Abstract
Background
Mortality in patients with HIV infection is increasingly due to comorbid medical conditions. Research on how adherence to medications for comorbidities relates to antiretroviral (ARV) medication adherence and how interrelations between illness perceptions and medication beliefs about HIV and comorbidities affect medication adherence is needed to inform adherence interventions.
Methods
HIV-infected adults with hypertension (n=151) or chronic kidney disease (CKD; n=41) were recruited from ambulatory practices at an academic medical center. Illness perceptions and medication beliefs about HIV and hypertension or CKD were assessed and adherence to one ARV medication and one medication for either hypertension or CKD was electronically monitored for ten weeks.
Results
Rates of taking, dosing, and timing adherence to ARV medication did not differ from adherence to medication for hypertension or CKD, with the exception that patients were more adherent to the timing of their ARV (78%) than to the timing of their anti-hypertensive (68%;p=0.01]. Patients viewed HIV as better understood, more chronic, having more negative consequences, and eliciting more emotions, compared to hypertension. Patients viewed ARVs as more necessary than medication for hypertension or CKD. Having a realistic view of the efficacy of ARVs (r=−0.20;p<0.05) and a high level of perceived HIV understanding (r=0.21;p<0.05) correlated with better ARV adherence.
Conclusions
HIV patients showed similar rates of adherence to ARVs as to medications for comorbidities, despite perceiving HIV as more threatening and ARVs as more important. This can be used in adapting existing interventions for ARV adherence to encompass adherence to medications for comorbid conditions.
Keywords: medication adherence, antiretrovirals, illness perceptions, medication beliefs, hypertension, chronic kidney disease
INTRODUCTION
Due to the advent of highly effective antiretroviral (ARV) therapy, HIV has been transformed into a chronic and manageable illness. Consequently, morbidity and mortality among persons living with HIV are increasingly due to the many co-occurring medical conditions and non-infectious complications.1 However, most prior studies of adherence in persons with HIV have focused on ARV adherence and have not investigated adherence to treatment for comorbid chronic medical conditions.
Hypertension (HTN) is among the three most prevalent medical comorbidities in HIV-infected individuals, with prevalence rates of 20-43%.2-8 Hypertension increases the risk for cardiovascular, cerebrovascular, and kidney disease. In a sample of over 33,000 HIV-infected veterans, HTN was the only medical comorbidity associated with higher CD4 lymphocyte count (CD4), leading the authors to hypothesize that long term ARV therapy may promote HTN.3 This effect of ARVs on blood pressure may be mediated through an increase in BMI.9
Chronic kidney disease (CKD) is another important comorbidity, which may be secondary to HIV infection, HIV treatment, or traditional CKD risk factors including HTN.10 The estimated prevalence of CKD among HIV-infected persons varies substantially among studies, depending on region, population, study design, and the definition of CKD used.10 In the HIV clinical setting in which the current study was conducted, the prevalence of CKD was previously reported to be between 11-15%.5,11 CKD is associated with poorer health outcomes in those with HIV infection, including increased rates of heart failure, cardiovascular disease, end-stage renal disease, and mortality.12,13
HIV-infected patients have been shown to have suboptimal levels of awareness of their diagnosed comorbid medical conditions including hypertension and CKD.5,6 Hypertension and CKD are commonly treated with medications, and patient adherence to the prescribed medication strongly predicts disease control in both conditions.14,15
Turner and colleagues found that primary care providers were less likely to intensify treatment for uncontrolled hypertension in patients with a greater number of comorbid medical conditions,16 demonstrating that increasing medical comorbidities are not only a challenge for patient but also for medical providers. Medication adherence research across multiple conditions has established that medication adherence is a rational behavior based on patient beliefs about their illness and prognosis.17 Consequently, patients taking medications for comorbid chronic illnesses may be differentially adherent to medications because they hold discordant beliefs about medications and the illnesses they treat. In contrast to this, in a sample of 361 urban HIV-infected patients, Monroe and colleagues found that poor HIV control correlated with poor control of hypertension and diabetes, possibly reflecting poor adherence across medication classes.18
To date, four studies have reported quantitative data on patient adherence to ARVs and concomitant medications for the treatment of chronic comorbid conditions. Wagner and colleagues19 reported that in a cohort of HIV-infected individuals suffering from mental illness, self-reported adherence to psychiatric medication was moderately correlated with self-reported and electronically monitored ARV adherence. Fumaz and colleagues 20 reported differences in the levels of self-reported adherence to ARVs and medication for Hepatitis C virus (HCV) co-infection during a 48 week course of HCV treatment, with higher adherence to HCV medications. Batchelder, Gonzalez and Berg21 examined self-reported medication adherence and illness beliefs in patients with comorbid HIV and type 2 diabetes using the same theoretical framework as that used in the current study. They found that adherence was related to patient beliefs, and that adherence was higher to ARVs than to diabetes medications. Langness and colleagues used pharmacy refill data to examine adherence rates for ARV and either anti-hypertensive or psychiatric medication in a large sample of 865 persons with HIV infection.22 They found that adherence to ARVs was better than adherence to both anti-hypertensive and psychiatric medication.
