Abstract
Lipidomics is a newly emerged discipline that studies cellular lipids on a large scale based on analytical chemistry principles and technological tools, particularly mass spectrometry. Recently, techniques have greatly advanced and novel applications of lipidomics in the biomedical sciences have emerged. This review provides a timely update on these aspects. After briefly introducing the lipidomics discipline, we compare mass spectrometry-based techniques for analysis of lipids and summarize very recent applications of lipidomics in health and disease. Finally, we discuss the status of the field, future directions, and advantages and limitations of the field.
Keywords: Lipid metabolism, lipidome, lipidomics, mass spectrometry, shotgun lipidomics, biomedical science
Lipidomics, a Newly Emerged Discipline in Bioscience
Lipids are crucial components of cellular membranes and lipid particles such as lipoproteins. Lipids play many essential roles in cellular functions, including cellular barriers, membrane matrices, signaling, and energy depots. Cellular lipids are highly complex; that is, there are tens to hundreds of thousands molecular species at concentrations ranging from amol to nmol/mg protein [1–3]. Cellular lipids are also highly dynamic; that is, they are changing constantly with physiological, pathological, and environmental conditions. Lipids are classified into a small number of classes and subclasses. Lipidomics (see Glossary) emerged in 2003 and has greatly advanced in recent years, largely due to the development of mass spectrometry (MS) [4, 5].
Although a variety of reviews on lipidomics have been published recently [6–22], these have had different focuses. The rapid advances in techniques and their applications make the timely reviewing of these aspects necessary. Here, we first review MS-based techniques for the analysis of lipids. These techniques are different in the absence or presence of liquid chromatography (LC) prior to mass spectrometric analysis. They can also be different in their analytical coverage (e.g., “targeted” vs “global” analysis). After comparing techniques, we discuss the applications of lipidomics for a variety of disease states, which have appeared in the last couple of years. Finally, we address how the current studies lead us to the accomplishments for tomorrow in the last section.
Lipidomics Workflow and Techniques
Workflow
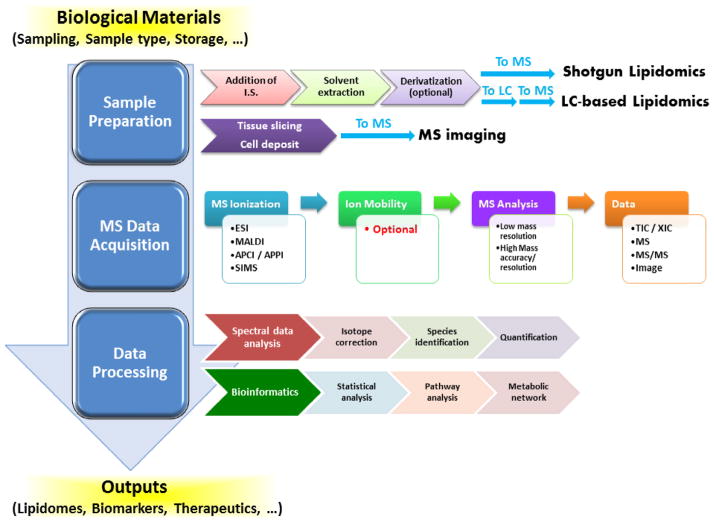
A typical workflow for lipidomic analysis of biological samples includes sample preparation, mass spectrometry-based analysis (i.e., MS data acquisition), and data processing (Figure 1).
Figure 1.
A typical workflow of lipidomic analysis of biological samples. Lipidomic analysis of biological samples includes sample preparation, mass spectrometry-based analysis (i.e., MS data acquisition), and data processing. MS: mass spectrometry; MS/MS: tandem mass spectrometry; LC: liquid chromatography; I.S.: internal standard; ESI: electrospray ionization; MALDI: Matrix assisted laser desorption/ionization; APCI: Atmospheric pressure chemical ionization; APPI: Atmospheric pressure photoionization; SIMS: Secondary ion mass spectrometry; TIC: total ion chromatogram; XIC: extracted ion chromatogram.
Prior to any lipidomic analysis, proper sampling and sample storage is mandatory. Factors affecting sampling conditions, sample preprocessing and storage, and selection of study subjects (particularly in clinical lipidomics studies), have been extensively reviewed recently [13, 23]. The properly collected samples are then prepared in a way that is suitable for the adapted techniques. Most lipidomics techniques utilize biological extracts [24, 25] while MS imaging largely uses non-extracted samples (e.g., tissue slices) [26]. During lipid extraction (see Box 1 for common extraction methods), the addition of appropriate internal standards is critical to quantitative lipidomic analysis [11]. Internal standards are commonly added by normalization to total protein, wet/dry tissue weight, or fluid volume for lipid quantitation (how to select internal standards has recently been extensively discussed [11]). Another critical factor is to achieve sufficient efficiency and unbiased recovery of individual lipid species from the biological materials [25].
Box 1. Common extraction methods used in lipidomics.
Modified Bligh & Dyer method: Chloroform/methanol/H2O (1:1:0.9, v/v/v) for extraction of a small amount of biological sample (e.g., < 50 mg of tissue). After phase separation, total lipids are present in the chloroform phase. This is a well-established standard method and broadly practiced. The disadvantages include the use of hazardous chloroform and the collection of chloroform extract from the bottom layer (which may cause the carry-over of water soluble impurities and difficulty in automation).
Modified Folch method: Chloroform/methanol (2:1, v/v) to extract biological tissue (e.g., ~ 0.1 g), then add water or 0.9% NaCl (0.2 volume) to wash the solvent extract. It has similar advantages and disadvantages to modified Bligh & Dyer method.
MTBE method: methyl tert-butyl ether (MTBE)/methanol/water (5:1.5:1.45, v/v/v). This method resolves some of the difficulties in chloroform-involved methods because MTBE is present in the top layer after phase separation, and therefore is more feasible for high throughput and automation. A drawback is that the MTBE phase contains a significant amount of aqueous component that may carry over water soluble contaminants.
BUME method: A volume of butanol/methanol (BUME, 3:1, v/v) to a small volume of aqueous phase, add an equal volume of heptane/ethyl acetate (3:1, v/v), and then add 1% acetic acid (equal volume to BUME) to induce phase separation. This method may compensate the above methods with less water soluble contaminants carried over in the organic phase. Its drawback is the difficulty in evaporation of butanol component in the organic phase.
After extraction, some optional steps may be applied prior to MS analysis. One option is to simplify the complexity of the extract, which is particularly important for a direct infusion-based shotgun approach because it applies no chromatography for separation or enrichment prior to MS analysis. This can be done through either physical approaches (e.g., liquid/liquid partitioning or solid phase extraction to separate polar vs. non-polar lipids) [27] or chemical approaches (e.g., base hydrolysis to enrich low abundance sphingolipids from complex lipid extracts containing high abundance phospholipids and/or glycerolipids) [28, 29]. The other option is to derivatize the extracts by chemically tagging specific functional groups of lipids [10]. This option is mostly useful when lipids of interest lack either inherent charged moieties (which prevents their efficient ionization), or lack characteristic or sensitive fragmentation patterns during tandem mass spectrometry (MS/MS) analysis (which limits the analytical sensitivity and specificity by precursor ion scan (PIS) or neutral loss scan (NLS) in shotgun approaches or by selected/multiple reaction monitoring (SRM/MRM) in LC-based approaches).
During MS analysis, lipid solutions are analyzed either by shotgun lipidomics [4, 30] or by chromatography-based lipidomics, particularly LC-based lipidomics [31–33]. Alternatively, the tissue slice or cell samples are directly submitted to MS imaging [26] (Figure 1). The most popular MS ionization techniques are listed in Box 2.
Box 2. Frequently used ionization techniques in modern mass spectrometry for lipidomics.
Electrospray ionization (ESI)
A soft ionization technique used in mass spectrometry that uses an electrospray produced by applying a strong electric field to a liquid passing through a capillary tube to create a fine aerosol from which ions are formed by desolvation.
Matrix-assisted laser desorption/ionization (MALDI)
A soft ionization technique used in mass spectrometry that allows the analysis of large and/or labile molecules (e.g., peptides, proteins, lipids, and polymers) and is particularly useful for MS imaging of tissue or cell samples. This technique involves embedding analytes in a matrix that absorbs energy at the wavelength of the laser. The pulsed laser irradiates the analytes, triggering ablation and desorption of the analytes and matrix material to facilitate the ionization of the analyte molecules in the hot plume of ablated gases.
Atmospheric pressure chemical ionization (APCI)
A soft ionization technique that utilizes gas-phase ion-molecule reactions at atmospheric pressure. Ionization occurs along a corona discharge electrode where the relative proton affinities of the reactant gas ions (e.g., evaporated mobile phase or solvent in most cases) and the gaseous analyte molecules allow either proton transfer/abstraction or adduct formation to produce the molecular ions.
Atmospheric pressure photoionization (APPI)
A useful alternative ionization technique for analysis of compounds that ionize poorly by ESI and APCI. This technique uses a vacuum-ultraviolet lamp designed for photoionization detection in gas chromatography as a source of 10-eV photons. The mixture of samples and solvent, after fully evaporated, is introduced into the photoionization region where the dopant photoions react to completion with solvent and analyte molecules because the ion source is at atmospheric pressure and the collision rate is high.
Secondary ion mass spectrometry (SIMS) employing silver or gold ions as primary ions
The most sensitive surface analytical technique that is used to analyze the composition of solid surfaces or thin films by bombarding the surface with a focused primary ion beam (e.g., silver, or gold ions) and collecting the ejected secondary ions that are introduced to a mass spectrometer for analysis.
