Abstract
BACKGROUND
There are contradicting reports on the associations between Apolipoprotein E4 (ApoE ε4) and brain outcomes in HIV with some evidence that relationships may be greatest in older age groups.
METHODS
We assessed cognition in 76 clinically stable HIV-infected participants over age 60 and genotyped ApoE. Sixty-one of these subjects underwent structural brain MRI and diffusion tensor imaging (DTI).
RESULTS
The median age of the participants was 64 years (range: 60–84) and the median estimated duration of HIV infection was 22 years. Apo ε4 carriers (n=19) were similar to non-carriers (n=57) in sex (95% vs. 96% male), and education (16.0 vs. 16.2 years) ApoE ε4 carriers demonstrated greater deficits in cognitive performance in the executive domain (p=0.045) and had reduced fractional anisotropy (FA) and increased mean diffusivity (MD) throughout large white matter tracts within the brain compared to non-carriers. Tensor Based Morphometry (TBM) analyses revealed ventricular expansion and atrophy in the posterior corpus callosum, thalamus, and brainstem among HIV-infected ApoE ε4 carriers compared to ε4-non-carriers.
CONCLUSION
In this sample of older HIV-infected individuals, having at least one ApoE ε4 allele was associated with decreased cognitive performance in the executive functioning domain, reduced brain white matter integrity, and brain atrophy. Brain atrophy was most prominent in the posterior corpus callosum, thalamus and brainstem. This pattern of cognitive deficit, atrophy and damage to white matter integrity was similar to that described in HIV, suggesting an exacerbation of HIV-related pathology; although emergence of other age-associated neurodegenerative disorders cannot be excluded.
Keywords: Acquired Immune Deficiency Syndrome, cognition disorders, AIDS Dementia Complex, aging, ApoE
INTRODUCTION
The demographic shifts associated with aging among the HIV-infected population in the U.S. are well-established.1 Around 50% of HIV cases reported to the U.S. Centers for Disease Control and Prevention (CDC) are over the age of 50.2 In 2008, there were more than 50,000 HIV-infected adults in the U.S. over age 603. Analyses of past cohort studies demonstrate extension of life expectancy with combination antiretroviral therapy (cART)4.
Risk for HIV-associated neurocognitive disorders (HAND) increases with age and, despite access to cART, HAND occurs in up to one-half of adults living with HIV.5 Older HIV-infected patients who are already at risk for HAND also face elevated risk of age-related neurodegenerative disorders with the chances of developing AD increasing with age, doubling every five years after age 65.6,7 It remains unknown if HIV impacts the frequency or severity of age-associated neurodegenerative disorders.
ApoE ε4 is a substantial genetic risk factor for AD, particularly late-onset AD (i.e., after age 65).8 ApoE ε4 has been linked with poorer cognitive performance in both healthy non-demented individuals and among individuals with mild cognitive impairment (MCI).9,10 The presence of ApoE ε4 is associated with poorer outcomes in other neurological conditions as well, including head injuries and seizures.11,12 Imaging studies reveal an association between ApoE ε4 and both medial temporal lobe and hippocampal atrophy in HIV-uninfected populations as well as evidence for disrupted brain network activity.8,13
Studies of ApoE ε4 in HIV-infected populations suggest this genotype plays a role in HIV neuropathology; a more complete review of these studies was recently published by Panos et al.14 Before cART use became widespread, HIV-infected individuals with at least one ApoE ε4 allele were twice as likely to have dementia compared to those without.15 In a more recent cohort study, HIV-infected individuals who are homozygous for ApoE ε4 have accelerated disease progression compared to those with the E3/e3 or E3/E4 genotype, although reported rates of dementia are reported to be similar by ApoE ε4 carrier status.16 In a small study of young HIV-infected South African adults, researchers report poorer performance on memory tasks and decreased fractional anisotropy (FA) of the corpus callosum in ApoE ε4 carriers compared to non-carriers.17
In older age, one cross-sectional study notes risk for HIV-associated dementia (HAD) in relation to ApoE ε4 carrier status (age >50 years); and, this is not seen in co-enrolled younger participants (age <40 years).18 Similarly, several recent reports among younger samples fail to identify risk. One such report from the CHARTER group (mean age = 40 years, 25% over age 50 years) finds no difference in HAND prevalence between ApoE ε4 carriers and non-carriers nor did they identify neuroimaging differences by carrier status.19,20 Similarly, investigations from the Multicenter AIDS Cohort Study (MACS, with mean ages at enrollment across groups between 30 to 40 years) do not identify an impact of ApoE ε4 on development of cognitive impairment among participants who tested normal at baseline.21 One group carefully tested the hypothesis that age may influence the association between ApoE ε4 carrier status and cognitive outcomes using data from the National NeuroAIDS Tissue Consortium (NNTC) noting detrimental effects on HAND diagnosis, executive functioning and information processing in older age (> 50 years) but not in those of younger age.14
Here, we investigate the risk of ApoE ε4 carrier status in a group of older HIV-infected participants where concern for neurodegenerative disorders is higher due to age. Our cross-sectional analyses examine neuropsychological testing performance, cognitive diagnoses, brain morphometry, and diffusion tensor imaging (DTI) data in relation to ApoE ε4 carrier status.
METHODS
Participant selection
We used broad community-based recruitment techniques to identify HIV- infected participants from the San Francisco Bay area ≥60 years of age, excluding individuals reporting neurological conditions known to impact cognition (e.g. stroke, opportunistic brain infection, loss of consciousness for <30 minutes, current (6 months) use of methamphetamine or cocaine). Participants were required to be English-speaking and to identify a proxy informant who would inform regarding functional abilities (despite this requirement, proxy contacts were made successfully in only 62/76).
