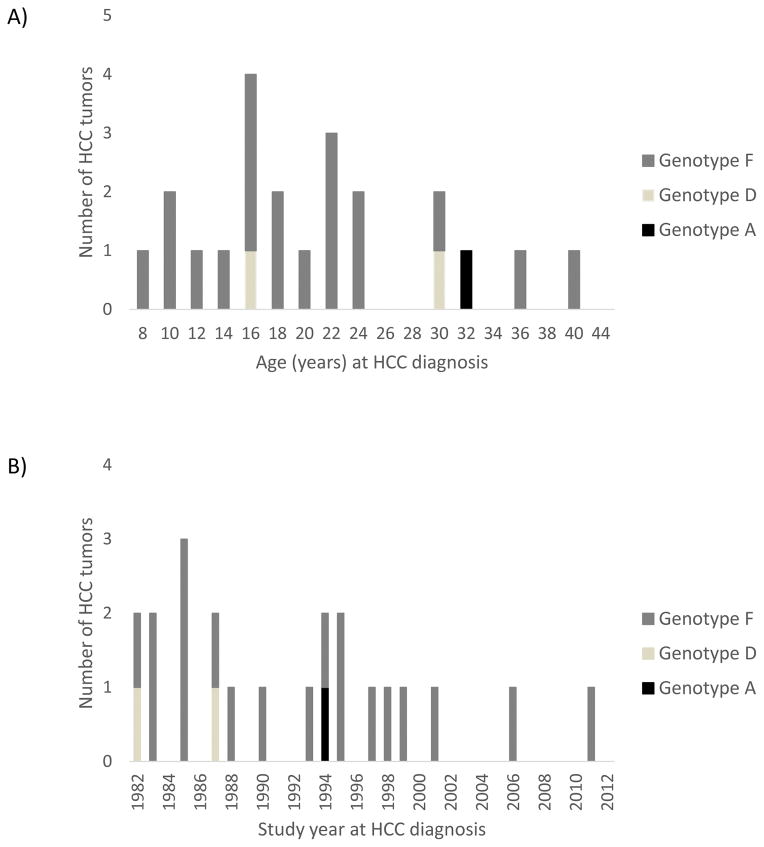
A total of 22 HCC cases were diagnosed in our study cohort of HBV-infected AN males aged <40 years and females aged <50 years (Table 1 and Figure 1, Panels A and B); pathologic confirmation. The majority of persons with HCC were male (68%), aged <10 years at initial positive HBsAg result (68%), and were infected with HBV genotype F (86%). No persons with HBV genotypes B6, C2 or an unknown genotype developed HCC. Among patients diagnosed with HCC, 9 had a family history of HCC (all with genotype F1) and 6 had cirrhosis (5 with genotype F1, and 1 with genotype D); 12 unique persons had a family history of HCC, cirrhosis or both. Among persons with genotype F1, the median age at HCC diagnosis was 19.9 years (minimum [min]–maximum [max]: 8.6–40.2 years) (Figure 1A).
Figure 1.
Hepatitis B Virus Infected Alaska Native Males Aged <40 Years and Females Aged <50 Years With Hepatocellular Carcinoma (HCC) By Age (A) and Study Year (B) at HCC Diagnosis and HBV Genotype — Alaska, 1982–2012. (N = 22)
Risk for HCC among persons in cohort
The overall HCC incidence in our cohort before males attained age 40 years or females attained age 50 years was 1.0/1,000 person-years of follow-up. There were no significant differences in the risk for HCC by sex or age at first HBsAg positive result (comparing age <10 years versus ≥10 years and age <18 years versus ≥18 years). The highest HCC incidence was observed among persons with HBV genotype F1 (4.4/1,000 person-years of follow-up); the HCC incidence among persons with genotype F1 after excluding persons with a family history of HCC, cirrhosis or both was 1.9/1,000 person-years of follow-up. Persons with HBV genotype F1 were more likely than persons with genotype A2, B6, C2 or D to develop HCC (risk ratio [RR]: 22.8; 95% confidence interval [CI]: 6.7–120). If all persons with an unknown genotype (accounting for 0 HCC cases and 2,483 person-years of follow-up) were assumed to have genotype F1, the HCC incidence rate for genotype F1 would be 2.8 and that risk for HCC remains significantly greater than all other persons in the cohort (RR: 14.8; 95% CI: 4.3–77.9). The increased HCC incidence among persons with HBV genotype F1 compared to persons with genotype A2, B6, C2 or D remained significant after excluding the 9 cohort persons with a family history of HCC (RR: 14.1; 95% CI: 3.6–79.5), the 6 cohort persons with cirrhosis (RR: 24.1; 95% CI: 6.7–26.4), or the 12 unique cohort persons with a family history of HCC, cirrhosis or both (RR: 17.0; 95% CI: 3.4–164). There was no trend towards increased or decreased HCC incidence with increasing duration of follow-up in the overall cohort (p-value for trend = 0.11) and among cohort persons with HBV genotype F1 infection (p-value for trend = 0.18).