Desorption ESI (DESI)
An ambient ionization technique that is a combination of ESI and desorption ionization methods. Ionization occurs by pneumatically directing a charged electrospray mist to the sample surface where subsequent splashed droplets carry desorbed, ionized analytes that then travel into the atmospheric pressure interface of the mass spectrometer.
Following the ionization of lipids, the ion mobility technique can be applied as an option for an additional dimension of ion manipulation and separation prior to MS analysis [34]. Next, either Full MS or MS/MS analysis (see Box 3 for MS/MS techniques) or both can be performed depending on whether a targeted or global analysis is desired. The MS analysis can be done using either low/unit mass resolution or high mass accuracy/mass resolution instruments [35]. It should be recognized that the mass resolution higher than 75,000 around m/z 800 appears required to avoid potential overlaps between lipid species and other complications [36]. Following MS analysis, data are displayed as MS spectra, MS/MS spectra, ion chromatogram (including total ion chromatogram (TIC) or extracted ion chromatogram (XIC); LC-based approaches only), or images (MS imaging only).
Box 3. Commonly used tandem mass spectrometric techniques in lipidomics.
Product ion scan
A tandem mass spectrometric technique where the first mass analyzer selects a specific precursor ion while the second mass analyzer detects all the resultant fragment ions from fragmentation of the selected precursor.
Precursor ion scan (PIS)
A tandem mass spectrometric technique where the first mass analyzer scans all the precursor ions while the second mass analyzer monitors only the selected fragment ion. The selected fragment ion corresponds to a common fragment ion of the precursors; therefore, all the precursors that produce the specified fragment ion during fragmentation are monitored.
Neutral loss scan (NLS)
A tandem mass spectrometric technique where the first mass analyzer scans all the precursor ions while the second mass analyzer scans the fragment ions set at an offset from the first mass analyzer. This offset corresponds to a common neutral loss from the precursor ions; therefore, all the precursors that undergo the loss of the specified neutral fragment are monitored.
Selected/multiple reaction monitoring (SRM/MRM)
A non-scanning tandem mass spectrometric technique used in targeted analysis that performs on triple quadrupole-like instruments and uses two mass analyzers as static mass filters to monitor a particular fragment ion of a selected precursor ion. The specific pair of m/z values associated with the precursor and fragment ions selected is referred to as a “transition”. Multiple reaction monitoring (MRM) is used to indicate the parallel acquisition of multiple SRM transitions.
After data acquisition, spectral data are processed for mandatory deisotoping (for correction of the effects of the presence of isotopic clusters on monoisotopic peak intensities) unless the presence of an internal standard and calibration curve for individual species in the case of LC-based lipidomics, followed by identification and quantification of individual lipid species based on the analytical approaches (i.e., shotgun, LC-MS, or imaging). Those qualitative and quantitative lipid data are further processed by bioinformatic data mining [37] to explore underlying mechanisms of lipid metabolism and its (dys)regulation in health vs. disease, leading to applications in the biomedical sciences (see below), including the discovery of biomarkers or drug targets for diseases as well as guidance in precision/personalized medicine and dietary intervention.
Techniques
As shown in Figure 1, lipids can be analyzed either directly from the biological materials (i.e., MS imaging) or after being extracted with organic solvents. For the latter, there are two categories of MS-based approaches: shotgun or LC-based lipidomics. These approaches are summarized in Table 1. The following is a brief comparison of the distinct and common features of shotgun and LC-based approaches.
Table 1.
Overview of the mass spectrometry-based techniques for lipidomics
| Technique | Types | Key features | Advantages | Limitations | Reviews |
|---|---|---|---|---|---|
| LC-based | LC-MS or MS/MS (ESI, APCI, or APPI MS) | Chromatographic separation with good reproducibility and high resolving power and chromatographic enrichment enabling comprehensive analysis of complex samples including trace level species; normal-phase LC, reversed-phase LC, HILIC and mix-mode LC satisfying different demands of various lipid classes/species | Separation of isomers and isobars; reduction of sample matrix effects; reduction of ion suppression; highly sensitive targeted analysis; offline or online 2D LC-MS for comprehensive profiling analysis | Low throughput; pre-knowledge required for targeted analysis (e.g., for estabilishment of SRM transitions); retention time drift; effects of mobile phase (e.g., composition, salts, or additives); complex data processing | [11, 21, 33, 77–81] |
| MALDI MS | Prior separation of lipid classes by offline LC to remove the signal suppressing lipid classes (e.g., PC) or overlapping species | Removal of prominent PC peaks facilitates analysis of other phospholipid classes | Low throughput; high ion source fragmentation | [21] | |
| Ion mobility MS | Separation of ionized molecules by size, shape, charge and mass based on their different ion mobilities in low or high electric fields; important supplement to the LC separation of molecules and MS separation of ions | Resolving chromato-graphically co-eluting chemical noise leading to an enhanced S/N ratio and increased peak capacity; separation and identification of isomeric and isobaric lipid species; alighing molecular ion spectra and fragment ion spectra at a give ion mobility drift time and chromatographic retention time to obtain a much cleaner fragment ion spectra | Loss of sensitivity; hard to compile with the time limit on the LC run | [33, 34, 82] | |
| Shotgun | Tandem MS | Addition of at least two internal standards for each lipid class of interest; requirement of a unique tandem MS analysis specific to the lipid class of interest | Straight-forward; simple and fast; semi-qualitative; quantitative | Selection of internal standards may be difficult; the specific MS/MS analysis may not be entirely specific to the class of interest; differential fragmentation kinetics of individual molecular species may affect accurate quantiation; structural identities and isobaric species may not be determined | [10, 11, 82] |
| High mass accuracy MS | Utilization of high mass resolution and mass accuracy instruments to sensitively acquire full MS spectra in the survey scan mode and to rapidly perform product ion scans step by step within an entire mass region of interest; multi-PIS or NLS extracted from the acquired data array of the product ion spectra to identify individual species and quantify identified species through the sum of the intensities of fragments of a molecular ion in comparison to that of internal standard; or data-dependent acquistion to acquire product ion spectra for identification and well-resolved high mass accuracy full mass spectra for quantification | Broad and sensitive analysis; Accurate measurment of the masses of molecular ions and fragment ions largely eliminating possible false positive identification | Quantitation from multi-PIS or NLS data by using the sum intensities of fragment ions may be affected by differentail fragmentation kinetics of individual species; differential ionization responses of different species particularly among non-polar lipid classes need to be corrected for accurate quantitation; linear dynamic range of quantification largely depends on the instrument under experimental conditions; unable to distinguish isomeric species having identical fragmentation patterns | [10, 11, 21, 77, 83] | |
| Multidimensional MS | Intrasource separation to reduce the complexity of lipid mixtures; identification of individual species by array analysis using tandem MS techniques (PIS and NLS) to determine building blocks of molecular ions; quantitation by two-step procedure enabling accurate quantitation of low abundance species using multiple standards including internal standard(s) and endogenous abundance species with concentrations pre-determined in the first step | Global and large scale analysis; intrasource separation minimizing the ion suppression effects; definite identification (head group and acyl chains through multiple building blocks, and regio-specificity); accurate quantitation (two-step procedure dramatically extending the linear dynamic range) | Not ideal for analysis of unknown or uncharacterized lipid classes without pre-determined building block knowledge; availability of sensitive MS/MS analysis required for sensitive identification and quantification; unable to distinguish isomeric species having identical fragmentation patterns | [10, 11, 21, 82, 84] | |
| MALDI MS | Appropriate MALDI matrix can obtain tremendous sensitivity and selectivity; analysis can be performed in a short time and with high tolerance to salts and other impurities in samples | Easy sample preparation; re-analysis of samples; high throughput; speed | Severe matrix background at low mass range; multiple adducts complicating spectra; heterogeneous sample spot; post-source decay | [82] | |
| Ion mobility MS | Separation of ionized molecules by size, shape, charge and mass based on their different ion mobilities in low or high electric fields | Separation of many isobaric and isomeric lipids that standard shotgun lipidomics is difficult to assess; reduction of the complexity of lipid mixtures to simplify the analysis of complex extracts; separation of endogenous matrix interferences to increase the selectivity | Loss of sensitivity; difficult for quantification | [34, 82, 85] | |
| MS imaging | MALDI MS | Appropriate MALDI matrix critical for high quality and sensitivity; being successfully applied to imaging lipid profiles of cells (including single cells), tissues, and even entire bodies | An established technique for visualization of lipid distribution and relative concentrations | Ion peaks corresponding to PC species prominent in the positive ion mode eliminating effective analysis of other positively charged lipid classes | [21, 82] |
| DESI MS | Untargeted analysis of lipids ionized from the near-surface region of samples under ambient conditions | Sensitive technique for 2D and 3D imaging of lipids from unmodified complex biological samples; definition of histology and tumor margins | Limited sensitivity | [21] | |
| SIMS | No prior sample treatment; determination of the molecular composition and individual compound localization on tissue section with high spatial resolution; single cell detection | Straightforward analysis without prior sample preparation; providing the closest possible to physiological conditions; high spatial resolution (submicrometer grade) | Difficult to detect intact lipids due to severe source fragmentation | [21] | |
| Ion mobility MS | Separation of ionized molecules by size, shape, charge and mass based on their different ion mobilities in low or high electric fields | Enhancing the data dimensionality in the imaging process; separation of the lipids ions of interest from the interfering background leading to a greater S/N and more accurate lipid localization | Very large dataset | [34, 82] |
Biological lipid extracts are typically complex, including the diversity in lipid classes/subclasses/molecular species and the vast dynamic range in the endogenous contents of individual species. Therefore, reduction of the complexity of lipid extracts is essential for reliable and accurate identification and quantification of individual species in the complex extract. LC-based lipidomics achieves this utilizing separation science in addition to enriching the low abundance species [33, 38]. In contrast, shotgun lipidomics is a high-throughput approach but at expense of preserving the complexity of a lipid extract because of the absence of pre-chromatographic separation. In the practice of shotgun lipidomics, the complexity has been strategically reduced either during sample preparation as aforementioned or during MS analysis via intrasource separation/selective ionization or both [4, 11]. In addition, monitoring head-group related fragments that are characteristic to a specific lipid class by MS/MS (Box 3) selectively detects molecular species within the class without interference from other coexisting classes.
