Abstract
Background
Depression affects 20-30% of people with HIV. Randomized controlled trials (RCTs) have demonstrated the effectiveness of interventions to improve depression among HIV-infected adults, but typically have highly selected populations which may limit generalizability. Inverse probability of sampling weights (IPSW) are a recently proposed method to transport (or standardize) findings from RCTs to a specific external target population.
Methods
We used IPSW to transport the 6-month effect of the Measurement-Based Care (MBC) intervention on depression from the SLAM DUNC trial to a population of HIV-infected depressed adults in routine care in the United States between 2010 and 2014.
Results
In the RCT, MBC was associated with an improvement in depression at 6 months of 3.6 points on the Hamilton Depression Rating scale (95% CI: -5.9, -1.3). When IPSW were used to standardize results from the trial to the target population, the intervention effect was attenuated by 1.2 points (mean improvement 2.4 points; 95% CI: -6.1, 1.3).
Conclusions
If implemented among HIV-infected depressed adults in routine care, MBC may be less effective than in the RCT but can still be expected to reduce depression. Attenuation of the intervention effect among adults in routine care reflects the fact that the trial enrolled a larger proportion of individuals for whom the intervention was more effective. Given the burden of depression among HIV-infected adults, more effective interventions to improve depression are urgently needed. However, examining the transportability of trial findings is essential to understand whether similar effects can be expected if interventions are scaled-up.
Keywords: depression, HIV, generalizability, transportability, depression treatment
Introduction
Depression affects 20-30% of people living with HIV (PLWH), making it the most commonly reported mental health condition among adults in HIV care in the United States.1,2 For PLWH, depression is a debilitating condition that negatively affects quality of life,3,4 antiretroviral (ART) adherence5-7 and viral suppression.8-10 Antidepressant medications are effective at treating severe depression in HIV-infected adults.11 However, identifying and effectively treating PLWH with depression remains a challenge.12 Less than 20% of HIV-infected persons with depression are estimated to receive any depression treatment, and just 9% to receive adequate treatment.13
Randomized controlled trials (RCTs) have demonstrated the efficacy of interventions to improve depression among both HIV-infected and uninfected adults.14,15 While RCTs typically have strong internal validity, they often have limited external validity.16,17 External validity is compromised when the study population enrolled in the RCT does not accurately represent the target population for implementation of the intervention and when the distribution of effect-measure modifiers between the trial and target populations differ.18 RCTs evaluating mental health outcomes in particular often have stringent eligibility criteria, which may result in under-representation of individuals with complicated or comorbid mental health conditions.19-21
A clear understanding of the external validity of RCT results is critical for practitioners and policymakers considering scaling-up mental health interventions. However, only recently have methods been developed to quantitatively assess the extent to which findings from RCTs can be generalized or ‘transported’ to a specific target population.18,22 In the current analysis, we report on the use of inverse probability of sampling weights (IPSW)18 to transport, or standardize, the effect estimate from a RCT of depression treatment among depressed, HIV-infected adults to a target population of HIV-infected depressed adults receiving HIV care at multiple clinical sites across the US.
Methods
The goal of the present analysis was to assess what effect the Measurement-Based Care (MBC) intervention might have on depression if implemented among HIV-infected, depressed adults in routine care in a multisite observational cohort. IPSW offer a way to quantitatively assess what the effect of an intervention tested in a RCT might be if implemented in the target population, by taking into account measured differences between the study and target populations. The concept of ‘transportability’ is closely related to generalizability. However, ‘transportability’ is preferred to distinguish settings in which the trial population is not a complete subset of the target population.23
Study population and data sources
Data for the present analysis come from two data sources: 1) the SLAM DUNC trial, which evaluated the efficacy of MBC on mental health and HIV outcomes24 and 2) the Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) cohort.