We also evaluated the HCC risk among our cohort patients by restricting follow-up to age <20 years. The HCC incidence/1000 person-years of follow-up among all cohort patients aged <20 years was 1.9; the incidence for patients with HBV genotype F1 was 8.3.
Survival among persons with HCC
The overall median survival among persons with HCC in our cohort was 10.7 years (min–max: 0.1–30.4 years) with 71% surviving >5 years after diagnosis (Table 2). There were no significant differences in median or 5-year survival among persons with HCC by sex, age at first positive HBsAg test result, or age at HCC diagnosis. Persons diagnosed with HCC at an early stage had a longer median survival than those diagnosed at a late stage (14.4 years versus 4.2; p-value = 0.01). Median survival after HCC diagnosis was longer among persons with HBV genotype F1 (14.0 years) compared with persons with HBV genotypes A2 (4.2 years) and D (0.2 years). Among persons with HCC, 5-year survival was 83% for persons HBV genotype F1 compared with 0% for genotypes A2 and D (p-value = 0.02).
Table 2.
Risk for HCC and Survival Among HBV-Infected Alaska Native Males Aged <40 Years and Females Aged <50 Years — Alaska, 1982–2012.
| Characteristics | HCC cases in cohort | HCC incidence/1,000 person-years follow-up | RR (95% CI) | Median survival, years (range) | p-valuea | % with 5-year survivalb | p-valuec |
|---|---|---|---|---|---|---|---|
| Total | 22 | 1.0 | N/A | 11.3 (0.1–30.4) | N/A | 15/21 (71%) | N/A |
| Sex | |||||||
| Female | 7 | 0.6 | Ref | 5.9 (1.1–24.3) | 0.65 | 4/6 (67%) | 1.0 |
| Male | 15 | 1.4 | 2.2 (0.8–6.3) | 11.8 (0.1–30.4) | 11/15 (73%) | ||
| Age at first HBsAg+ | |||||||
| <10 years | 15 | 1.5 | Ref | 14.2 (1.1–29.3) | 0.13 | 12/14 (86%) | 0.12 |
| ≥10 years | 7 | 0.6 | 0.4 (0.1–1.1) | 4.2 (0.1–30.4) | 3/7 (43%) | ||
| <18 years | 20 | 1.3 | Ref | 12.9 (0.1–30.4) | 0.36 | 14/19 (74%) | 0.50 |
| ≥18 years | 2 | 0.3 | 0.2 (0.03–1.0) | 5.0 (3.4–6.6) | 1/2 (50%) | ||
| Age at HCC diagnosis | |||||||
| <20 years | 11 | -- | -- | 17.7 (0.3–30.4) | 0.06 | 9/11 (82%) | 0.36 |
| ≥20 years | 11 | 5.9 (0.1–27.9) | 6/10 (60%) | ||||
| HCC tumor staged | |||||||
| Early | 15 | -- | -- | 14.4 (1.1–30.4) | 0.01 | 12/14 (86%) | 0.12 |
| Late | 7 | 4.2 (0.1–11.8) | 3/7 (43%) | ||||
| HBV genotype/subgenotype | |||||||
| A2 | 1 | 0.4 | Ref | 4.2 | 0.02f | 0/1 (0%) | 0.02f |
| B6 | 0 | 0.0 | N/A | N/A | |||
| C2 | 0 | 0.0 | N/A | N/A | |||
| D | 2 | 0.2 | 0.2 (0.1–0.3) | 0/2 (0%) | |||
| F1 | 19 | 4.4 | 22.8 (6.7–120)e | 14.0 (1.1–30.4) | 15/18 (83%) |
Abbreviations: CI, confidence interval; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; N/A, not applicable; RR, risk ratio.