In addition to the difference in reducing the complexity of lipid extract, shotgun and LC-based lipidomics are also different in their data output and, subsequently, the requisite data analysis. The data acquired in shotgun platforms are solely mass spectra including full MS and MS/MS spectra. Shotgun lipidomics identifies lipid species using either product ion spectra or building block-related NLS/PIS spectra. Since identification of a large number of lipid species requires the acquisition of numerous product ion spectra but a significantly smaller number of building block-related NLS/PIS spectra, the latter is much more efficient. The data outputted from LC-MS platforms include both chromatograms (e.g., TIC or XIC) and mass spectra (e.g., full MS and MS/MS spectra including product ion and SRM/MRM data). LC-MS lipidomics identify lipid species from both chromatogram (i.e., retention time) and mass spectral data (e.g., product ion spectra of selected precursor ions, or SRM/MRM from pre-knowledge of targeted precursors). Retention time adds a valuable feature to the species identification in LC-MS approaches. However, the time restriction originating from the “on-the-fly” chromatographic analysis markedly limits the number of the selected precursor ions that can be fragmented during a single LC run. In contrast, all precursor ions of interest are subjected to numerous MS/MS scans, beneficial from the “unlimited” time in shotgun lipidomics.
For quantification, LC-MS platforms largely use the chromatogram data (i.e., peak areas) while shotgun platforms use ion peak intensities detected in either full MS or MS/MS spectral data or both. The advantages and limitations of both approaches have been extensively reviewed [39].
Although shotgun and LC-MS lipidomics approaches are different in multiple aspects, they share common features too. One of the common features is that ESI- and MALDI-MS are both applicable to these approaches although LC-MALDI-MS is operated off-line and not as commonly used as LC-ESI-MS. Another common feature is that shotgun and LC-MS approaches are both compatible to ion mobility MS [34] which adds an additional dimension of separation. The third feature is that after data acquisition and processing for species identification and quantification, same bioinformatics tools can be applied to the data from both platforms [37, 40].
Applications of Lipidomics for the Biomedical Sciences
Metabolic syndrome
Metabolic syndrome is a serious health condition because the risk factors together significantly raise the chances for metabolically related diseases, such as cardiovascular diseases, diabetes, stroke, and non-alcoholic fatty liver disease, compared to any one factor alone. Moreover, metabolic syndrome is becoming more common due to a rise in obesity rates. Lipidomics can play a key role in mechanistic studies, risk prediction, and therapeutic monitoring for metabolic syndrome-related diseases given the tight association of lipids with these diseases [41]. Lipidomics has long been used for diabetes and obesity research and these applications have been extensively reviewed elsewhere (Table 2). Lipidomics of plasma and lipoprotein fractions has provided insights into the complexities of the high-density lipoprotein (HDL) lipidome, unraveled the controversies surrounding HDL-based therapies to attenuate vascular disease [42, 43], and identified phospholipids as a major bioactive component of HDL [42, 44]. The application of lipidomics in vascular health research and ischemic heart disease has demonstrated utilities in population profiling, pathogenesis studies, identification of biomarkers, and monitoring of therapeutic responses through the comprehensive and systematic quantitative analysis of multiple lipid classes including oxidized lipids [45].
Table 2.
Selected applications of lipidomics for biological and biomedical researcha
| Application | Type | Technique | Source of lipids | Findings and implications | Reviews |
|---|---|---|---|---|---|
| Metabolic syndrome | Cardiovascular disease (CVD) | LC-based [86]; shotgun; liquid extraction surface analysis [87]; GC-MS [88] | Plasma and tissue (e.g., plaque) | New insights into the association of molecular lipids with CVD [89, 90]; new approach to risk stratification for unstable CAD [41, 86]; unraveling the lipid heterogeneity within atherosclerotic lesions [87]; revealing biomarkers of atherosclerosis [88, 90]; understanding obesity risk factor of CVD [91]; Identifying lipidomic and metabolomic risk markers of vascular diseases [15, 92] | 2016 [7, 15] 2015 [90] 2014 [41, 89, 91] 2013 [45] |
| Inflammatory diseases (e.g., atherosclerosis) | LC-MS/MS | Serum, plasma, and variety of tissues | Understanding of the mechanisms of inflammatory diseases [93]; identifying bioactive metabolites with potent anti-inflammatory property [94], and lipid mediators in promotion and resolution of inflammation [95, 96]; eicosanoids in infection and inflammation [97] | 2015 [93, 95, 97, 98] 2014 [94] |
|
| HDL-based therapies in atherosclerotic vascular disease | LC-based; shotgun | Plasma HDL | Expanding our knowledge of cardiovascular and metabolic diseases; possible mechanisms of the atheroprotective role of HDL-associated PS; potential applications of PS and other negatively charged phospholipids to HDL-based therapies | 2016 [7, 42, 44] 2013 [43] |
|
| Diabetes and obesity | LC-MS/MS [99–102]; flow injection MS [103, 104]; shotgun [105]; GC [106] | Plasma | Improving the performance of classification models [99]; positive association of plasma lipids with obesity [100]; association of plasma lipidome with type 2 diabetes [102, 104, 106, 107] and similar association present in prediabetes [101, 105]; new mechanistic insights into islet physiology and pathophysiology, and the etiologies of diabetes [108]; exploring the role of metabolomic analysis in diabetes research relating to the development of diabetes in children [109] and changes in obese children with weight loss [103] | 2016 [7, 108, 109] 2014 [41] |
|
| Neurological disorders | Alzheimer’s disease [110–112]; Huntington’s diease [113]; multiple sclerosis [114] | LC-MS and MS/MS [112–114]; shotgun [110, 111]; | CSF [111, 114]; brain tissue [110, 111, 113]; plasma [110, 112]; serum [114] | Alterations in phospholipids [113]; increased levels of DG and others [110, 111]; reduced cholesteryl esters [112]; changed lipid mediators [114]; platelet activiating factors as potential biomarkers in inflammation and neurodegeneration [115] | 2014 [116] 2013 [115, 117] |
| Cancer | Breast [118, 119]; prostate [120]; lung [121]; ovary [122]; esophagus [123]; kidney [124]; skin [125] | LC-MS and MS/MS [118, 120, 122–125]; shotgun [121]; MALDI imaging [121]; DESI-MS imaging [123] | Plasma [118, 122]; tumor tissue [120, 123–125]; human and mouse squamous cell carcinoma [121] | A panel of lipids as biomarkers [118, 119]; accumulation of cholesteryl esters [120]; acyl chain elongation [121]; high lysoPC and lower PC and TG [122]; changed phospholipids [123]; Increased content if species containing PUFA [124]; the role of cycloxyenase-2 in tumorigenesis [125] | 2016 [126] 2014 [127] |
| Eye diseases | Vision research | Shotgun | Tear and tissue | Application in ophthalmology and ocular research | 2013 [128] |
| Tear analysis in ocular surface diseases | LC-MS/MS [129] | Tear fluid | Revealing structure-specific lipid aberrations in dry eye syndrome [129] | 2013 [50] | |
| Meibomian gland secretion (MGS) and tear film in ocular surface protection | LC-MS; GC-MS | MGS and tear film | Finding reliable markers of ocular diseases; identifying physiological role of meibomian lipids and most relevant targets for future biomedical ocular studies | 2013 [130] | |
| Inflammation and neuroprotection | LC-MS; shotgun; MALDI imaging | Retina | Defining the actions of DHA and its neuroprotective derivative for intervention in ocular neuro-degenerative diseases; characterization of PUFA-containing phospholipids for a better understanding of the pathogenesis in photoreceptor membranes; lipid localization within retina sections; lipid-based therapy | 2015 [52] 2013 [131] |
|
| Nutrition | Dietary intervention | LC-MS; shotgun | Plasma or tissue | Short-term over-nutrition influential in plasma lipids of healthy humans [132]; plasma lipids used as surrogates for dietary intake supporting a negative association beteen dairy food consumption and risk of type 2 diabetes in metabolic syndrome individuals [133]; response to cholesterol-lowing dietary intake; metabolic response to caloric restriction diet; dietary lipid transport and signaling; dietary lipids in the regulation of intestinal immunity [14]; identifying novel biomarkers in nutritional epidemiology [54] | 2016 [14] 2015 [54] 2014 [41] 2013 [53, 134] |
| Health benefits of dietary ω-3 PUFA | LC-MS/MS | cardiomyocyte culture and tissue, hepatic tissue, plasma and other tissues | Analysis of bioactive mediators derived from dietary ω-3 PUFA to investigate the molecular mechanisms underlying their health benefits or controversy effects [56]; incorporation of nutritional DHA into tissue [135, 136] | 2015 [57] 2013 [56] |
|
| Drug discovery/screen | Target discovery; candidate drugs; preclinic/clinical testing; toxicity; biomarkers (see below); personalized medicine (see below); tranditional Chinese medicine (TCM) [137] | LC-based; shotgun | Cancer cells [138] or animal models [137] treated with different compounds | Companion diagnostic in several steps in drug development process; useful for studying the action mechanisms of TCM [137]; potential of de novo lipogenesis inhibitors as anticancer drugs [58]; lipid biomarkers for drug efficacy [139]; plasma lipidomics in cardiovascular drug discovery [60] | 2015 [138, 140] 2014 [58, 59] |
| Multi-omics and personalized medicine | Type 1 diabetes (T1D) [61]; asthma [141]; chronic pain [142]; global lipid metabolism [72]; systems and dynamical medicine [140]; diet effects [143] | LC-based; shotgun [65] | Variety of biological samples | Metabolic modeling as a pormising new avenue for the interaction of metabolic and immune system in T1D [61]; omics leading to the molecular signatures and standardized phenotyping of asthma [141] and chronic pain [142]; translational bioinformatics converting voluminous omics data into predictive models [140]; proteome and lipidome dynamics analysis revealing functional regulation of global lipid metabolism [72]; effects of diet on multi-omic measurement and relationship between genetic variants and diet in modulationg cardiometabolic risk [143] | 2016 [61] 2014 [140] 2013 [65] |
This list is not exhaustive but provides just an overview of some recent applications.