The SLAM DUNC trial has been described in detail elsewhere.24 Briefly, HIV-infected persons receiving medical care at one of four US infectious disease clinics (2 of which – University of North Carolina, Chapel Hill (UNC) and University of Alabama, Birmingham (UAB) - are also CNICS sites) were eligible to participate if they were English speaking, ages 18-64, screened positive for depression (score ≥10) on the Patient Health Questionnaire-9 (PHQ-9)25, and were confirmed to have a current major depressive disorder on the Mini International Neuropsychiatric Interview (MINI) that was not treatment resistant.26 Exclusion criteria included history of bipolar or psychotic disorder, failure of> 2 adequate antidepressant trials in the current depressive episode, or psychiatric presentation requiring acute intervention. Participants were randomized to receive either enhanced usual care for depression or the MBC intervention.27 The MBC intervention consisted of a clinically supervised Depression Care Manager (DCM) who provided evidence-based antidepressant treatment recommendations to the HIV provider. DCM recommendations focused on initiation of antidepressants and dose adjustment based on algorithm-centered depressive symptom response and tolerability; HIV providers made all final decisions about treatment. Participants randomized to the enhanced usual care arm could have also received depression treatment from their HIV provider or other sources, but no in-clinic decision support was provided by the DCM. All participants provided written informed consent, and ethical approval was provided by Duke University, UNC and UAB.
The CNICS cohort includes over 30,000 PLWH in routine clinical care at 8 sites in the United States.28 CNICS collects detailed information on demographics, ART, antidepressant medications, HIV/AIDS clinical events, co-morbid conditions, HIV-related lab values and vital status on patients who consent to participate. Between 2005 and 2011, CNICS sites began collecting self-administered socio-behavioral questionnaires, called Patient-Reported Measures and Outcomes (PROs), approximately every 4-6 months. The PROs include a validated measure of depression (PHQ-9). Ethical approval for the use of routinely collected clinical data was provided by all CNICS sites.
Data for the present analysis includes all SLAM DUNC participants (n=304; intervention arm n=149, usual care arm n=155). Patients in CNICS were included if they met modified SLAM DUNC inclusion criteria, including being age 18-64, having a PHQ-9 ≥10 between March 31, 2010 and December 1, 2014 (enrollment period for SLAM DUNC), and no bipolar or psychotic disorder history (n=3,176). Baseline for SLAM DUNC participants was defined as study enrollment and for CNICS participants as the first visit with a PHQ-9 measure> 10 in the study period. The outcome of interest was depression severity at 6 months, measured using the Hamilton Depression Rating Scale (HAM-D).29
Weights estimation
We created two types of weights to address two separate issues. First, we created inverse probability of observation weights (IPOW) to address possible selection bias from missing HAM-D outcomes (n=119) in the SLAM DUNC trial population only. Second, we created IPSW to standardize the effect of the MBC intervention on depression at 6 months among trial participants to our target population of HIV-infected, depressed adults in CNICS. We explored the bias-variance trade-off of progressively truncating the weights between percentiles 0 to 5 and 95 to 100 and chose stabilized weights with a mean as close to 1.00 as possible and a reasonable range (Supplemental Digital Content AppendixTable 1).
IPOW were created using data from SLAM DUNC participants only. An indicator variable for having an observed versus missing 6-month HAM-D measurement was created and the probability of having an observed outcome measure was estimated as a function of a participant's baseline adaptive coping30,31, viral load suppression (<50 copies/mL), self-efficacy,32 self-reported ART adherence, HAM-D score, appointment adherence (0-100%) and alcohol use (Table 1). Predictors were identified based on previous investigations of predictors of missing information in these data.15 IPOW were stabilized by the marginal probability of having an observed outcome and were truncated at the 5th and 95th percentiles to improve stability.
Table 1. Measurement of Variables Investigated to Construct IPSW in SLAM DUNC and CNICS.
| Variable | SLAM DUNC Measure | CNICS measure |
|---|---|---|
| Same measure | ||
| Baseline PHQ-9a | PHQ-9, continuous | PHQ-9, continuous |
| Gendera | female; male | female; male |
| Race/ethnicitya | white, non-Hispanic; black, non-Hispanic; Hispanic or other | white, non-Hispanic; black, non-Hispanic; Hispanic or other |
| Risk group for HIV acquisitiona | IDU; MSM, no IDU; heterosexual, no MSM or IDU; other | IDU; MSM, no IDU; heterosexual, no MSM or IDU; other |
| Baseline CD4 counta | lab value from medical record, continuous | lab value from medical record, continuous |
| Baseline viral load suppressiona | lab value from medical record, <50 copies/mL | lab value from medical record, <50 copies/mL |
| Years in HIV care | from medical record | from medical record |
| Baseline ART use and adherencea | If on ART (yes/no) any self-reported missed doses in the last week | If on ART (yes/no) any self-reported missed doses in the last week |
| Different measures | ||
| Panic disordera | current panic disorder on the MINI | endorsement of any of 5 core symptoms of panic disorder |
| Drug use | meets criteria for drug use or dependence on the MINI | any self-reported drug use in the past 3 months, excluding marijuana |
| High risk alcohol use | meets criteria for alcohol abuse or dependence on the MINI | > 4 on the AUDIT for men and > 3 for women |
Abbreviations: PHQ-9 = Patient health questionnare-9; IDU = intravenous drug user; MSM = men who have sex with men; MINI = Mini International Neuropsychiatric Interview; AUDIT = Alcohol Use Disorders Identification Test.