Given the paucity of research in this area, we examined the relationships of patient illness perceptions and medication beliefs with electronically monitored adherence to ARVs and medications for comorbid HTN or CKD.
METHODS
Participants
HIV-infected adults with HTN and/or CKD were recruited from ambulatory practices affiliated with a single academic medical center in New York City. All study and consent procedures were reviewed and approved by the Institutional Review board at Icahn School of Medicine at Mount Sinai, and all study participants provided written informed consent. Subjects were evaluated at study baseline, at which time they were given the electronic monitoring devices. Subjects were seen for one follow-up interview ten weeks later.
Two cohorts of participants were enrolled consecutively. All participants were age 21 years or older and were currently prescribed ARV therapy and at least one medication for the management of HTN or CKD, with no anticipated changes in therapy within the three months after enrollment. Because of the adherence burden associated with short-term HCV therapy, patients currently receiving or expected to begin treatment for HCV co-infection within the following three months were excluded. For similar reasons, patients currently on dialysis were excluded from the HIV and HTN cohort. Because the standardized study instruments were administered by two separate teams of trained research staff, study eligibility was restricted to English-speaking (both cohorts) or Spanish-speaking individuals (HIV and HTN cohort only). Participants with concerns about electronic monitoring of adherence were excluded from the HIV and HTN study.
HIV and HTN Cohort
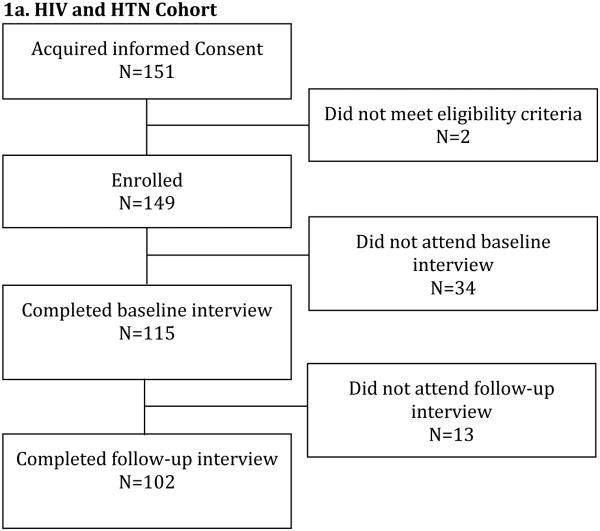
Participants with HIV and HTN were recruited from three clinical practices providing HIV primary care to >2500 patients (see Figure 1a). Data were collected between February 2011 and November 2012. HTN was defined as a documented diagnosis of HTN in the electronic medical record. Eligible participants were currently prescribed at least one antihypertensive medication.
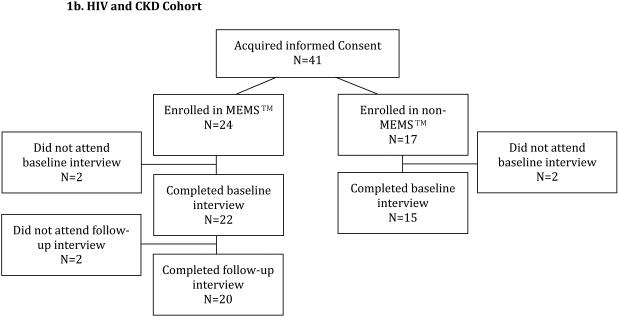
Figure 1.
Flowchart for Study Participation
HIV and CKD Cohort
Participants were recruited from three clinical practices, including one of the HIV primary care practices described above, a nephrology clinic, and an outpatient hemodialysis unit (see Figure 1b). Data were collected between September 2013 and August 2014. CKD was defined as estimated glomerular filtration rate [eGFR] < 60mL/min/1.73m2 (as calculated by the Modification of Diet in Renal Disease equation) or the presence of albuminuria or proteinuria (defined as urine albumin-to-creatinine ration 30 mg/g or greater or urine protein-to-creatinine ratio 200 mg/g or greater), persisting for >3 months, or end-stage renal disease (ESRD) on dialysis. Eligible participants were currently prescribed medications to delay CKD progression or manage CKD complications. Patients with concerns about electronic monitoring of adherence were eligible to participate in the baseline interview. This change in eligibility criteria reflects lessons learned during the HIV and HTN study and was aimed at maximizing.