Neurological disorders
The brain contains the highest amount of lipids. Naturally, neurological disorders are associated with lipid signaling, metabolism, trafficking, and homeostasis. Lipidomics can be used to investigate these aspects and to develop biomarkers for early diagnosis and prognosis of these disorders. In fact, lipidomics has been employed to study brain complications since its development [46]. Some recent representative studies on neurological diseases are summarized in Table 2. Previous studies on brain disorders by lipidomics can be found in the cited review articles (Table 2).
Cancer
Lipids play many key roles in all of the basic processes essential for tumor development [47]. For example, lipids play roles in cell growth and metabolism, which are essential for rapidly proliferating cancer cells: non-esterified fatty acids are the major building blocks for lipid biosynthesis and remodeling; cholesterol, phospholipids, and sphingolipids represent the major structural components of cellular membranes; and triglycerides serve as the energy storage depot, which, along with acyl CoA and acylcarnitine, are involved in energy metabolism and ATP production. In addition, bioactive lipids, such as lysophospholipids, play important roles in signaling, functioning as second messengers and as hormones in cancer cells to promote cell proliferation, survival, and migration [48]. Similarly, hydrolysis products of phosphatidylinositol and its phosphorylated derivatives are second messengers to activate the PI3K/AKT signaling pathway [49]. The significance of this pathway in chemotherapy and radiotherapy for human cancers is well recognized [49]. It is not surprising that cancer cells undergo profound changes in lipid metabolism and homeostasis, thus offering new diagnostic and therapeutic opportunities that could be unraveled by lipidomics. Body fluids serve as a source of biomarkers for early diagnosis of cancers, as their lipid profiles reflect the general condition of the whole organism. In addition to identifying biomarkers, qualitative and quantitative assessment of lipids in blood and other body fluids may be also useful in monitoring the efficacy and toxicity of anticancer treatment. Some representative lipidomics studies on cancer research from the past couple of years are tabulated (Table 2).
Eye diseases
Lipidomics has enabled analysis of lipids in vision research and ophthalmology to understand and diagnose eye disease (Table 2). Human meibomian lipids, after mixing with aqueous tears, form the tear film to protect delicate ocular structures from desiccating. Lipidomics has provided insights into the stability of tear film and biomarkers for diagnosis, prognosis and treatment of ocular surface diseases [50, 51]. Lipidomics has also enabled effective lipid-based therapy to replenish tear film lipids for modulating ocular surface inflammation and disease [52]. In addition, mediator lipidomics that focuses on the analysis of lipid-derived bioactive molecules such as lipid second messengers and lipid regulators has targeted ophthalmological processes. Some recent applications of lipidomics on eye diseases are summarized in Table 2.
Nutrition
Nutritional lipidomics provides a comprehensive view of lipids in nutrition, but its translation to practice via nutritional interventions is still in its infancy [53]. Lipidomics can be effectively used in nutrition research to understand diet-dependent changes in the structure, composition, and function of cellular lipids. Lipidomics can also be used in nutrition study to assign the functions of lipids as signaling molecules, nutrient sensors, and intermediates of metabolic pathways, and to elucidate the interactions between nutrients and human metabolism (Table 2). In addition, lipidomics can evaluate the dietary intake in a more standardized and precise way for monitoring the acute, medium term, and chronic effects of dietary components, and may provide nutritional advice to guarantee lifelong optimized health [54, 55]. Lipidomics has provided insights into the molecular mechanism underlying the health benefits of dietary ω-3 polyunsaturated fatty acids (PUFA) and the regulatory role of ω-3 and -6 fatty acids in the inflammatory response [56, 57]. The wise use of lipidomics should be an essential part of future ω-3 trials [57].
Drug discovery and screens
Lipidomics provides new insights into pharmaceutical research through implementing lipidomics in drug target discovery, screening, toxicity evaluation, preclinical testing, prediction and monitoring of response, and personalized medicine (Table 2). For example, a major interest in de novo lipogenesis inhibitors is their proapoptotic effects on cancerous cells. Lipidomics can be used to screen a large variety of candidate anticancer drugs for those that inhibit de novo lipid synthesis [58] and to identify novel drug efficacy biomarkers [59, 60].
Multi-omics and personalized medicine
A new direction for omics in the biomedical sciences is the reclassification of diseases from a molecular perspective thus playing a key role in personalized medicine [60]. The complexity in health and disease requires multi-omics with each complementing the information provided by the others to understand the biology of the whole system [61]. The elucidation of the crosstalk among different systems can contribute to the discovery of accurate and robust biomarkers at various disease stages for the development of systems medicine [62]. Novel biomarkers can improve risk stratification and patient selection for better treatment response [15], allow the development of the next generation of therapeutics, and help in the prediction and monitoring of treatment efficacy and response to therapeutic measures [15, 60, 63, 64]. Among multi-omics (mainly represented by genomics, proteomics and metabolomics), metabolomics (including lipidomics) is the latest addition to the omics family. Distinct from the other omics, metabolomics measures the metabolites that are closely related to the phenotypes and thereby are directly connected with biological conditions and disease states [61, 65]. Integration of multiple omics techniques is moving the biomedical field towards personalized medicine as the ultimate paradigm of responsible clinical practice [66] (Table 2). Translational bioinformatics plays an essential role in this transition by bridging the gap among different knowledge domains for the translation of the enormous data set collected from multi-omics into the simulation of complex systems and predictive models for achieving predictive, preventive and personalized medicine [60, 62].
Outcomes and Perspectives
Summary of the state of current knowledge: advantages and limitations
Since the emergence of the lipidomics discipline in 2003, the advancing analytical technologies have greatly driven the field to essentially all biological and biomedical areas (Table 2). These technologies include soft ionization methods and other techniques (e.g., ion mobility) in mass spectrometry, separation science such as ultra-performance LC and nanomaterials, analysis of direct infusion (e.g., shotgun lipidomics and MS imaging), and novel bioinformatic strategies and libraries (Figure 1 and Table 1). Lipidomics has led us to identify new signaling molecules, reveal the underlying mechanisms responsible for patho(physio)logical conditions, discover potential biomarkers for early diagnosis and prognosis of diseases, screen drug targets and/or test drug efficacy, guide nutritional intervention, and achieve personalized medicine. These accomplishments are due to not only technique development, but also to the nature of lipidomics in being able to comprehensively analyze hundreds to thousands of lipid species at its current development [10, 32, 67, 68] and to study lipid metabolism [69].
Regardless of the tremendous advances made in recent years, a few areas of technological progress are still desirable. First, whether individual lipid species can be accurately quantified with current methods is still under debate [11]. Currently, the identification and quantification of individual signaling lipid species, including chiral isomers for eicosanoids, positional isomers of polyphosphoinositides, phospholipids carrying modified fatty acids, a variety of sphingoids, and numerous intermediate metabolites, are still not fully achievable. In addition, coverage of the entire cellular lipidome is still in dream. Moreover, bioinformatics for interpreting large sets of lipidomics data is largely limited to the levels of lipid classes for the construction of metabolic pathways and network. Currently, the access to the levels of individual lipid species in pathway mapping remains problematic. Finally, a definitive unraveling of the biochemical mechanisms responsible for a disease state is still rare. Accordingly, great efforts are needed for all of these areas.
The most productive avenues for future research
Lipidomics, at its current stage, has been developed in two directions: either targeted or global analysis. The former is mostly used for studying signal processing, while the latter is very powerful for studying lipid metabolism, molecular mechanisms and biomarker discovery. Derivatization appears to be very useful for the development of targeted approaches for both shotgun and LC-based lipidomics [10]. In global analysis, an increase in the coverage of lipid classes and molecular species is critical. The broader the coverage is, the better the approach allows us to map the entire metabolic pathways of lipid classes/subclasses and individual species of a system and to better understand the inter-relationship between these classes and species within a metabolic pathway or between the metabolic networks. Luckily, different approaches have already demonstrated their power for “visualizing” and understanding the changes of hundreds to thousands of individual species [3, 40, 67, 68]. However, further increases in the coverage of lipid classes and individual species (particularly for those very low abundance species) using an automated, high throughput manner in any platform remain demanding.