Included in IPSW.
To create IPSW to assess transportability, we first considered covariates available in both data sources likely to be associated with trial participation and to modify the effect of the intervention on depression.18 Not all covariates associated with selection into the trial were also effect-measure modifiers (Figure 2 and Figure 3). For parsimony, only the following baseline covariates were used to create the IPSW: PHQ9 score, panic disorder, ART use and adherence, gender, race/ethnicity, risk group for HIV acquisition, age, CD4 count, viral load suppression and years in care (Table 1). After harmonizing variable categorization, the two data sources were combined. IPSW were estimated as the inverse odds of inclusion in SLUM DUNC, as a function of included covariates. One-way interactions were considered, but taken out of the final IPSW model to improve model stability. IPSW were stabilized by the marginal odds of selection into SLAM DUNC and truncated at the 1st and 99th percentiles.18
Figure 2.
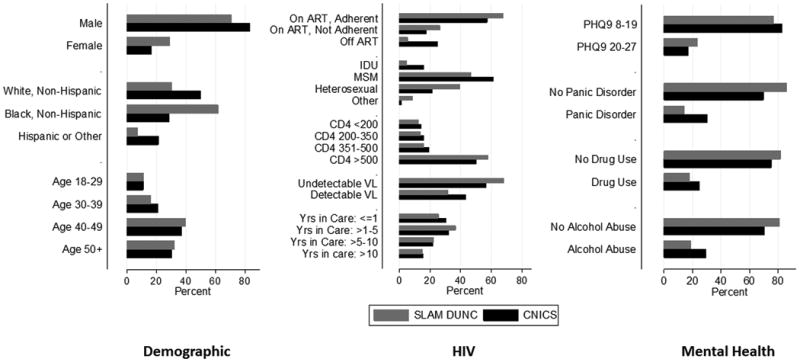
Distribution of demographic, HIV-related and mental health covariates in SLAM DUNC versus CNICS.
Figure 3.
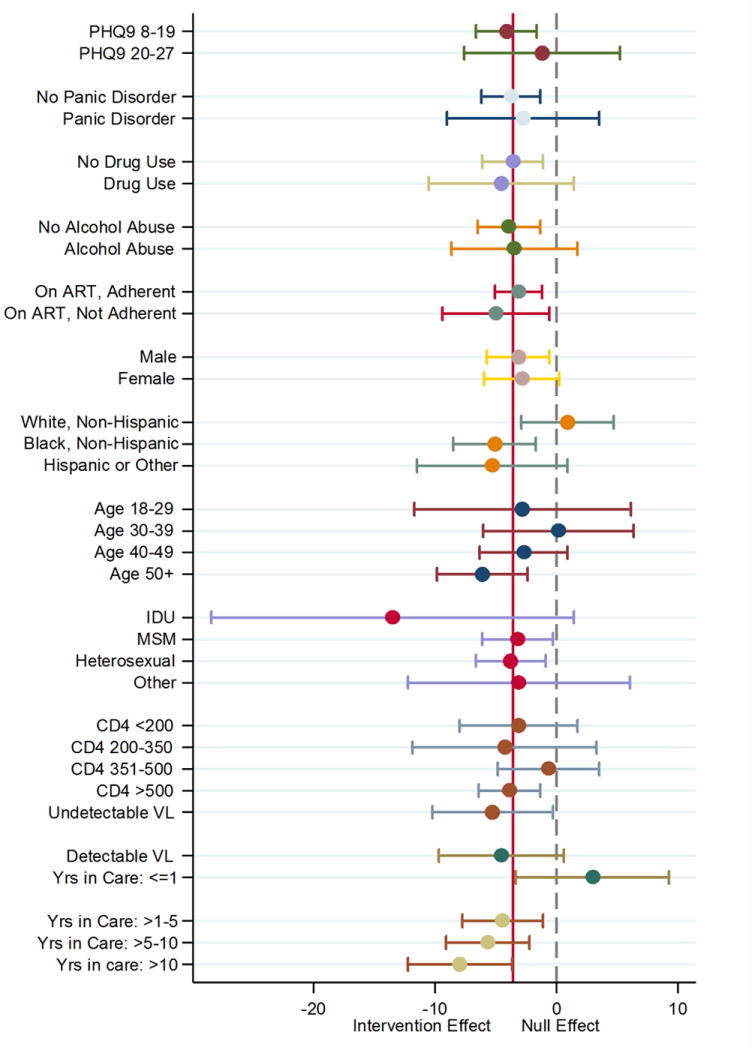
Stratum-specific estimates of the effect of the MBC intervention on depression scores at 6 months.