Notes: Statistically significant results highlighted in bold; -- indicates analysis not done.
Log rank test comparing median years of survival within category.
Number surviving ≥5 years after HCC diagnosis; 1 person followed for <5 years after HCC diagnosis and excluded from 5-year survival analysis.
Fishers exact test comparing % surviving ≥5 year after HCC diagnosis within category.
Early-stage tumor if single tumor ≤5 cm in diameter or ≤3 tumors ≤3 cm in diameter; otherwise late-stage tumor. Tumor size unknown for persons refusing additional evaluation.
Comparing persons with HBV genotype F with all persons with a known genotype.
Comparing persons with HBV genotype F with persons with HBV genotype A and D.
Among the 22 cohort persons with HCC, 0% (0/3) persons with HBV genotypes A2 or D were diagnosed at an early-stage compared with 79% (15/19) of persons with HBV genotype F1; 1 person with HBV genotype F1 progressed to late-stage between diagnosis and treatment and received transarterial radioembolization (Table 3). Of the 19 persons with HBV genotype F1 who developed HCC, 51% (9) had a first-degree relative with HCC and 26% (5) had cirrhosis. Survival >5 years after HCC diagnosis among persons with HBV genotype F1 was 88% (15/17) if their HCC tumor was amenable to resection/ablation compared with 0% (0/2) of persons treated with embolization. Among the 22 cohort persons with HCC, 19 (86%) were diagnosed with HCC prior to 2001 when the ability to test for HBV DNA levels and AST/ALT was not routinely available for all patients. A total of 8 cohort persons received treatment for HBV after their HCC diagnosis (1 person was treated with interferon prior to 2001, 8 person treated with nucleos(t)ide analogues after 2001). Of the 3 cohort persons diagnosed with HCC after 2001, 1 person received HBV treatment prior to HCC diagnosis (data not shown).
Table 3.
Characteristics of HBV-Infected Alaska Native Males Aged <40 Years and Females Aged <50 Years with HCC — Alaska, 1982–2012.
| Patient No. | Age range (years)a | Sex | HCC FHb | Cirrhosisa,c | HBV genotype | Tumor stagea,d | Initial HCC Treatment | HCC recurrence | Time to HCC recurrence (years) | Survival after initial HCC diagnosis (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18–50 | F | No | No | A2 | Late | Resection | Yes | <5 | 1–4 |
| 2 | 18–40 | M | No | Yes | D | Late | None | No | <1 | |
| 3 | 5–17 | M | No | Unknown | D | Late | None | No | <1 | |
| 4 | 5–17 | M | No | No | F1 | Early | Resection | Yes | >10 | >10 |
| 5 | 5–17 | F | Yes | No | F1 | Early | Resection | Yes | >10 | >10 |
| 6 | 18–50 | F | Yes | No | F1 | Early | TAREe | No | 1–4 | |
| 7 | 18–50 | F | Yes | Yes | F1 | Late | Resection | Yes | <5 | 5–10 |
| 8 | 18–40 | M | Yes | No | F1 | Early | Resection | Yes | >10 | >10 |
| 9 | 18–40 | M | No | No | F1 | Late | Resection | No | >10 | |
| 10 | 18–40 | M | Yes | No | F1 | Early | Resection | Yes | 5–10 | >10 |
| 11 | 18–50 | F | No | No | F1 | Early | Resection | No | >10 | |
| 12 | 5–17 | M | No | No | F1 | Early | Resection | No | >10 | |
| 13 | 18–40 | M | No | Yes | F1 | Early | Resection | Yes | 5–10 | >10 |
| 14 | 18–50 | F | No | Yes | F1 | Early | Resection | Yes | <5 | >10 |
| 15 | 18–50 | F | Yes | Unknown | F1 | Late | TACE | Yes | 1–4 | |
| 16 | 5–17 | M | No | No | F1 | Early | Resection | No | >10 | |
| 17 | 18–40 | M | Yes | No | F1 | Early | Resection | Yes | <5 | 1–4 |
| 18 | 5–17 | M | Yes | Yes | F1 | Early | Ablation | Yes | 5–10 | >10 |
| 19 | 18–40 | M | No | No | F1 | Early | Resection | No | >10 | |
| 20 | 18–40 | M | Yes | Yes | F1 | Early | Resection | Yes | <5 | 1–4 |
| 21 | 5–17 | M | No | No | F1 | Early | Resection | No | >10 | |