The usefulness and power of bioinformatics for interpreting lipidomics data based on mass spectral simulation or dynamic modeling has already been demonstrated [70, 71]. However, these successes are only in isolated studies on different clustered pathways. Similar modeling or novel approaches for analysis of a more comprehensive network or ideally for the entire cellular lipidome are still warranted. Furthermore, as the technologies for lipidomics advance, interweaving of this discipline with other fields becomes demanding. Integration of lipidomics with other omics strategies could maximize the power of lipidomics for understanding the molecular mechanisms underpinning diseases [72, 73]. Thus, in addition to broadening the coverage of lipid analysis, one of the logical future directions in the field should be to integrate lipidomics data with genetic, transcriptional, and enzyme data to perform metabolic pathway reconstruction and flux analyses. The reconstruction of lipid metabolism pathways require novel strategies for pathway mapping of lipid data at the molecular species levels instead of at lipid class/subclass levels [40]. Given the structural diversity of lipid classes and species, these tasks are challenging and require a combination of novel and existing bioinformatics resources.
Studying the dynamic changes of a limited set of lipid classes and/or species via lipidomics, through simple stable isotope labeling, has been well practiced [74, 75]. However, studies on assessing enzymatic activities, lipid turnover kinetics, and the effects of individual enzyme activation on lipid homeostasis in a metabolic pathway and/or network are missing. This type of research apparently requires lipidomics techniques possessing sensitive, high throughput, and broad coverage capabilities. Furthermore, more complex studies on the fluxomics scale are needed to reveal the reaction rates in lipid metabolism. These kinds of studies in lipidomics should enable the comprehensive determination of lipid metabolism at the molecular level and provide a true understanding of the roles of lipids in the biomedical sciences.
Finally, as advanced in the sensitivity of modern MS instrumentation, the lower limit of detection at the concentration of amol/mg protein of tissue or cell, or amol/ml of body fluid has been frequently reported in the literature. This concentration is within the range of that in single cells. Therefore, the interest in single cell lipidomics has been greatly raised at the recent related conferences and workshops. In fact, single cell lipidomics is held back more from the obstacles of sample preparation than MS detection [76]. It can be foreseen that this goal is achievable in the near future.
Outstanding Questions.
Can we broaden the analysis coverage of lipid classes and individual molecular species to achieve full coverage of the cellular lipidome?
Although advances in mass spectrometry have dramatically increased analytical sensitivity and specificity, and therefore greatly broadened coverage including of lower abundance species, there is still a long way to go to uncover the entire lipidome of certain organisms. Coverage improvements are needed for both known entities (that are difficult to analyze largely because of their very low abundance, isomer differentiation, and variety of acyl chain modifications) and unknown entities.
Can we construct the entire metabolic network at the level of lipid classes and individual molecular species?
Bioinformatics has shown its use and power in interpreting lipidomics data through simulation, modeling, pathway mapping, and network construction. However, the establishment of the entire metabolic network at the lipid molecular species level is still in its infancy and will require contributions from multidisciplinary scientific fields.
How do lipid dynamics and metabolism translate into in-depth insights into the roles of lipids in the biomedical sciences?
Although the dynamics of lipid classes and molecular species have been studied (e.g., through metabolic flux analysis), an evaluation of lipid turnover kinetics and the effects of enzyme (de)activation after perturbation of lipid homeostasis on metabolic pathways/networks is still missing. This is largely due to the need for high sensitivity, high throughput, and broad coverage lipidomics techniques, which are still under development.
Can we integrate lipidomics with other omics strategies to maximize understanding of the molecular mechanisms underlying diseases?
Metabolomics (including lipidomics) measures metabolites that are closely related to the phenotypes of pathophysiological conditions. The complexity of human disease requires multi-omics approaches where each omics dataset complements the information provided by the others to elucidate systems-level biology. Given the structural diversity of lipid classes and species, integration of lipidomics with other omics is challenging and demands novel bioinformatics resources.
Trends.
‘Lipidomics’ applies to studying lipid metabolism on a broad scale. Lipidomics may elucidate the biochemical mechanism(s) underlying specific changes in lipid metabolism.
Advances in mass spectrometry have greatly accelerated the lipidomics field. Chemical derivatization has shown its broad use in improving analytical sensitivity and specificity in lipidomics.
Multi-omic data integration is challenging but necessary to uncover the mechanism(s) responsible for the lipid metabolism changes of a biological system after perturbation.
Dynamic lipidomics for assessing lipid turnover kinetics and the effects of individual enzyme (de)activation on lipid homeostasis remains challenging.
Single cell lipidomics characterizes the unique chemical composition of individual cells, and is on the agenda of advancing lipidomics.
Acknowledgments
This work was partially supported by National Institute of General Medical Sciences Grant R01 GM105724, intramural institutional research funds (X.H.), and National Institute of Diabetes And Digestive And Kidney Diseases Grant P30DK056341 (Nutrition Obesity Research Center) (K.Y.).
Glossary
- Biomarker
a characteristic that is objectively measured and evaluated accurately and reproducibly as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.
- Deisotoping
the removal of mass spectral complications due to the presence of isotopic clusters.
- Fluxomics
metabolic flux analysis that integrates in vivo measurements of metabolic fluxes with stoichiometric network models to allow determining absolute flux through large metabolic networks.
- Global analysis of lipids
an untargeted analysis that comprehensively analyzes the entire or partial cellular lipidome and aims at detecting every lipid species present in the sample.
- Intrasource separation/selective ionization
separation of the distinct lipid classes that occur in the electrospray ion source through electric field-induced selective ionization of molecular species possessing differential charge propensities.
- Ion mobility spectrometry (IMS)
an analytical technique that separates and identifies ionized molecules in the gas phase based on their mobility in a carrier buffer gas. The methods include drift-time, aspiration, differential, and traveling-wave IMS.
- Lipid extraction
a procedure that separates cellular or fluid lipids from the other constituents but also preserves these lipids for further analyses. Most procedures exploit the high solubility of lipids in organic solvents.
- Lipidomics
the large-scale study of pathways and networks of cellular lipids in biological systems. Lipidomics research studies the structure and function of the complete set of lipids (the lipidome) in a given cell or organism as well as their interactions with other cellular components.
- Liquid chromatography (LC)-based lipidomics
the lipidomics approaches that employ LC to separate the complex lipid classes and/or individual lipid species prior to MS analysis.
- Mass spectrometry imaging (MS imaging)
an in situ technique used in MS to visualize the spatial distribution of chemical compositions by their molecular masses and/or characteristic fragment ions.
- Metabolic syndrome
a complication due to a group of risk factors for metabolically related diseases. The factors include a large waistline, a high triglyceride level, a low high-density lipoprotein (HDL) cholesterol level, high blood pressure, and high fasting blood sugar.
- Metabolic network
the complete set of metabolic processes that determine the biochemical properties of a cell. Analysis of metabolic network identifies the relative activities of its individual branches (metabolic pathways) and provides insights into the molecular mechanisms.
- Precision/Personalized medicine
providing the right patient with the right drug at the right dose at the right time. More broadly, it may be thought of as the tailoring of medical treatment to the individual characteristics, needs and preferences of a patient during all stages of care, leading to better individual treatment.
- Shotgun lipidomics
direct-infusion based lipidomics approaches that analyze lipids without pre-chromatographic separation of lipids prior to mass spectrometry. A unique feature of shotgun lipidomics is analyzing lipids under constant concentration conditions.
- Tandem mass spectrometry (tandem MS or MS/MS or MS2)
the MS technique that involves fragmentation of precursor ions and monitoring of both precursor ions and resultant product (or fragment) ions (see Box 3 for details).