Statistical analysis
We conducted an intent-to-treat (ITT) analysis and used linear regression to estimate the effect of the MBC intervention on mean HAM-D score at 6 months. All models included fixed effects for design variables (site and physician's depression treatment experience) and used robust variance estimators. Standard errors were bootstrapped (n=1,000) to obtain 95% confidence intervals (CIs). We compared unweighted and weighted effect estimates.
The weighted analysis proceeded in four steps. First, we performed multiple imputation using chained equations33 (n=20 imputations) to fill in missing data for all covariates in both SLAM DUNC and CNICS that were used to create the IPOW or IPSW. Second, using imputed data from SLAM DUNC participants only, we calculated and applied IPOW to reweight those with observed outcomes to represent the entire SLAM DUNC study population. Third, using imputed data from SLAM DUNC and CNICS combined, we calculated and applied IPSW to standardize effect estimates from SLAM DUNC to our target population in CNICS. In the final analysis, we multiplied the IPOW and IPSW together to address both missing outcomes and transportability.
We conducted two sensitivity analyses. Due to the low number of persons in certain categories in the SLAM DUNC study population (those not on ART at study start, Hispanic/other ethnicity, and intravenous drug users (IDUs)), we conducted a sensitivity analysis excluding these groups from both samples. We also included only CNICS sites from the same geographic region as SLAM DUNC study sites (UNC and UAB) in order to understand whether changes in transported effects were largely attributable to differences in the distribution of the HIV epidemic in the Southeast United States, compared with the rest of the US. All analyses were performed using Stata 13 (StataCorp, College Station, TX).
Results
A total of 3,176 patients in CNICS met the modified inclusion criteria for SLAM DUNC and were included in the analysis. Of the 304 participants enrolled in SLAM DUNC, n=190 (63%; intervention arm n=92, usual care n=98) had HAM-D measurements at 6 months (Supplemental Digital Content Appendix Table 2). Missing data was a challenge in the SLAM DUNC trial; 77% of participants completed at least one follow-up at either 3, 6, 9 or 12 months but interim missing data were common.15 Extensive investigation of missing data for the primary analysis of the trial showed that missingness with respect to both HIV and mental health outcomes was balanced by arm. Additionally, methods to correct for missing data34 in the analysis of the primary findings from trial did not yield meaningful changes in effect estimates.15 In line with these findings, there was little difference between the entire SLAM DUNC study population and those with a HAM-D measurement at 6 months among previously identified predictors of missing data in our analysis (Figure 1).
Figure 1.