Measures
Illness Perceptions and Medication Beliefs
The guiding theoretical framework for this study is Leventhal’s Common Sense Model, which posits that patients’ management of chronic illness is directly related to how they perceive aspects of the illness, both from a cognitive and emotional perspective.23-25 Horne has expanded upon this disease management model to include the patient’s view of medications used to treat the illness.25
At baseline, participants in each cohort completed the Illness Perception Questionnaire-Revised (IPQ-R) and the Beliefs about Medicines Questionnaire (BMQ) for HIV and either HTN or CKD. Both tools have been validated in multiple chronic illness populations and have shown good validity and reliability.25,26 The IPQ-R measures illness perceptions along discrete domains: (1) “Control/cure” involves the perception that either treatment or one’s own behavior can influence the course of the illness; (2) “Timeline” is the perceived course of the illness (chronic or cyclical); (3) “Consequences” reflect the perceived outcome of having the illness; (4) “Identity” refers to the labeling of an illness in terms of symptoms the patient believes are caused by this illness or by its treatment; (5) “Emotional Representations” are the patient’s affective responses to the illness; and (6) “Illness Coherence” is the degree to which the patient believes that he or she understands the illness. The BMQ was used to assess patients’ beliefs about medications along two domains 1) “Necessity” (patient beliefs about the need for medications) and 2) “Concerns” (patient concerns about the potential adverse effects of medications). Participants were asked these questions specifically about ARV medications and about medications used to treat HTN or CKD. To avoid bias, we alternated the order of question administration of the IPQ-R and BMQ for HIV and HTN or CKD.
Questions in the IPQ-R and BMQ are scored on a 5-point Likert scale with higher scores reflecting greater degree of agreement or stronger beliefs. Scores for each domain are calculated as the mean of the scores for questions relevant to that domain. Additionally, a necessity-concern differential is calculated from the BMQ results by subtracting the concerns score from the necessity score.
Medication Adherence
Medication adherence was measured using the Medication Event Monitoring System (MEMS™ AARDEX Group Ltd) caps over a ten-week period. A single medication in each category (ARV and HTN or CKD) was selected for electronic monitoring. In the HIV and HTN cohort, the highest dosing frequency possible was chosen so that the dosing frequency for both the ARV and HTN medication were the same. If not possible to match the ARV and HTN dosing frequency (for example if there was a twice daily ARV and only a once daily HTN medication), then the highest frequency for each one was used. When there were multiple ARV or HTN medications at a given dosing frequency, the medication to be monitored was chosen by a random number generator. In contrast, in the HIV and CKD cohort, the CKD medication to be monitored was chosen according to the following prioritization 1) Medications targeting underlying causes of CKD; 2) Medications targeting complications of CKD; 3) Angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB); 4) Anti-diabetic medications; 5) Other antihypertensive medications. An ARV medication that had the same dosing frequency as the chosen CKD medication was then selected. The same procedures as described above were used to handle multiple medication choices and to match dosing frequencies.
MEMS™ caps employ a pressure-activated microprocessor in the cap of the medication bottle that records the date, time, and duration of the bottle’s opening. Although there is no 'gold-standard' for assessing adherence, MEMS™ caps have emerged as the most valid and sensitive measure and have been cross-validated in multiple studies comparing self-report, assay pill count, and collateral report.27,28 Metrics for summarizing MEMS™ adherence data include “Taking Adherence” [(number of device activations during a certain period divided by number of tablets prescribed during the same period) × 100], interpreted as the percentage of pills taken as prescribed over a specified time period; “Dosing Adherence” [(number of days with the correct number of device activations based on the prescribed regimen divided by the number of days during the same period) × 100], which reflects the percentage of days on which doses are taken correctly; and “Timing Adherence” [(number of device activations at 24±6 h for once-daily, 12±3 h for twice-daily, 8±2 for thrice-daily, or 6±1.5 h for four-times-daily medication regimens during a certain period divided by the number of days during the same period) × 100], reflecting the percentage of doses taken at the correct inter-dose intervals. “Therapeutic Coverage” was defined as the percentage of time the patient has drug coverage based on the dose events recorded and the time window set based on dosing frequency for drug duration of action. PowerView software (Version 3.5.2; AARDEX, Inc.) was used to retrieve data from MEMS™ caps and to calculate these metrics. The MEMS™ adherence data were censored in line with recommended guidelines.29
While virologic suppression is possible with less than 95% adherence to ARV therapy, adherence below that level has been associated with increased viral replication, development of drug-resistance, and adverse medical outcomes.30,31 Thus, consistent with prior research, a minimum of 95% adherence remains the clinical goal for HIV therapy.32 Taking medication correctly at least 80% of the time has been the most commonly used definition of medication adherence in the general chronic disease management adherence literature.33 Thus, participants were categorized as adherent to HIV and HTN or CKD medications based on the 95% and 80% taking adherence thresholds, respectively.
Illness Control
The relationship between electronically monitored adherence and illness control was examined in the HIV and HTN cohort. HIV virologic control was defined as having two consecutive measurements of HIV viral load <48 copies/ml. Blood pressure control was defined as having blood pressure at the baseline interview of <140 mmHg systolic and <90 mmHg diastolic (based on the average of two measurements).