- Targeted analysis of lipids
the lipidomics approach that focuses on known lipids, and develops a specific method with a high sensitivity for quantitative analysis of these lipids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yetukuri L, et al. Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst Biol. 2007;1:12. doi: 10.1186/1752-0509-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han X, Jiang X. A review of lipidomic technologies applicable to sphingolipidomics and their relevant applications. Eur J Lipid Sci Technol. 2009;111:39–52. doi: 10.1002/ejlt.200800117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang K, et al. Automated lipid identification and quantification by multi-dimensional mass spectrometry-based shotgun lipidomics. Anal Chem. 2009;81:4356–4368. doi: 10.1021/ac900241u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han X, et al. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom Rev. 2012;31:134–178. doi: 10.1002/mas.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanksby SJ, Mitchell TW. Advances in mass spectrometry for lipidomics. Annu Rev Anal Chem. 2010;3:433–465. doi: 10.1146/annurev.anchem.111808.073705. [DOI] [PubMed] [Google Scholar]
- 6.Witting M, Schmitt-Kopplin P. The Caenorhabditis elegans lipidome: A primer for lipid analysis in Caenorhabditis elegans. Arch Biochem Biophys. 2016;589:27–37. doi: 10.1016/j.abb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, et al. Recent advances in lipidomics for disease research. J Sep Sci. 2016;39:38–50. doi: 10.1002/jssc.201500899. [DOI] [PubMed] [Google Scholar]
- 8.Crick PJ, Guan XL. Lipid metabolism in mycobacteria-Insights using mass spectrometry-based lipidomics. Biochim Biophys Acta. 2016;1861:60–67. doi: 10.1016/j.bbalip.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Sethi S, et al. Analytical approaches for lipidomics and its potential applications in neuropsychiatric disorders. World J Biol Psychiatry. 2016 doi: 10.3109/15622975.15622015.11117656. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, et al. Novel advances in shotgun lipidomics for biology and medicine. Prog Lipid Res. 2016;61:83–108. doi: 10.1016/j.plipres.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, et al. Selection of internal standards for accurate quantification of complex lipid species in biological extracts by electrospray ionization mass spectrometry-What, how and why? Mass Spectrom Rev. 2016 doi: 10.1002/mas.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keereetaweep J, Chapman KD. Lipidomic analysis of endocannabinoid signaling: Targeted metabolite identification and quantification. Neural Plast. 2016;2016:2426398. doi: 10.1155/2016/2426398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyotylainen T, Oresic M. Bioanalytical techniques in nontargeted clinical lipidomics. Bioanalysis. 2016;8:351–364. doi: 10.4155/bio.15.244. [DOI] [PubMed] [Google Scholar]
- 14.Kunisawa J, Kiyono H. Sphingolipids and epoxidized lipid metabolites in the control of gut immunosurveillance and allergy. Front Nutr. 2016;3:3. doi: 10.3389/fnut.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laaksonen R. Identifying new risk markers and potential targets for coronary artery disease: The value of the lipidome and metabolome. Cardiovasc Drugs Ther. 2016;30:19–32. doi: 10.1007/s10557-016-6651-8. [DOI] [PubMed] [Google Scholar]
- 16.Michaelson LV, et al. Plant sphingolipids: Their importance in cellular organization and adaption. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbalip.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolim AE, et al. Lipidomics in the study of lipid metabolism: Current perspectives in the omic sciences. Gene. 2015;554:131–139. doi: 10.1016/j.gene.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Brugger B. Lipidomics: analysis of the lipid composition of cells and subcellular organelles by electrospray ionization mass spectrometry. Annu Rev Biochem. 2014;83:79–98. doi: 10.1146/annurev-biochem-060713-035324. [DOI] [PubMed] [Google Scholar]
- 19.Dowhan W. Lipids and extracellular materials. Annu Rev Biochem. 2014;83:45–49. doi: 10.1146/annurev-biochem-010314-112017. [DOI] [PubMed] [Google Scholar]
- 20.Vaz FM, et al. Principles and practice of lipidomics. J Inherit Metab Dis. 2015;38:41–52. doi: 10.1007/s10545-014-9792-6. [DOI] [PubMed] [Google Scholar]
- 21.Li M, et al. Analytical methods in lipidomics and their applications. Anal Chem. 2014;86:161–175. doi: 10.1021/ac403554h. [DOI] [PubMed] [Google Scholar]
- 22.Ghaste M, et al. Applications of Fourier transform ion cyclotron resonance (FT-ICR) and orbitrap based high resolution mass spectrometry in metabolomics and lipidomics. Int J Mol Sci. 2016;17:816. doi: 10.3390/ijms17060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyotylainen T, Oresic M. Optimizing the lipidomics workflow for clinical studies--practical considerations. Anal Bioanal Chem. 2015;407:4973–4993. doi: 10.1007/s00216-015-8633-2. [DOI] [PubMed] [Google Scholar]
- 24.Seppanen-Laakso T, Oresic M. How to study lipidomes. J Mol Endocrinol. 2009;42:185–190. doi: 10.1677/JME-08-0150. [DOI] [PubMed] [Google Scholar]
- 25.Furse S, et al. Isolation of lipids from biological samples. Mol Membr Biol. 2015;32:55–64. doi: 10.3109/09687688.2015.1050468. [DOI] [PubMed] [Google Scholar]
- 26.Murphy RC, et al. Imaging of lipid species by MALDI mass spectrometry. J Lipid Res. 2009;50(Suppl):S317–S322. doi: 10.1194/jlr.R800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han X, et al. Metabolomics in early Alzheimer’s disease: Identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS ONE. 2011;6:e21643. doi: 10.1371/journal.pone.0021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merrill AH, Jr, et al. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–224. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, et al. Alkaline methanolysis of lipid extracts extends shotgun lipidomics analyses to the low abundance regime of cellular sphingolipids. Anal Biochem. 2007;371:135–145. doi: 10.1016/j.ab.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stahlman M, et al. High throughput oriented shotgun lipidomics by quadrupole time-of-flight mass spectrometry. J Chromatogr B. 2009;877:2664–2672. doi: 10.1016/j.jchromb.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 31.Dennis EA, et al. A mouse macrophage lipidome. J Biol Chem. 2010;285:39976–39985. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fauland A, et al. A comprehensive method for lipid profiling by liquid chromatography-ion cyclotron resonance mass spectrometry. J Lipid Res. 2011;52:2314–2322. doi: 10.1194/jlr.D016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cajka T, Fiehn O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Analyt Chem. 2014;61:192–206. doi: 10.1016/j.trac.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paglia G, et al. Applications of ion-mobility mass spectrometry for lipid analysis. Anal Bioanal Chem. 2015;407:4995–5007. doi: 10.1007/s00216-015-8664-8. [DOI] [PubMed] [Google Scholar]
- 35.Junot C, et al. High resolution mass spectrometry based techniques at the crossroads of metabolic pathways. Mass Spectrom Rev. 2014;33:471–500. doi: 10.1002/mas.21401. [DOI] [PubMed] [Google Scholar]
- 36.Wang M, et al. Accurate mass searching of individual lipid species candidates from high-resolution mass spectra for shotgun lipidomics. Rapid Commun Mass Spectrom. 2014;28:2201–2210. doi: 10.1002/rcm.7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Checa A, et al. Lipidomic data analysis: tutorial, practical guidelines and applications. Anal Chim Acta. 2015;885:1–16. doi: 10.1016/j.aca.2015.02.068. [DOI] [PubMed] [Google Scholar]
- 38.Zhao YY, et al. Ultra-performance liquid chromatography-mass spectrometry as a sensitive and powerful technology in lipidomic applications. Chem Biol Interact. 2014;220:181–192. doi: 10.1016/j.cbi.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 39.Yang K, Han X. Accurate quantification of lipid species by electrospray ionization mass spectrometry - Meets a key challenge in lipidomics. Metabolites. 2011;1:21–40. doi: 10.3390/metabo1010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyotylainen T, Oresic M. Systems biology strategies to study lipidomes in health and disease. Prog Lipid Res. 2014;55:43–60. doi: 10.1016/j.plipres.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Meikle PJ, et al. Lipidomics: potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacol Ther. 2014;143:12–23. doi: 10.1016/j.pharmthera.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Darabi M, et al. Therapeutic applications of reconstituted HDL: When structure meets function. Pharmacol Ther. 2016;157:28–42. doi: 10.1016/j.pharmthera.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Kontush A, et al. Unraveling the complexities of the HDL lipidome. J Lipid Res. 2013;54:2950–2963. doi: 10.1194/jlr.R036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darabi M, Kontush A. Can phosphatidylserine enhance atheroprotective activities of high-density lipoprotein? Biochimie. 2016;120:81–86. doi: 10.1016/j.biochi.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 45.Rasmiena AA, et al. Metabolomics and ischaemic heart disease. Clin Sci (Lond) 2013;124:289–306. doi: 10.1042/CS20120268. [DOI] [PubMed] [Google Scholar]
- 46.Han X. Lipid alterations in the earliest clinically recognizable stage of Alzheimer’s disease: Implication of the role of lipids in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:65–77. doi: 10.2174/1567205052772786. [DOI] [PubMed] [Google Scholar]
- 47.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 48.Murph M, et al. Liquid chromatography mass spectrometry for quantifying plasma lysophospholipids: potential biomarkers for cancer diagnosis. Methods Enzymol. 2007;433:1–25. doi: 10.1016/S0076-6879(07)33001-2. [DOI] [PubMed] [Google Scholar]