| 22 | 18–40 | M | No | No | F1 | Late | Resection | Yes | <5 | 5–10 |
Abbreviations: FH, family history; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; No., number; TACE, transarterial chemoembolization; TARE, transarterial radioembolization
Age range at time of initial HCC diagnosis presented rather than exact age in order to protect study participants’ confidentiality
First-degree relative with HCC
Ishak fibrosis score = 5–6 on liver histology
Early-stage tumor if single tumor ≤5 cm in diameter or ≤3 tumors ≤3 cm in diameter; otherwise late-stage tumor. Tumor size unknown for persons refusing additional evaluation.
Person progressed from early- to late-stage between diagnosis and treatment
Bold indicates alive at by end of study period; all except one deceased person died of HCC
DISCUSSION
No recommendations for HCC surveillance exist for children and young adults with chronic HBV infection because data on HCC incidence in this population are lacking. Data from a population-based cohort of HBV-infected AN persons followed prospectively for up to 30 years allowed for a unique opportunity to examine HCC risk among males aged <40 years and females aged <50 years, a population not currently recommended to receive HCC surveillance. We demonstrated that persons with HBV genotype F1 infection are at increased risk for HCC independent of having a family history of HCC or cirrhosis. Furthermore, the HCC incidence in persons with HBV genotype F1 and without a family history of HCC or cirrhosis approached 0.2%/year, the threshold above which AASLD practice guidelines consider HCC surveillance to be cost-effective (2). A majority of persons with HBV genotype F1 were diagnosed with HBV as children aged ≤18 years. Therefore, HCC surveillance should be considered for all persons with HBV genotype F1 infection regardless of age, especially since HCC surveillance could reduce HCC mortality (14, 15).
There were several unusual features among persons in our cohort infected with HBV genotype F1 who developed HCC. First, a large proportion of cohort persons with HBV genotype F1 and HCC were first-degree relatives of other persons with HCC. However, we demonstrated that the HCC risk remained significantly elevated among persons with HBV genotype F1 compared with persons with other genotypes even after excluding persons with a family history of HCC and cirrhosis. In addition, persons with HBV genotype F1 and HCC had a high 5-year survival rate. The long survival can be partly explained by the fact that the majority of persons had tumors that were amenable to surgical resection or ablation. Studies have demonstrated that 5-year survival after surgical resection can exceed 70% (16, 17). The tendency for HCC to occur in children aged ≤18 years infected with HBV genotype F1 independent of other known risk factors combined with the good prognosis associated with surgical resection or ablation further argues for HCC surveillance in all persons with F1 regardless age.
Outside of Alaska, HBV genotype F (subgenotypes F1–F4) predominantly circulates among the Amerindians of Central and South America (18). The prevalence of HBV infection in Central and South America ranges from 0.5–8%; genotype F is the predominant genotype in many of those regions (19). However, few studies have reported on the risk of HCC associated with HBV genotype F infection outside of Alaska (18). In the absence of evidence on the risk of HCC for persons with genotype F, it might be prudent to consider HBV genotype testing early in the course of disease in HBV-infected residents of Central and South America or HBV-infected persons who have emigrated from these countries so that HCC surveillance can be initiated.