Statistical Analysis
Demographic data are presented as proportions and means [standard deviations (SD)], while data on illness perceptions and medications beliefs are presented as medians [interquartile ranges (IQR)]. Taking adherence was analyzed as both a dichotomous and a continuous variable. Categorical variables (sex, race, virologic control, blood pressure control, GFR category) were examined using chi-squared tests and continuous variables (age, CD4, eGFR) were examined using t-tests and ANOVA to compare subgroups within each study cohort. The associations between adherence for HIV and HTN or CKD; between illness perceptions and medications beliefs for each condition; and between adherence measures and illness perceptions and medications beliefs for each condition, were measured by Spearman’s correlation coefficient. The differences between these variables were tested with the Wilcoxon signed rank sum. The associations between taking adherence and illness control were tested with the Mann-Whitney test. All data analyses were conducted using SPSS version 21. A p-value <0.05 was considered statistically significant.
RESULTS
Baseline Demographic Characteristics
Participant enrollment in the two cohorts is presented in Figures 1a and 1b. Demographic and clinical characteristics for cohort participants who completed the baseline interview are presented in Table 1. Participants in both cohorts had mean age of 54 (9) years, were mostly male (57% and 59%), and were predominantly black or Hispanic (90% and 92%). In the HIV and HTN cohort, 68% of participants had virologic control and 63% had blood pressure control at baseline; whereas in the HIV and CKD cohort, 88% of participants had HIV virologic control but only 46% had blood pressure control at baseline. Participants in both cohorts had mean CD4 close to 600 cells/mm3.
Table 1.
Baseline Demographic and Clinical Characteristics
| HIV and HTN Cohort (n=115) | HIV and CKD Cohort (n=37) | |
|---|---|---|
| Age – mean years (SD) | 53.5 (8.5) | 54.4 (9.2) |
| Sex - male | 68 (59.1%) | 21 (56.8%) |
| Race – black / not Hispanic |
66 (57.4%) | 25 (67.6%) |
| Hispanic | 37 (32.2%) | 9 (24.3%) |
| white | 12 (10.4%) | 1 (2.7%) |
| Asian | 0 | 1 (2.7%) |
| Other | 0 | 1 (2.7%) |
| HIV viral load undetectable (<48 copies/ml) |
78 (67.8%) | 28 (87.5%)* |
| CD4+ cells/mm3 - mean (SD) | 602.3 (277.8) | 588.6 (263.4) |
| Well-controlled blood pressure | 72 (62.6%) | 17 (45.9%) |
| eGFR | ||
| ≥ 60 mL/min/1.73m2 | 83 (72.2%) | 3 (8.1%) |
| 30-59 mL/min/1.73m2 | 28 (24.3%) | 21 (56.8%) |
| 15-29 mL/min/1.73m2 | 4 (3.5%) | 4 (10.8%) |
| <15 mL/min/1.73m2 or dialysis | Not eligible | 9 (24.3%) |
HTN = hypertension
CKD = chronic kidney disease
GFR= glomerular filtration rate
Data missing on 5 subjects with end-stage renal disease
Participants who completed both the baseline and follow-up study visits in the HIV and HTN cohort (n=102) were significantly more likely to have HIV virologic control at baseline as compared to participants who did not complete both study visits (n=47; 93.1% vs. 78.7%, p=0.023; see Supplemental Table 1), but the groups did not differ on other baseline demographic or clinical characteristics. In the HIV and CKD cohort, there were no significant differences in baseline characteristics among participants who completed both study visits in the MEMS™ group (n=20), participants who were enrolled in the non-MEMS™ group and who completed the baseline interview (n=15), and participants who did not adhere fully to the study protocol (n=6; see Supplemental Table 2).
Illness Perceptions and Medications Beliefs: HIV and HTN Cohort
Participants in the HIV and HTN cohort attributed greater chronicity to their HIV illness (z=−4.7; p<0.01) and considered HTN to have a more cyclical nature (z=−3.6; p<0.01). HIV evoked stronger emotional responses (z=−5.1; p<0.01), was perceived to be better understood (z=−4.1; p<0.01), and was viewed as having greater negative consequences than HTN (z=−6.3; p<0.01). Participants attributed more symptoms to their HIV illness and its medications than to HTN (z=−5.2; p<0.01) and its medications (z=−5.7; p<0.01); they were more concerned about the potential adverse effects of HIV medications than of HTN medications (z=−4.1; p<0.01), but also expressed a stronger belief in the necessity of HIV medications than of HTN medications (z=−7.2; p<0.01). There were no differences in participants’ perceptions of having personal control over HIV and HTN, or of the illnesses being controllable by treatment (Table 2).
Table 2.