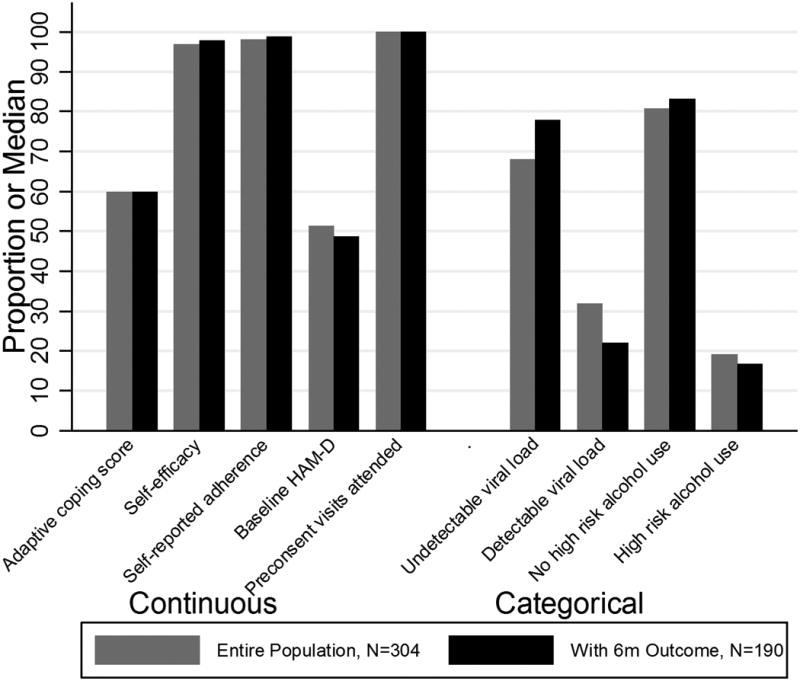
Distribution of covariates in entire SLAM DUNC sample (n=304), to those with a 6-month HAM-D (n=190).
Compared with participants in SLAM DUNC, CNICS participants were more likely to have panic disorder (30% versus 14%), use drugs (25% versus 18%) and not be on ART at baseline (25% versus 6%). SLAM DUNC participants were more likely to be female (29% versus 17%), black, non-Hispanic (62% versus 29%), to have contracted HIV through heterosexual transmission (40% versus 22%) and to have more severe depression (PHQ-9 20-27; 23% versus 17%) (Figure 2).
In the unweighted ITT analysis, MBC was associated with a mean decrease (e.g. improvement) of 3.6 points on the HAM-D scale (95% CI: -5.9, -1.3) at 6 months (Table 2). When the effect of MBC on HAM-D scores at 6 months was investigated within sub-groups, the effect of the intervention was attenuated among those with higher PHQ-9 scores (-1.2; 95% CI: -7.6, 5.3), white, non-Hispanics (0.9; 95% CI -2.9, 4.7), individuals aged 30-39 (0.2; 95% CI -6.0, 6.3), those with CD4 count 351-500 (-0.7; 95% CI: -4.8, 3.5) and those in care< 1 year (2.9; 95% CI: -3.3, 9.2) (Figure 3). The effect of the intervention was stronger among those on ART and not adherent at baseline (-5.0; 95% CI: -9.4, -0.6), black, non-Hispanics (-5.1; 95% CI: -8.5, -1.7), Hispanic or other race/ethnicity (-5.3; 95% CI: -11.5, 0.9), those who acquired HIV through IDU (-13.5; 95% CI: -28.4, 1.4) and individuals in care >10 years (-8.0; 95% CI: -12.2, -3.7) (Figure 3).
Table 2. The Effect of Measurement-Based Care on Hamilton Depression Rating Scale (HAM-D) Scores 6 months.
| Restricted to non-IDUs, those on ART and non-Hispanic/Other ethnicity | Restricted to CNICS sites in the Southeast United Statesa | ||
|---|---|---|---|
| Mean Change (95% CI) in HAM-D at 6 months | Mean Change (95% CI) in HAM-D at 6 months | Mean Change (95% CI) in HAM-D at 6 months | |
| Unweighted | -3.6 (-5.9, -1.3) | -3.2 (-5.6, -0.8) | n/a |
| Weighted | |||
| IPOW | -3.6 (-6.0, -1.3) | -3.4 (-5.9, -0.9) | n/a |
| IPSW | -2.4 (-6.1, 1.3) | -1.5 (-5.3, 2.3) | -1.5 (-5.3, 2.3) |
| Weights combined | -1.9 (-5.3, 1.5) | -1.6 (-4.0, 0.8) | -1.4 (-4.5, 1.8) |
Abbreviations: IDU= Intravenous drug users.