In general, HCC incidence and mortality increases with age among HBV infected persons (1, 20); however, the risk for adverse outcomes associated with HBV infection varies by genotype (7). Therefore, it is possible that previous studies did not detect a substantial increase in HCC risk among persons aged <50 years because the majority of those study participants had HBV genotypes B and C (21), whereas the majority of our study participants had HBV genotypes D and F1. Similar to previous studies documenting increasing HCC risk with age that were conducted in Asian populations, the patients in our study cohort also likely acquired HBV infection by perinatal or by horizontal transmission in early childhood (22). Consequently, it is unknown how much of the increased risk for HCC is the result of advancing age versus increasing duration of HBV infection. We evaluated the risk for HCC among persons who were diagnosed with HBV at ages <10 years and <18 years compared with ages ≥10 and ≥18 years, respectively, and did not detect a difference in HCC incidence based on age of diagnosis. Likewise, our evaluation of the HCC risk according to duration of infection also did not detect a trend towards increasing incidence with increasing duration of infection among males aged <40 years and females aged <50 years.
Our study has limitations. First, we were unable to comprehensively account for other HCC risk factors (e.g., HIV- or HCV-coinfection, cirrhosis or family history of HCC) because that information was unavailable for many cohort patients who did not develop HCC. However, the prevalence of HIV and HCV infection in the study population is low, and persons with known HIV- or HCV-coinfection were excluded from analysis. Additionally, it is notable that HBV genotype F1 was still significantly associated with HCC after excluding patients with a family history of HCC or cirrhosis from the numerator but not the denominator of our incidence calculations. Second, we calculated the person-years of follow-up (estimated duration of HBV infection) based on the date of the first positive HBsAg result. Previous studies in this population have shown that most persons with genotype C likely acquired HBV infection perinatally because of the long period of HBeAg antigenemia, and most persons infected with other HBV genotypes acquired HBV infection horizontally in the first 5 years of life (23–25). Thus, not including the person-years of follow-up between the date of infection and date of first positive HBsAg could result in an overestimate of the HCC incidence. Fourth, the relatively small number of persons with HCC in our cohort limited our ability to evaluate the risk for HCC in persons infected with an HBV genotype other than genotype F1. Finally, the increased survival among persons with HBV genotype F1 compared with other genotypes could partly be the result of length-time bias associated with HCC surveillance. In other words, it is possible that HCC tumors associated with HBV genotype F1 infection were slower growing and more amenable to detection at an early curative stage than tumors caused by other genotypes (26). It is unlikely, however, that the difference in survival after HCC diagnosis between HBV genotypes is attributable to lead-time bias. Lead-time bias refers to diagnosing HCC to an earlier point in time during the course of disease without altering the date of death (26). In our study, there is no reason to suspect that persons with genotype F infection were differentially susceptible to lead-time bias compared with persons with other genotypes because all study participants received the same HCC surveillance and had the potential to be diagnosed at the same earlier stage of disease.
Nonetheless, our study has considerable strengths. First, a majority of the AN population was screened for HBV infection and most of those AN persons with a positive HBsAg result consented to participate in the prospective study from which our cohort was selected (4). As a result, we are able to provide population-based estimates of the risk of HCC. In addition, the presence of multiple HBV genotypes in our cohort allowed for the direct comparison of HCC risk by genotype. Finally, it is unlikely any HCC cases were missed in our cohort because most AN people receive care through the Alaska Tribal Health system. We also cross-referenced the names of persons in our cohort with the Alaska Native Tumor Registry to identify HCC cases potentially diagnosed outside the Alaska Tribal Health System (11). Thus, we are able to provide a comprehensive HCC incidence estimate.
Our results can be strengthened if an increased risk for HCC can be demonstrated in other populations with a high prevalence of HBV genotype F infection. A cost-effectiveness analysis evaluating the benefits of HCC surveillance for all persons with HBV genotype F infection can inform policymakers on the optimal HCC surveillance approach for their region.
Acknowledgments
Financial Support: This work was supported by the Centers for Disease Control and Prevention, NCHHSTP, Division of Viral Hepatitis (CA# 1U01PS004113). The study sponsor did not have a role in 1) study design; 2) the collection, analysis, and interpretation of data; 3) the writing of the report; and 4) the decision to submit the manuscript for publication.
We thank Chriss Homan his assistance in preparing the data for analysis. Mr. Homan does not have any conflicts of interest to disclose.