Illness Perceptions and Medication Beliefs
| HIV and HTN Cohort (n=115) | HIV and CKD Cohort (n=37) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scale | HIV M (IQR) |
HTN M (IQR) |
Correlation Coefficient |
p- value |
Z score |
p- value |
HIV M (IQR) |
CKD M (IQR) |
Correlation Coefficient |
p- value |
Z score |
p- value |
| Treatment Control |
3.8 (0.5) |
4.0 (0.5) |
0.26 | 0.01 | −2.6 | 0.10 | 3.5 (0.8) |
3.5 (0.8) |
0.21 | 0.21 | −0.63 | 0.53 |
| Personal Control | 4.0 (0.7) |
4.0 (1.0) |
0.53 | 0.000 | −0.06 | 0.94 | 4.0 (0.7) |
3.8 (0.7) |
0.26 | 0.12 | −1.90 | 0.057 |
| Timeline – Chronic |
3.3 (1.3) |
2.7 (1.3) |
0.36 | 0.000 | −4.7 | <0.01 | 3.2 (1.3) |
2.7 (1.3) |
0.40 | 0.015 | −0.44 | 0.66 |
| Timeline – Cyclical |
3.0 (1.5) |
3.5 (1.5) |
0.35 | 0.000 | −3.6 | <0.01 | 3.0 (1.4) |
3.0 (1.4) |
0.41 | 0.013 | −0.08 | 0.94 |
| Consequences | 3.3 (1.0) |
2.8 (0.8) |
0.31 | 0.001 | −6.3 | <0.01 | 3.3 (0.9) |
3.0 (0.9) |
0.39 | 0.019 | −1.62 | 0.11 |
| Illness Identity | 2.0 (6.0) |
0.0 (2.0) |
0.50 | 0.000 | −5.2 | <0.01 | 0.5 (3.0) |
0.5 (3.0) |
0.59 | 0.000 | −1.10 | 0.27 |
| Medication Identity |
1.0 (4.0) |
0.0 (0.0) |
0.42 | 0.000 | −5.7 | <0.01 | 1.0 (3.8) |
0.0 (0.0) |
0.63 | 0.000 | −3.00 | <0.01 |
| Emotional Representations |
2.8 (1.4) |
2.0 (0.8) |
0.37 | 0.000 | −5.1 | <0.01 | 2.4 (1.6) |
2.5 (1.0) |
0.44 | 0.008 | −1.03 | 0.30 |
| Illness Coherence | 4.0 (0.5) |
4.0 (1.0) |
0.47 | 0.000 | −4.1 | <0.01 | 4.0 (1.3) |
3.8 (1.5) |
0.19 | 0.27 | −1.63 | 0.10 |
| Medication Necessity |
4.0 (0.8) |
3.6 (0.9) |
0.46 | 0.000 | −7.2 | <0.01 | 4.0 (0.8) |
3.9 (0.6) |
0.30 | 0.07 | −2.62 | <0.01 |
| Medication Concerns |
2.4 (0.7) |
2.2 (0.5) |
0.63 | 0.000 | −4.1 | <0.01 | 2.6 (0.7) |
2.4 (0.6) |
0.23 | 0.17 | −1.82 | 0.069 |
| Necessity- Concerns Differential |
1.7 (1.0) |
1.3 (1.0) |
0.57 | 0.000 | −4.5 | <0.01 | 1.4 (1.1) |
1.3 (0.7) |
0.35 | 0.038 | −0.66 | 0.51 |
IQR = Interquartile Range; HTN = hypertension; CKD = chronic kidney disease
Illness Perceptions and Medications Beliefs: HIV and CKD Cohort
Participants attributed more symptoms to their HIV medications than to their CKD medications (z=−3.0; p<0.01) and expressed stronger belief in the necessity of medications for HIV than for CKD (z=−2.6; p<0.01). Illness perceptions and medication beliefs did not differ significantly for other domains in the HIV and CKD cohort (Table 2).
Electronically Monitored Adherence
Of the 102 participants in the HIV and HTN cohort with electronic monitoring data, 92 were monitored for a once daily ARV medication and 10 for a twice daily ARV medication; 96 were monitored for once daily anti-hypertensive medication and 6 for twice daily anti-hypertensive medication. Based on the established thresholds for taking adherence of 95% and 80% for HIV and HTN medications, respectively, 61% of HIV and HTN cohort participants were adherent to HIV medications and 80% were adherent to HTN medications (p=0.22); for comparison, 82% of participants met the 80% threshold for HIV medications and 57% met the 95% threshold for HTN medications. While this difference was not statistically significant, the 19% difference in meeting the adherence cutoff for HTN medications as compared to HIV medications may be considered by some to be clinically significant.
Among the 20 participants in the HIV and CKD cohort who completed the MEMS™ portion of the study, eighteen had once daily and two had twice daily ARV medication monitored. Eighteen participants had once daily CKD medication monitored, one twice daily, and one three times daily. In 17 of the 20 participants, the CKD medication selected for electronic monitoring was an ACE inhibitor, angiotensin receptor blocker, or other antihypertensive agent. Based on conventional thresholds used in the literature, taking adherence of ARV and CKD medication did not differ significantly, with 65% and 80% of participants adherent to HIV (95% threshold) and CKD (80% threshold) medications, respectively (p=0.23). For comparison, 75% of participants reported at least 80% adherence to HIV medications and 60% reported 95% adherence to CKD medications.