University of North Carolina, Chapel Hill and University of Alabama-Birmingham
In the weighted analysis, when only IPOW were used the intervention effect was similar to the unweighted ITT analysis (mean decrease -3.6; 95% CI: -6.0, -1.3), suggesting that missing HAM-D outcomes in SLAM DUNC did not induce an appreciable amount of selection bias in the clinical trial (Table 2). However, the mean of the IPOW weight was 1.06 (mean 1.06-1.07 for un-truncated and all truncated weights, see Supplemental Digital Content Appendix Table 1), rather than 1.00, which may indicate a degree of misspecification of the weight model or non-positivity.35 When IPSW were used to standardize the effect of the intervention in SLAM DUNC to the target population in CNICS, the effect of the intervention was attenuated (mean decrease -2.4; 95% CI: -6.1, 1.3). When both IPOW and IPSW were combined, the effect of the intervention was further attenuated (mean decrease -1.9; 95% CI: -5.3, 1.5). Similar trends were seen when Hispanics and other race/ethnicity, those not on ART and IDUs were excluded and when only CNICS sites in the Southeast United States were included (Table 2).
Discussion
In our analysis, the effect of MBC on depression at 6 months remained protective, but was attenuated when transported from the SLAM DUNC trial to a target population of depressed, HIV-infected adults in CNICS. In the trial, participants in the intervention arm experienced a 3.6 point improvement on average in their depression on the HAM-D scale. When transported to CNICS, we estimated between a 1.9 and 2.4 point improvement in depression. Some clinicians consider a 3 point change on the HAM-D scale to be clinically significant,36 suggesting the intervention may still be helpful if implemented in CNICS but have less of an impact on clinical depression than among trial participants. Similar results were seen when analyses excluded populations with limited data in SLAM DUNC (Hispanic/other ethnicity, IDU, and those not on ART at baseline).
One reason the intervention effect may have been attenuated was that the trial population reflected the HIV epidemic in the Southeast United States.37 Compared to CNICS, more black, non-Hispanics, women, and people who contracted HIV through heterosexual contact were included in the trial (Figure 2). However, the effect of the intervention was stronger only among black, non-Hispanics; being female or having heterosexual transmission did not modify the intervention effect (Figure 3). Further, when we restricted analyses to CNICS sites within the Southeast United States only, we observed similar attenuation in the effect of MBC on depression. These results suggest that differences in demographic characteristics do not fully account for why the effect of MBC may be attenuated if implemented in CNICS.
SLAM DUNC also enrolled a larger proportion of older participants who had been in care longer and had more severe depression, compared with CNICS. The MBC intervention was more effective for adults> 50 years of age and less effective for adults 30-39 years of age. Stratified estimates also suggested that the effectiveness of the intervention increased the longer a participant had been in care (Figure 3). This may reflect the fact that participants newly entering care may be dealing with the grief of a recent HIV diagnosis, rather than an episode of clinical depression. It is possible that more participants in SLAM DUNC had clinical depression, and thus the MBC intervention was more effective for these individuals.
Interestingly, fewer participants in SLAM DUNC experienced panic or were high risk alcohol or drug users than in CNICS. With the exception of drug use, these differences persisted when trial participants were compared to CNICS participants only in the SE United States. While these differences may reflect actual discrepancies between trial and CNICS participants, it is likely that they also reflect a degree of misclassification since measurement of these variables was not identical between SLAM DUNC and CNICS (Table 1). These measures represent the investigators' best efforts to match the constructs measured in both studies. However, the more stringent specifications in SLAM DUNC likely contributed to the smaller proportion of participants in the trial with panic disorder, drug and alcohol use. More generally, differences in how factors were measured highlights the importance of having reasonably similar measures to transport effect estimates.
Transporting an effect estimate from a RCT population to a target population rests on several assumptions. First, the distribution of effect-measure modifiers must be similar between the two populations.38 IPSW reweight the trial population to account for differences in the distribution of effect-measure modifiers in the target population. This approach relies on the assumption that all effect-measure modifiers (and determinants of selection into the trial) have been correctly measured and modeled. In our analysis, we were able to measure a number of variables associated with selection into the trial that also modified the treatment effect. However, some important effect-measure modifiers may have been misclassified (discussed above), not measured (e.g. adherence to antidepressant medication), or not measured consistently in the two populations (e.g. anxiety).
A second assumption of transporting effect estimates is that there is no interference. Interference affects the transportability of results to the extent that intervening on one individual in a given population affects the intervention status of another individual in that population.38 For example, in populations where more people tell their friends about starting depression treatment, the intervention could appear more effective because more individuals seek depression screening. In the SLAM DUNC trial all clinics implemented routine depression screening.15 Therefore, all patients had an equal probability of having their depression diagnosed and we assume negligible interference when transporting our results to CNICS.