- 49.Fresno Vara JA, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Zhou L, Beuerman RW. Tear analysis in ocular surface diseases. Prog Retin Eye Res. 2012;31:527–550. doi: 10.1016/j.preteyeres.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Pieragostino D, et al. Unraveling the molecular repertoire of tears as a source of biomarkers: beyond ocular diseases. Proteomics Clin Appl. 2015;9:169–186. doi: 10.1002/prca.201400084. [DOI] [PubMed] [Google Scholar]
- 52.Lim A, et al. Lipid-based therapy for ocular surface inflammation and disease. Trends Mol Med. 2015;21:736–748. doi: 10.1016/j.molmed.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Smilowitz JT, et al. Nutritional lipidomics: molecular metabolism, analytics, and diagnostics. Mol Nutr Food Res. 2013;57:1319–1335. doi: 10.1002/mnfr.201200808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corella D, Ordovas JM. Biomarkers: background, classification and guidelines for applications in nutritional epidemiology. Nutr Hosp. 2015;31(Suppl 3):177–188. doi: 10.3305/nh.2015.31.sup3.8765. [DOI] [PubMed] [Google Scholar]
- 55.Jungnickel H, Luch A. A personalized life: biomarker monitoring from cradle to grave. Experientia Suppl. 2012;101:471–498. doi: 10.1007/978-3-7643-8340-4_17. [DOI] [PubMed] [Google Scholar]
- 56.Maskrey BH, et al. Emerging importance of omega-3 fatty acids in the innate immune response: molecular mechanisms and lipidomic strategies for their analysis. Mol Nutr Food Res. 2013;57:1390–1400. doi: 10.1002/mnfr.201200723. [DOI] [PubMed] [Google Scholar]
- 57.Visioli F. Lipidomics to assess omega 3 bioactivity. J Clin Med. 2015;4:1753–1760. doi: 10.3390/jcm4091753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamaziere A, et al. How lipidomics provides new insight into drug discovery. Expert Opin Drug Discov. 2014;9:819–836. doi: 10.1517/17460441.2014.914026. [DOI] [PubMed] [Google Scholar]
- 59.Vihervaara T, et al. Lipidomics in drug discovery. Drug Discov Today. 2014;19:164–170. doi: 10.1016/j.drudis.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Puri R, et al. The emerging role of plasma lipidomics in cardiovascular drug discovery. Expert Opin Drug Discov. 2012;7:63–72. doi: 10.1517/17460441.2012.644041. [DOI] [PubMed] [Google Scholar]
- 61.Marinkovic T, Oresic M. Modeling strategies to study metabolic pathways in progression to type 1 diabetes--Challenges and opportunities. Arch Biochem Biophys. 2016;589:131–137. doi: 10.1016/j.abb.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Yan Q. Translational bioinformatics approaches for systems and dynamical medicine. Methods Mol Biol. 2014;1175:19–34. doi: 10.1007/978-1-4939-0956-8_2. [DOI] [PubMed] [Google Scholar]
- 63.Zhao YY, et al. Lipidomics applications for discovering biomarkers of diseases in clinical chemistry. Int Rev Cell Mol Biol. 2014;313:1–26. doi: 10.1016/B978-0-12-800177-6.00001-3. [DOI] [PubMed] [Google Scholar]
- 64.Heinonen MT, et al. New insights and biomarkers for type 1 diabetes. Scand J Immunol. 2015;82:244–253. doi: 10.1111/sji.12338. [DOI] [PubMed] [Google Scholar]
- 65.Di Girolamo F, et al. The role of mass spectrometry in the “omics” era. Curr Org Chem. 2013;17:2891–2905. doi: 10.2174/1385272817888131118162725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merched AJ, Chan L. Nutrigenetics and nutrigenomics of atherosclerosis. Curr Atheroscler Rep. 2013;15:328. doi: 10.1007/s11883-013-0328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Almeida R, et al. Comprehensive lipidome analysis by shotgun lipidomics on a hybrid quadrupole-orbitrap-linear ion trap mass spectrometer. J Am Soc Mass Spectrom. 2015;26:133–148. doi: 10.1007/s13361-014-1013-x. [DOI] [PubMed] [Google Scholar]
- 68.Quehenberger O, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han X. Lipidomics for studying metabolism. Nat Rev Endocrinol. 2016 doi: 10.1038/nrendo.2016.1098. [DOI] [PubMed] [Google Scholar]
- 70.Kiebish MA, et al. Dynamic simulation of cardiolipin remodeling: greasing the wheels for an interpretative approach to lipidomics. J Lipid Res. 2010;51:2153–2170. doi: 10.1194/jlr.M004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han RH, et al. Simulation of triacylglycerol ion profiles: bioinformatics for interpretation of triacylglycerol biosynthesis. J Lipid Res. 2013;54:1023–1032. doi: 10.1194/jlr.M033837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casanovas A, et al. Quantitative analysis of proteome and lipidome dynamics reveals functional regulation of global lipid metabolism. Chem Biol. 2015;22:412–425. doi: 10.1016/j.chembiol.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Loizides-Mangold U. On the future of mass-spectrometry-based lipidomics. FEBS J. 2013;280:2817–2829. doi: 10.1111/febs.12202. [DOI] [PubMed] [Google Scholar]
- 74.Postle AD, Hunt AN. Dynamic lipidomics with stable isotope labelling. J Chromatogr B. 2009;877:2716–2721. doi: 10.1016/j.jchromb.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 75.Ecker J, Liebisch G. Application of stable isotopes to investigate the metabolism of fatty acids, glycerophospholipid and sphingolipid species. Prog Lipid Res. 2014;54:14–31. doi: 10.1016/j.plipres.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Rubakhin SS, et al. Profiling metabolites and peptides in single cells. Nat Methods. 2011;8:S20–S29. doi: 10.1038/nmeth.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jung HR, et al. High throughput quantitative molecular lipidomics. Biochim Biophys Acta. 2011;1811:925–934. doi: 10.1016/j.bbalip.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 78.Sokol E, et al. Profiling of lipid species by normal-phase liquid chromatography, nanoelectrospray ionization, and ion trap-orbitrap mass spectrometry. Anal Biochem. 2013;443:88–96. doi: 10.1016/j.ab.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 79.Myers DS, et al. Quantitative analysis of glycerophospholipids by LC-MS: acquisition, data handling, and interpretation. Biochim Biophys Acta. 2011;1811:748–757. doi: 10.1016/j.bbalip.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brouwers JF. Liquid chromatographic-mass spectrometric analysis of phospholipids. Chromatography, ionization and quantification. Biochim Biophys Acta. 2011;1811:763–775. doi: 10.1016/j.bbalip.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 81.Smith R, et al. LC-MS alignment in theory and practice: a comprehensive algorithmic review. Brief Bioinform. 2015;16:104–117. doi: 10.1093/bib/bbt080. [DOI] [PubMed] [Google Scholar]
- 82.Wang C, et al. Applications of mass spectrometry for cellular lipid analysis. Mol Biosyst. 2015;11:698–713. doi: 10.1039/c4mb00586d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Papan C, et al. Systematic screening for novel lipids by shotgun lipidomics. Anal Chem. 2014;86:2703–2710. doi: 10.1021/ac404083u. [DOI] [PubMed] [Google Scholar]
- 84.Wang M, Han X. Multidimensional mass spectrometry-based shotgun lipidomics. Methods Mol Biol. 2014;1198:203–220. doi: 10.1007/978-1-4939-1258-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lintonen TP, et al. Differential mobility spectrometry-driven shotgun lipidomics. Anal Chem. 2014;86:9662–9669. doi: 10.1021/ac5021744. [DOI] [PubMed] [Google Scholar]
- 86.Meikle PJ, et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:2723–2732. doi: 10.1161/ATVBAHA.111.234096. [DOI] [PubMed] [Google Scholar]
- 87.Stegemann C, et al. Comparative lipidomics profiling of human atherosclerotic plaques. Circ Cardiovasc Genet. 2011;4:232–242. doi: 10.1161/CIRCGENETICS.110.959098. [DOI] [PubMed] [Google Scholar]
- 88.Chen X, et al. Plasma metabolomics reveals biomarkers of the atherosclerosis. J Sep Sci. 2010;33:2776–2783. doi: 10.1002/jssc.201000395. [DOI] [PubMed] [Google Scholar]
- 89.Hinterwirth H, et al. Lipidomics: quest for molecular lipid biomarkers in cardiovascular disease. Circ Cardiovasc Genet. 2014;7:941–954. doi: 10.1161/CIRCGENETICS.114.000550. [DOI] [PubMed] [Google Scholar]
- 90.Kolovou G, et al. Lipidomics in vascular health: current perspectives. Vasc Health Risk Manag. 2015;11:333–342. doi: 10.2147/VHRM.S54874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ban RH, et al. Lipidomic profiling at the interface of metabolic surgery and cardiovascular disease. Curr Atheroscler Rep. 2014;16:455. doi: 10.1007/s11883-014-0455-8. [DOI] [PubMed] [Google Scholar]
- 92.Hoefer IE, et al. Novel methodologies for biomarker discovery in atherosclerosis. Eur Heart J. 2015;36:2635–2642. doi: 10.1093/eurheartj/ehv236. [DOI] [PubMed] [Google Scholar]
- 93.Spickett CM, Pitt AR. Oxidative lipidomics coming of age: advances in analysis of oxidized phospholipids in physiology and pathology. Antioxid Redox Signal. 2015;22:1646–1666. doi: 10.1089/ars.2014.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Isobe Y, Arita M. Identification of novel omega-3 fatty acid-derived bioactive metabolites based on a targeted lipidomics approach. J Clin Biochem Nutr. 2014;55:79–84. doi: 10.3164/jcbn.14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kasuga K, et al. Bioanalytical insights into mediator lipidomics. J Pharm Biomed Anal. 2015;113:151–162. doi: 10.1016/j.jpba.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 96.Li M, et al. A not-stop-flow online normal-/reversed-phase two-dimensional liquid chromatography-quadrupole time-of-flight mass spectrometry method for comprehensive lipid profiling of human plasma from atherosclerosis patients. J Chromatogr A. 2014;1372:110–119. doi: 10.1016/j.chroma.2014.10.094. [DOI] [PubMed] [Google Scholar]
- 97.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Astarita G, et al. Targeted lipidomic strategies for oxygenated metabolites of polyunsaturated fatty acids. Biochim Biophys Acta. 2015;1851:456–468. doi: 10.1016/j.bbalip.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wong G, et al. Inclusion of plasma lipid species improves classification of individuals at risk of type 2 diabetes. PLoS One. 2013;8:e76577. doi: 10.1371/journal.pone.0076577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weir JM, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res. 2013;54:2898–2908. doi: 10.1194/jlr.P035808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meikle PJ, et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS One. 2013;8:e74341. doi: 10.1371/journal.pone.0074341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shui G, et al. Polar lipid derangements in type 2 diabetes mellitus: potential pathological relevance of fatty acyl heterogeneity in sphingolipids. Mretabolomics. 2013;9:786–799. [Google Scholar]