Participants in the HIV and HTN cohort had greater timing adherence for HIV than HTN medications (78.3% vs. 68.1%, p=0.01; Table 3). There were no statistically significant differences for dosing adherence or therapeutic coverage in either cohort, and no difference in timing adherence in the HIV and CKD cohort (Table 3). All 4 adherence metrics were strongly correlated between ARVs and medications for HTN or CKD (all correlation coefficients >0.8, all p<0.01; Table 3).
Table 3.
Electronically Monitored Adherence Data
| HIV and HTN Cohort (n=102) | HIV and CKD Cohort (n=20) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adherence Measure, Median (IQR) |
HIV med |
HTN med |
Correlation Coefficient |
p-value | Z score | p-value | HIV med | CKD med | Correlation Coefficient |
p-value | Z score | p- value |
| Taking Adherence |
97.1 (11.8) |
97.1 (15.7) |
0.80 | <0.01 | −1.2 | 0.22 | 97.9 (17.3) | 96.4 (8.6) | 0.90 | < 0.01 | −1.2 | 0.23 |
| Dosing Adherence |
92.9 (19.0) |
92.9 (20.0) |
0.81 | <0.01 | −1.5 | 0.15 | 92.9 (32.2) | 92.9 (26.1) |
0.88 | < 0.01 | −0.1 | 0.94 |
| Timing Adherence |
78.3 (40.7) |
68.1 (40.7) |
0.84 | < 0.01 | −2.6 | 0.01 | 87.7 (33.3) | 86.3 (50.9) |
0.83 | < 0.01 | −0.1 | 0.91 |
| Therapeutic Coverage |
97.2 (9.7) |
95.8 (11.9) |
0.82 | < 0.01 | −1.3 | 0.18 | 98.0 (9.0) | 97.4 (6.0) | 0.85 | < 0.01 | −1.0 | 0.30 |
IQR = Interquartile Range; HTN = hypertension; CKD = chronic kidney disease
Relationship between Electronically Monitored Adherence and Illness Perceptions/Medications Beliefs
In the HIV and HTN cohort, perceiving HIV as less controllable by treatment and having a better perceived understanding of HIV were correlated with better taking adherence to HIV medications (r=−0.198, p=0.046 and r=0.207, p=0.036, respectively). No significant correlations were found between the other illness perception domains or participants’ beliefs about HIV medications and their adherence to HIV medications, or between participants’ perceptions of HTN and HTN medications and adherence to HTN medications (all p values >0.05). In the HIV and CKD cohort, there was no apparent relationship between participants’ illness perceptions/ medication beliefs and adherence to medications for HIV or CKD (all p values >0.05). The high levels of adherence in these samples may have limited the identification of relationships between illness perceptions/medication beliefs and adherence that may be identified in sample with more variability in adherence levels.
Relationship between HIV and HTN illness control at follow-up
The relationship between HIV and HTN illness control was evaluated at ten-week follow-up. Virologic control was achieved by 88% (90/102) of participants and blood pressure was controlled in 60% (61/102) of participants. Blood pressure control and HIV control at follow-up were not correlated [χ2(1,N=104)=0.597,p=0.76].
Discussion
Using electronic monitoring of medication adherence for a ten week period, we did not find significant differences in participants’ adherence to their HIV medications and to their medications for a comorbid condition, with the exception that ARV timing adherence was significantly higher than antihypertensive timing adherence. Participants’ adherence to their HIV medication was highly correlated to their adherence to their medication for comorbid HTN or CKD for all adherence metrics assessed.
Overall, participants in this study demonstrated very high levels of adherence, with median dosing adherence levels to HIV, HTN and CKD medications all being above 90%. Participants in the HIV and HTN cohort who did not complete study visits were more likely to have detectable HIV viral load at baseline (greater than 48 copies/ml), suggesting that the requirements of study participation may have biased the sample with medication adherence data towards a more adherent patient population (see Supplemental Table 1). As a result of this bias in the sample who completed the study, the longitudinal findings of this study may not be generalizable to those who have poor adherence.
The results of the four other studies that have examined ARV adherence and adherence to another comorbid condition,19-22 have been inconsistent, with two studies finding higher rates of adherence to ARVs than to medications for a comorbid condition,21,22 one finding similar rates,19 and one finding lower rates of adherence to ARVs.20 The current study reports on a sample with higher ARV adherence rates than these prior studies, and the findings can be interpreted to indicate that in a sample of highly adherent HIV-positive patients, the adherence behavior mastered for HIV may be transferred to self-management of other comorbid conditions, while the impact of illness perceptions and medication beliefs may be less relevant to determining adherence in this population.