Abbreviations
- AN
Alaska Native
- ANTHC
Alaska Native Tribal Health Consortium
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- HCC
hepatocellular carcinoma
Footnotes
Conflict of interest: None of the authors have any conflicts to disclose. No honoraria, grants, or other forms of payment were given to any of the authors to produce the manuscript.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M American Association for the Study of Liver, D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasley A, Kruszon-Moran D, Kuhnert W, Simard EP, Finelli L, McQuillan G, et al. The Prevalence of Hepatitis B Virus Infection in the United States in the Era of Vaccination. Journal of Infectious Diseases. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 4.McMahon BJ, Rhoades ER, Heyward WL, Tower E, Ritter D, Lanier AP, et al. A comprehensive programme to reduce the incidence of hepatitis B virus infection and its sequelae in Alaskan natives. Lancet. 1987;2:1134–6. doi: 10.1016/s0140-6736(87)91557-1. [DOI] [PubMed] [Google Scholar]
- 5.McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842–6. doi: 10.1053/jhep.2000.17914. [DOI] [PubMed] [Google Scholar]
- 6.McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759–68. doi: 10.7326/0003-4819-135-9-200111060-00006. [DOI] [PubMed] [Google Scholar]
- 7.McMahon BJ. Natural history of chronic hepatitis B. Clinics in liver disease. 2010;14:381–96. doi: 10.1016/j.cld.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Ching LK, Gounder PP, Bulkow L, Spradling PR, Bruce M, Negus S, et al. Incidence of Hepatocellular Carcinoma According to Hepatitis B Virus Genotype in Alaska Native People. Liver international: official journal of the International Association for the Study of the Liver. 2016 doi: 10.1111/liv.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livingston SE, Simonetti JP, McMahon BJ, Bulkow LR, Hurlburt KJ, Homan CE, et al. Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J Infect Dis. 2007;195:5–11. doi: 10.1086/509894. [DOI] [PubMed] [Google Scholar]
- 10.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–9. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 11.Kelly J, Schade T, Starkey B, White S, Ashokkumar R, Lanier A. 1969–2008 40-Year Report. Anchorage, AK: Alaska Native Tribal Health Consortium, Alaska Native Epidemiology Center; 2012. Cancer in Alaska. [Google Scholar]
- 12.About the SEER registries: National Cancer Institute Surveillance Research Program. [Available from: http://seer.cancer.gov/registries/
- 13.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 14.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–22. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 15.Yeh YP, Hu TH, Cho PY, Chen HH, Yen AM, Chen SL, et al. Evaluation of abdominal ultrasonography mass screening for hepatocellular carcinoma in Taiwan. Hepatology. 2014;59:1840–9. doi: 10.1002/hep.26703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–16. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 18.Roman S, Jose-Abrego A, Fierro NA, Escobedo-Melendez G, Ojeda-Granados C, Martinez-Lopez E, et al. Hepatitis B virus infection in Latin America: a genomic medicine approach. World journal of gastroenterology : WJG. 2014;20:7181–96. doi: 10.3748/wjg.v20.i23.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramvis A, Kew M, Francois G. Hepatitis B virus genotypes. Vaccine. 2005;23:2409–23. doi: 10.1016/j.vaccine.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 20.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–33. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 21.Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. Journal of gastroenterology and hepatology. 2011;26:628–38. doi: 10.1111/j.1440-1746.2011.06695.x. [DOI] [PubMed] [Google Scholar]
- 22.McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 23.Livingston SE, Simonetti JP, Bulkow LR, Homan CE, Snowball MM, Cagle HH, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology. 2007;133:1452–7. doi: 10.1053/j.gastro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 24.McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. Journal of Infectious Diseases. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 25.Estroff DT, Greenman JA, Heyward WL, McMahon BJ, Hardison HH, Bender TR. In: Increased prevalence of hepatitis B surface antigen in pregnant Alaska Eskimos. Fortuine R, editor. Seattle, Washington USA: University of Washington Press; 1985. [Google Scholar]
- 26.Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: lessons from history and study design issues. Seminars in oncology. 2010;37:202–15. doi: 10.1053/j.seminoncol.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]