- 103.Reinehr T, et al. Changes in the serum metabolite profile in obese children with weight loss. Eur J Nutr. 2015;54:173–181. doi: 10.1007/s00394-014-0698-8. [DOI] [PubMed] [Google Scholar]
- 104.Floegel A, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62:639–648. doi: 10.2337/db12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Han X, et al. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: A shotgun lipidomics study. Biochemistry. 2007;46:6417–6428. doi: 10.1021/bi7004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Forouhi NG, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2:810–818. doi: 10.1016/S2213-8587(14)70146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stahlman M, et al. Clinical dyslipidaemia is associated with changes in the lipid composition and inflammatory properties of apolipoprotein-B-containing lipoproteins from women with type 2 diabetes. Diabetologia. 2012;55:1156–1166. doi: 10.1007/s00125-011-2444-6. [DOI] [PubMed] [Google Scholar]
- 108.Gooding JR, et al. Metabolomics applied to the pancreatic islet. Arch Biochem Biophys. 2016;589:120–130. doi: 10.1016/j.abb.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frohnert BI, Rewers MJ. Metabolomics in childhood diabetes. Pediatr Diabetes. 2016;17:3–14. doi: 10.1111/pedi.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wood PL, et al. Targeted Lipidomics of Fontal Cortex and Plasma Diacylglycerols (DAG) in Mild Cognitive Impairment and Alzheimer’s Disease: Validation of DAG Accumulation Early in the Pathophysiology of Alzheimer’s Disease. J Alzheimers Dis. 2015;48:537–546. doi: 10.3233/JAD-150336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wood PL, et al. Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer’s disease subjects. Acta Neuropsychiatr. 2015;27:270–278. doi: 10.1017/neu.2015.18. [DOI] [PubMed] [Google Scholar]
- 112.Proitsi P, et al. Plasma lipidomics analysis finds long chain cholesteryl esters to be associated with Alzheimer’s disease. Transl Psychiatry. 2015;5:e494. doi: 10.1038/tp.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vodicka P, et al. Mass spectrometry analysis of wild-type and knock-in Q140/Q140 huntington’s disease mouse bains reveals changes in glycerophospholipids including alterations in phosphatidic acid and lyso-phosphatidic acid. J Huntingtons Dis. 2015;4:187–201. doi: 10.3233/JHD-150149. [DOI] [PubMed] [Google Scholar]
- 114.Pruss H, et al. Proresolution lipid mediators in multiple sclerosis - differential, disease severity-dependent synthesis - a clinical pilot trial. PLoS One. 2013;8:e55859. doi: 10.1371/journal.pone.0055859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mazereeuw G, et al. Platelet activating factors in depression and coronary artery disease: a potential biomarker related to inflammatory mechanisms and neurodegeneration. Neurosci Biobehav Rev. 2013;37:1611–1621. doi: 10.1016/j.neubiorev.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 116.Wood PL. Mass spectrometry strategies for clinical metabolomics and lipidomics in psychiatry, neurology, and neuro-oncology. Neuropsychopharmacology. 2014;39:24–33. doi: 10.1038/npp.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Enriquez-Algeciras M, Bhattacharya SK. Lipidomic mass spectrometry and its application in neuroscience. World J Biol Chem. 2013;4:102–110. doi: 10.4331/wjbc.v4.i4.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen X, et al. Plasma lipidomics profiling identified lipid biomarkers in distinguishing early-stage breast cancer from benign lesions. Oncotarget. 2016 doi: 10.18632/oncotarget.19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang L, et al. Comprehensive lipid profiling of plasma in patients with benign breast tumor and breast cancer reveals novel biomarkers. Anal Bioanal Chem. 2015;407:5065–5077. doi: 10.1007/s00216-015-8484-x. [DOI] [PubMed] [Google Scholar]
- 120.Li J, et al. Integration of lipidomics and transcriptomics unravels aberrant lipid metabolism and defines cholesteryl oleate as potential biomarker of prostate cancer. Sci Rep. 2016;6:20984. doi: 10.1038/srep20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marien E, et al. Phospholipid profiling identifies acyl chain elongation as a ubiquitous trait and potential target for the treatment of lung squamous cell carcinoma. Oncotarget. 2016;7:12582–12597. doi: 10.18632/oncotarget.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y, et al. High resolution mass spectrometry coupled with multivariate data analysis revealing plasma lipidomic alteration in ovarian cancer in Asian women. Talanta. 2016;150:88–96. doi: 10.1016/j.talanta.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 123.Abbassi-Ghadi N, et al. A comparison of DESI-MS and LC-MS for the lipidomic profiling of human cancer tissue. J Am Soc Mass Spectrom. 2016;27:255–264. doi: 10.1007/s13361-015-1278-8. [DOI] [PubMed] [Google Scholar]
- 124.Cifkova E, et al. Lipidomic differentiation between human kidney tumors and surrounding normal tissues using HILIC-HPLC/ESI-MS and multivariate data analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1000:14–21. doi: 10.1016/j.jchromb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 125.Jiao J, et al. Targeted deletion and lipidomic analysis identify epithelial cell COX-2 as a major driver of chemically induced skin cancer. Mol Cancer Res. 2014;12:1677–1688. doi: 10.1158/1541-7786.MCR-14-0397-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li S, et al. Lipidomic analysis of epidermal lipids: a tool to predict progression of inflammatory skin disease in humans. Expert Rev Proteomics. 2016;13:451–456. doi: 10.1080/14789450.2016.1177462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Q, Wakelam MJ. Lipidomics in the analysis of malignancy. Adv Biol Regul. 2014;54:93–98. doi: 10.1016/j.jbior.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 128.Bhattacharya SK. Recent advances in shotgun lipidomics and their implication for vision research and ophthalmology. Curr Eye Res. 2013;38:417–427. doi: 10.3109/02713683.2012.760742. [DOI] [PubMed] [Google Scholar]
- 129.Lam SM, et al. Lipidomic analysis of human tear fluid reveals structure-specific lipid alterations in dry eye syndrome. J Lipid Res. 2014;55:299–306. doi: 10.1194/jlr.P041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Butovich IA. Tear film lipids. Exp Eye Res. 2013;117:4–27. doi: 10.1016/j.exer.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gordon WC, Bazan NG. Mediator lipidomics in ophthalmology: targets for modulation in inflammation, neuroprotection and nerve regeneration. Curr Eye Res. 2013;38:995–1005. doi: 10.3109/02713683.2013.827211. [DOI] [PubMed] [Google Scholar]
- 132.Heilbronn LK, et al. The effect of short-term overfeeding on serum lipids in healthy humans. Obesity (Silver Spring) 2013;21:E649–659. doi: 10.1002/oby.20508. [DOI] [PubMed] [Google Scholar]
- 133.Nestel PJ, et al. Specific plasma lipid classes and phospholipid fatty acids indicative of dairy food consumption associate with insulin sensitivity. Am J Clin Nutr. 2014;99:46–53. doi: 10.3945/ajcn.113.071712. [DOI] [PubMed] [Google Scholar]
- 134.Hyotylainen T, et al. Lipidomics in nutrition and food research. Mol Nutr Food Res. 2013;57:1306–1318. doi: 10.1002/mnfr.201200759. [DOI] [PubMed] [Google Scholar]
- 135.Lamaziere A, et al. Comparison of docosahexaenoic acid uptake in murine cardiomyocyte culture and tissue: significance to physiologically relevant studies. Prostaglandins Leukot Essent Fatty Acids. 2015;94:49–54. doi: 10.1016/j.plefa.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 136.Lamaziere A, et al. Lipidomics of hepatic lipogenesis inhibition by omega 3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88:149–154. doi: 10.1016/j.plefa.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 137.Li W, et al. Comparative metabolomics analysis on hematopoietic functions of herb pair Gui-Xiong by ultra-high-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry and pattern recognition approach. J Chromatogr A. 2014;1346:49–56. doi: 10.1016/j.chroma.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 138.Dehairs J, et al. Lipidomics in drug development. Drug Discov Today Technol. 2015;13:33–38. doi: 10.1016/j.ddtec.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 139.Janis MT, et al. Beyond LDL-C lowering: distinct molecular sphingolipids are good indicators of proprotein convertase subtilisin/kexin type 9 (PCSK9) deficiency. Atherosclerosis. 2013;228:380–385. doi: 10.1016/j.atherosclerosis.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 140.Yan SK, et al. “Omics” in pharmaceutical research: overview, applications, challenges, and future perspectives. Chin J Nat Med. 2015;13:3–21. doi: 10.1016/S1875-5364(15)60002-4. [DOI] [PubMed] [Google Scholar]
- 141.Wagener AH, et al. Toward composite molecular signatures in the phenotyping of asthma. Ann Am Thorac Soc. 2013;10(Suppl):S197–S205. doi: 10.1513/AnnalsATS.201302-035AW. [DOI] [PubMed] [Google Scholar]
- 142.Antunes-Martins A, et al. Systems biology approaches to finding novel pain mediators. Wiley Interdiscip Rev Syst Biol Med. 2013;5:11–35. doi: 10.1002/wsbm.1192. [DOI] [PubMed] [Google Scholar]
- 143.Ferguson JF, et al. Nutrigenomics, the microbiome, and gene-environment interactions: New directions in cardiovascular disease research, prevention, and treatment: A scientific statement from the American Heart Association. Circ Cardiovasc Genet. 2016;9:291–313. doi: 10.1161/HCG.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]