The finding that perceiving HIV as less controllable by treatment is correlated with better ARV adherence appears counterintuitive. One of the four questions making up the IPQ-R treatment control scale is “My treatment will be effective in curing my HIV infection.” Those who strongly endorsed this item and had the highest levels of perceived HIV treatment control may have an overly optimistic, unrealistic view of the power of HIV medications to control the illness, and less appreciation of the importance of their own behavior. This suggests that HIV adherence education should continue to emphasize the ability of HIV medications to successfully suppress the HIV virus (as oppose to cure HIV) and the essential role for the patient in optimally adhering to medication. Our findings also suggest that patients have successfully integrated the importance of timing adherence specifically in relation to their HIV medications.
Participants hold very different views of HIV than of HTN and similarly of ARVs than of anti-hypertensives; we observed significant differences in all domains of illness perceptions and medication beliefs measured, with the exception of treatment control and personal control. While these findings were not as clearly demonstrated in the HIV and CKD sample, this may have been due to the relatively small sample size in this cohort and the fact that the cohort included a broad spectrum of CKD severity. Larger studies are needed to evaluate differential illness perceptions and medication adherence in patients with more advanced CKD.
Conclusions
Whereas HIV illness control is very tightly related to ARV adherence, illness control in hypertension and in CKD is more likely to be influenced by additional behavioral (e.g., diet and physical activity) and medical factors, making it challenging for providers to know how much to attribute to poor medication adherence when faced with poor control of HTN or CKD in their HIV patients. The similar rates of adherence to ARVs and adherence to comorbid conditions found in this study suggest that the determinants of adherence to each class of medications do not differ widely within patients. It appears that the strategies HIV patients develop to adhere to their ARVs are in turn applied to medications for their comorbid medical conditions. It seems likely that HIV providers can use the patient’s ARV adherence as a surrogate marker of adherence to their HTN or CKD medications in patients with high levels of adherence.
This is the largest study to date examining medication adherence to ARVs and to medications for a comorbid medical condition in patients with HIV infection using electronic adherence monitoring. Future research in this area may be most informative by focusing on the patients who are nonadherent to their ARVs and/or to medications for their comorbid medical conditions. This is an inherently challenging population to retain in research studies and innovative strategies will be needed in order to successfully do so.35
Supplementary Material
Acknowledgements
We thank the study participants for their time and willingness to share their views of living with HIV and other comorbid medical conditions and for permitting us to monitor their medication adherence. We thank our colleagues who referred participants to the study. We gratefully acknowledge funding for this research from the National Institutes of Health, National Institute of Nursing Research (NINR), NR012648 and the American Society of Nephrology Student Scholar Grant Program. The opinions expressed in this article are the authors’ own and do not necessarily reflect the view of the sponsors.
Funding Sources: Grant #R21NR012648 from the National Institute of Nursing Research (NINR) and American Society of Nephrology Student Scholar Grant Program
Footnotes
Parts of this data were presented at the 8th International Conference on HIV Treatment and Prevention Adherence, Miami, Florida, 2013 and ASN Kidney Week Annual Meeting, Philadelphia, Pennsylvania, 2014.
Disclosures: Jeffrey Weiss is a consultant for AbbVie, Inc. For the remaining authors none were declared.
References
- 1.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D: A: D): a multicohort collaboration. The Lancet. 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 2.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clinical Infectious Diseases. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 3.Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clinical Infectious Diseases. 2007;45:1593–601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilbourne AM, Justice AC, et al. General medical and psychiatric comorbidity among HIV-infected veterans in the post-HAART era. Journal of Clinical Epidemiology. 2001;54:S22–S28. doi: 10.1016/s0895-4356(01)00443-7. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JJ, Osorio G, Ryan E, et al. Prevalence and patient awareness of medical comorbidities in an urban AIDS clinic. AIDS Patient Care and STDs. 2010;24:39–48. doi: 10.1089/apc.2009.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeSocio GV, Ricci E, Maggi P, et al. Prevalence, awareness, treatment and control rate of hypertension in HIV-infected patients: The HIV-HY study. Am J Hypertens. 2014;27:222–228. doi: 10.1093/ajh/hpt182. [DOI] [PubMed] [Google Scholar]