Finally, the version of an intervention an individual receives may affect the transportability of results if multiple versions of the intervention are available. In our analysis, we assume that any variation in the way MBC is delivered did not affect the outcome of an individual. In considering scaling-up the MBC intervention to all CNICS sites, treatment-variation irrelevance seems reasonable, given that MBC is a well-defined intervention that has been implemented in multiple settings.39-41 However, these results are based on an implicit assumption that MBC could be implemented in the target population with the same fidelity as it was in SLAM DUNC.
If implemented among HIV-infected depressed adults in routine care, MBC may be less effective than in the SLAM DUNC trial but can still be expected to reduce depression. Attenuation of the intervention effect reflects the fact that the trial enrolled a larger proportion of individuals for whom the intervention was more effective. These results highlight the heterogeneity in the effect of depression interventions across groups. Further research is needed to identify groups most likely to benefit from the MBC intervention, and those that may need alternative or additional interventions (e.g. substance abuse treatment). Given the considerable burden of depression among HIV-infected adults, interventions to help HIV providers better diagnose and treat depression are urgently needed. Intervention which combine antidepressant treatment with counseling may prove move effective for PLWH. As such interventions are developed and tested in RCTs, examining the transportability of trial findings is essential to understand whether similar effects can be expected if interventions are scaled-up.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health [grants R01MH086362, R01MH100970, R24AI067039, T32AI007001]; the Centers for AIDS Research at Duke, University of North Carolina at Chapel Hill, and University of Alabama at Birmingham [grants P30-AI50410, P30-AI064518 and P30-AI027767]; and by the National Institutes of Health Office of the Director and theEunice Kennedy Shriver National Institute of Child Health and Human Development [grant DP2-HD-08-4070 to D.W.].
Funding: This work was supported by the National Institutes of Health [grant numbers R01MH086362, R01MH100970, R24AI067039, T32AI007001] and the Centers for AIDS Research at Duke, University of North Carolina at Chapel Hill, and University of Alabama at Birmingham [grant numbers P30-AI50410, P30-AI064518 and P30-AI027767]. Dr. Westreich was supported by the National Institutes of Health Office of the Director and theEunice Kennedy Shriver National Institute of Child Health and Human Development [grant number DP2-HD-08-4070].
Footnotes
Presentation: A version of this work was presented at the 48th Annual Society for Epidemiologic Research Meeting, Denver, Colorado, June 2015 and the IAPAC 10th International Conference on HIV Treatment and Prevention Adherence, Miami, Florida, June 2015.
Conflicts of Interest: The authors report no conflicts of interest.
References
- 1.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 2.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 3.Bengtson AM, Pence BW, O'Donnell J, et al. Improvements in depression and changes in quality of life among HIV-infected adults. AIDS Care. 2015;27(1):47–53. doi: 10.1080/09540121.2014.946386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimpel RR, Fleck MP. Depression as a major impact on the quality of life of HIV-positive Brazilians. Psychology, Health & Medicine. 2013 doi: 10.1080/13548506.2013.772302. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–187. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):384–390. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- 7.Mugavero M, Ostermann J, Whetten K, et al. Barriers to antiretroviral adherence: the importance of depression, abuse, and other traumatic events. AIDS Patient Care STDS. 2006;20(6):418–428. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- 8.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. Jama. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 9.Ironson G, O'Cleirigh C, Fletcher MA, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosomatic medicine. 2005;67(6):1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leserman J, Jackson ED, Petitto JM, et al. Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosomatic medicine. 1999;61(3):397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Himelhoch S, Medoff DR. Efficacy of antidepressant medication among HIV-positive individuals with depression: a systematic review and meta-analysis. AIDS Patient Care STDS. 2005;19(12):813–822. doi: 10.1089/apc.2005.19.813. [DOI] [PubMed] [Google Scholar]