- 7.Myerson M, Poltavskiy E, Armstrong EJ, et al. Prevalence, treatment and control of dyslipidemia and hypertension in 4278 HIV outpatients. J Acquir Immune Defic Syndr. 2014;66:370–377. doi: 10.1097/QAI.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 8.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infection. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane HM, Rompaey VS, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. Aids. 2006;20:1019–1026. doi: 10.1097/01.aids.0000222074.45372.00. [DOI] [PubMed] [Google Scholar]
- 10.Mallipattu SK, Salem F, Wyatt CM. The changing epidemiology of HIV-related chronic kidney disease in the era of antiretroviral therapy. Kidney International. 2014;86:259–265. doi: 10.1038/ki.2014.44. [DOI] [PubMed] [Google Scholar]
- 11.Wyatt CM, Winston JA, Malvestutto CD, et al. Chronic kidney disease in HIV infection: an urban epidemic. AIDS. 2007;21:2101–2103. doi: 10.1097/QAD.0b013e3282ef1bb4. [DOI] [PubMed] [Google Scholar]
- 12.Choi AI, Li Y, Deeks SG, et al. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121:651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas GM, Cozzi-Lepri A, Wyatt CM, et al. HIV Med. 2014;15:116–123. doi: 10.1111/hiv.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdine S, Arslan E. Monitoring treatment adherence in hypertension. Curr Hypertens Rep. 2013;15:269–272. doi: 10.1007/s11906-013-0369-9. [DOI] [PubMed] [Google Scholar]
- 15.Burnier M, Priujm M, Wuerzner G, et al. Drug adherence in chronic kidney diseases and dialysis. Nephrol Dial Transplant. 2015;30:39–44. doi: 10.1093/ndt/gfu015. [DOI] [PubMed] [Google Scholar]
- 16.Turner BJ, Hollenbeak CS, Weiner M, et al. Effect of unrelated comorbid conditions on hypertension management. Annals of Internal Medicine. 2008;148:578–586. doi: 10.7326/0003-4819-148-8-200804150-00002. [DOI] [PubMed] [Google Scholar]
- 17.McHorney CA, Gadkari AS. Individual patients hold different beliefs to prescription medications to which they persist vs nonpersist and persist vs nonfulfill. Patient Preference and Adherence. 2010;4:187. doi: 10.2147/ppa.s10603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monroe AK, Chander G, Moore RD. Control of medical comorbidities in individuals with HIV. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;58:458. doi: 10.1097/QAI.0b013e31823801c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner G, Kanouse D, Koegel P, et al. Adherence to HIV Antiretrovirals among Persons with Serious Mental Illness. AIDS Patient Care and STDs. 2003;17:179–186. doi: 10.1089/108729103321619782. [DOI] [PubMed] [Google Scholar]
- 20.Fumaz CR, Muñoz-Moreno JA, Ballesteros AL, et al. Influence of the type of pegylated interferon on the onset of depressive and neuropsychiatric symptoms in HIV-HCV coinfected patients. AIDS Care. 2007;19:138–45. doi: 10.1080/09540120600645539. [DOI] [PubMed] [Google Scholar]
- 21.Batchelder AW, Gonzalez JS, Berg KM. Differential medication nonadherence and illness beliefs in co-morbid HIV and type 2 diabetes. Journal of Behavioral Medicine. 2014;37:266–275. doi: 10.1007/s10865-012-9486-1. [DOI] [PubMed] [Google Scholar]
- 22.Langness J, Gill J, et al. Comparison of adherence rates for antiretroviral, blood pressure, or mental health medicatons for HIV-positive patients at an academic medical center outpatient pharmacy. J Manag Care Pharm. 2014;20:809–814. doi: 10.18553/jmcp.2014.20.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diefenbach MA, Leventhal H. The common-sense model of illness representation: Theoretical and practical considerations. Journal of Social Distress and the Homeless. 1996;5:11–38. [Google Scholar]
- 24.Leventhal H, Nerenze DR, Steele DJ. Illness representation and coping with health threats. In: Baum A, Taylor SE, Singer JE, editors. Handbook Of Psychology And Health, Handbook Of Psychology And Health. Lawrence Erlbaum; Hillsdale, NJ: 1984. pp. 219–252. [Google Scholar]
- 25.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychology and Health. 1999;14:1–24. [Google Scholar]
- 26.Ashley L, Smith AB, Keding A, et al. Psychometric evaluation of the Revised Illness Perception Questionnaire (IPQ-R) in cancer patients: Confirmatory factor analysis and Rasch analysis. Journal of Psychosomatic Research. 2013;75:556–562. doi: 10.1016/j.jpsychores.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clinical Infectious Diseases. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 29.Fennie KP, Bova CA, Williams AB. Adjusting and censoring electronic monitoring device data: implications for study outcomes. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;43:S88–S95. doi: 10.1097/01.qai.0000248336.97814.2f. [DOI] [PubMed] [Google Scholar]
- 30.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 31.Shuter J, Sarlo JA, Kanmaz TJ, et al. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95% JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;45:4–8. doi: 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- 32.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Annals of Internal Medicine. 2012;156:817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen RA, Kim MM, Song L, et al. Comparison of methods to assess medication adherence and classify nonadherence. Annals of Pharmacotherapy. 2009;43:413–422. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 35.Zweben A, Fucito LM, O’Malley SS. Effective strategies for maintaining research participation in clinical trials. Drug Information Journal. 2009;43:459–467. doi: 10.1177/009286150904300411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.