- 12.Asch SM, Kilbourne AM, Gifford AL, et al. Underdiagnosis of depression in HIV: who are we missing? J Gen Intern Med. 2003;18(6):450–460. doi: 10.1046/j.1525-1497.2003.20938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pence BW, O'Donnell JK, Gaynes BN. The depression treatment cascade in primary care: a public health perspective. Current psychiatry reports. 2012;14(4):328–335. doi: 10.1007/s11920-012-0274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 15.Pence BW, Gaynes BN, Adams JL, et al. The effect of antidepressant treatment on HIV and depression outcomes: the SLAM DUNC randomized trial. Aids. 2015 doi: 10.1097/QAD.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365(9453):82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 18.Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: The ACTG 320 trial. Am J Epidemiol. 2010;172(1):107–115. doi: 10.1093/aje/kwq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braslow JT, Duan N, Starks SL, Polo A, Bromley E, Wells KB. Generalizability of studies on mental health treatment and outcomes, 1981 to 1996. Psychiatr Serv. 2005;56(10):1261–1268. doi: 10.1176/appi.ps.56.10.1261. [DOI] [PubMed] [Google Scholar]
- 20.Stirman SW, Derubeis RJ, Crits-Christoph P, Rothman A. Can the randomized controlled trial literature generalize to nonrandomized patients? Journal of consulting and clinical psychology. 2005;73(1):127–135. doi: 10.1037/0022-006X.73.1.127. [DOI] [PubMed] [Google Scholar]
- 21.Wisniewski SR, Rush AJ, Nierenberg AA, et al. Can phase III trial results of antidepressant medications be generalized to clinical practice? A STAR*D report. Am J Psychiatry. 2009;166(5):599–607. doi: 10.1176/appi.ajp.2008.08071027. [DOI] [PubMed] [Google Scholar]
- 22.Greenhouse JB, Kaizar EE, Kelleher K, Seltman H, Gardner W. Generalizing from clinical trial data: a case study. The risk of suicidality among pediatric antidepressant users. Stat Med. 2008;27(11):1801–1813. doi: 10.1002/sim.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuart EA, Bradshaw CP, Leaf PJ. Assessing the generalizability of randomized trial results to target populations. Prev Sci. 2015;16(3):475–485. doi: 10.1007/s11121-014-0513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pence BW, Gaynes BN, Williams Q, et al. Assessing the effect of Measurement-Based Care depression treatment on HIV medication adherence and health outcomes: rationale and design of the SLAM DUNC Study. Contemp Clin Trials. 2012;33(4):828–838. doi: 10.1016/j.cct.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- 27.Adams JL, Gaynes BN, McGuinness T, Modi R, Willig J, Pence BW. Treating depression within the HIV “medical home”: a guided algorithm for antidepressant management by HIV clinicians. AIDS Patient Care STDS. 2012;26(11):647–654. doi: 10.1089/apc.2012.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carver CS. You want to measure coping but your protocol's too long: consider the brief COPE. Int J Behav Med. 1997;4(1):92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 31.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56(2):267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 32.Shively M, Smith T, Bormann J, Gifford A. Evaluating Self-Efficacy for HIV Disease Management Skills. AIDS and Behavior. 2002;6(4):371–379. [Google Scholar]
- 33.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter H, Kenward M. A practical guide. London: The London School of Hygeine and Tropical Medicine; 2007. Missing data in randomised controlled trials. [Google Scholar]
- 35.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Excellence NIfC. Depression: management of depression in primary and secondary care. London: National Institute for Clinical Excellence; 2004. [Google Scholar]
- 37.Reif S, Pence BW, Hall I, Hu X, Whetten K, Wilson E. HIV Diagnoses, Prevalence and Outcomes in Nine Southern States. J Community Health. 2015;40(4):642–651. doi: 10.1007/s10900-014-9979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernan MA, VanderWeele TJ. Compound treatments and transportability of causal inference. Epidemiology. 2011;22(3):368–377. doi: 10.1097/EDE.0b013e3182109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Culpepper L. Improving patient outcomes in depression through guideline-concordant, measurement-based care. J Clin Psychiatry. 2013;74(4):e07. doi: 10.4088/JCP.12075tx1c. [DOI] [PubMed] [Google Scholar]
- 40.Pence BW, Gaynes BN, Atashili J, et al. Feasibility, safety, acceptability, and preliminary efficacy of measurement-based care depression treatment for HIV patients in Bamenda, Cameroon. AIDS Behav. 2014;18(6):1142–1151. doi: 10.1007/s10461-014-0727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warden D, Trivedi MH, Carmody T, et al. Adherence to antidepressant combinations and monotherapy for major depressive disorder: a CO-MED report of measurement-based care. J Psychiatr Pract. 2014;20(2):118–132. doi: 10.1097/01.pra.0000445246.46424.fe. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