We screened 116 people and 97 chose to participate. Four were excluded during the entry visit (intoxicated at visit, MRI evidence of past major stroke (n=1) or broad white matter injury later learned to be present prior to HIV (n=1), withdrew consent). Of the remaining 93 cases, 87 completed sufficient baseline evaluations to be included and 76 of those were genotyped for ApoE ε4. All participants provided IRB-approved informed consent.
Clinical and neuropsychological characterization
All participants completed standard evaluations that included a structured neurological exam, medical history, and clinical proxy informant interview for functional status related to cognition. We accepted either a self- or proxy-report of functional limitations due to cognition.
All participants completed a 90-minute neuropsychological testing battery that assessed multiple cognitive domains including memory, executive function, psychomotor speed, visuospatial and motor abilities, and attention as previously described.22 Raw neuropsychological scores were transformed into z-scores using published age and education stratified normative data or internal normative data, when indicated (Supplemental Table 1 shows full battery by cognitive domain with normative sources). Summary neuropsychological scores (NPZ) for cognitive domains were defined by averaging individual z-scores within each of the domains and a global NPZ score was calculated by averaging z-scores from tests across all domains. Cases underwent multidisciplinary case conference review with at least one behavioral neurologist and a neuropsychologist to assign consensus diagnoses based on clinical acumen applying the 2007 “Frascati” criteria as a guide.23
We captured clinical HIV variables using a structured physician interview, which included a self-report of estimated duration since diagnosis of HIV and nadir HIV CD4 t-lymphocyte cell count. We measured CD4+ t-lymphocyte subsets and plasma HIV RNA levels at local laboratories, if not available through clinical care within 3 months of the evaluation. Participants completed the Geriatric Depression Scale questionnaire (GDS) to evaluate mood.
Genotyping
Genomic DNA was extracted from peripheral blood using standard protocols (Gentra PureGene Blood Kit, QIAGEN, Inc. USA, Valencia, CA). ApoE genotyping was obtained from SNPs rs429358 and rs7412, by real-time PCR on an Applied Biosystems 7900HT Real Time PCR machine (Applied Biosystems, Foster City, CA), using Taqman SNP Genotyping Assays (#C_3084793_20 and C_904973_10 for rs429358 and rs7412, respectively). Assays were run in triplicate. The SDS version 2.3 software was used to analyze the raw data and to call the genotypes.
Imaging acquisition and analysis
Willing participants who did not have contraindication (n=61/76, twelve were ApoE ε4 carriers) completed a 3 Tesla brain MRI on one Siemens 3T TIM Trio platform that included a whole-brain 3D-T1 MP-RAGE sequence (1.0×1.0×1.0 mm3 matrix, RT=2300ms, TE=2.98ms) and DTI (64 diffusion weighted image directions (b=2000 s/mm2), FOV=220mm, voxel size = 2.2 × 2.2 × 2.2 mm3; TR/TE = 8000/109 ms]. All 61 T1-weighted MR and DWI images passed visual quality checks to ensure lack of excessive motion and/or artifacts.
Quantification of brain volumes
We employed dual complementary approaches to quantify volumes and assess volumetric differences by groups. First, brain volumes were calculated using the conventional quantification techniques of FreeSurfer software, employing a workflow that involved human quality checks followed by reprocessing, as recommended.24 We also employed tensor-based morphometry (TBM) techniques. Briefly, a study-specific minimal deformation template (MDT) was created from the participants’ spatially aligned corrected T1-weighted anatomical volumes, as previously described.25,26 Each subject’s pre-processed T1-weighted scan was then registered to the MDT. This warp leads to an estimate of spatial deformations needed to map one brain to the MDT. The determinants of the Jacobian at each voxel then relates to the amount of volumetric change needed to fit the images together at that location. The two approaches are complementary with TBM unbiasly testing all regions with only those that meet strict statistical thresholds being reported whereas the Freesurfer approach tests a prior chosen functional units such as deep grey matter structures.
Diffusion tensor imaging (DTI)
We performed image processing for DTI that included calculation of scalar metrics (FA and mean, axial, and radial diffusivity [MD, AD, RD]) on a voxel-wide basis as previously described.27 Briefly, images were pre-processed using FSL tools (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) to correct for motion and eddy current distortions. The b0 image for each subject was then registered to the subject’s corresponding T1-weighted image to account for possible EPI-induced susceptibility artifacts, and the resulting transformations were applied to the full distortion corrected DWI set. FSL was used to calculate the tensors from which maps of fractional anisotropy (FA) and diffusion scalars were created. Another MDT was created from the FA images of the same subjects that were used for the T1-weighted template. All subject’s FA images were then registered to the template. All FA-based registrations were then also applied to the diffusivity maps to ensure the same voxelwise correspondence across subjects.
Voxelwise statistics were performed on DTI-derived measures focusing on white matter regions where the tensors are believed to be less noisy (defined as those voxels with FA >0.2 in the minimal deformation template (MDT). The statistical model remained the same, with the DTI measure being the outcome variable and ε4 carrier status being the predictor of interest, with age and sex as covariates. We corrected for multiple comparisons across all voxels using the false discovery rate procedure, controlling the rate of false discoveries at 0.05.
Statistical approach
We defined ApoE ε4 carriers as having at least one ApoE ε4 allele. We used chi-squared tests to analyze the distribution of ApoE alleles across diagnostic groups and general linear models for associations with NPZ scores controlling for CD4 count, CD4 nadir, and plasma HIV RNA level. Duration of infection was initially included despite a high p-value (p=0.360), but was later removed, as it did not add to the models. Sensitivity analyses followed, that excluded cases with detectable plasma HIV RNA. These statistical analyses were performed in Stata v 13.2.
Analyses of TBM involved a voxelwise linear regression with age and sex added to the model. We used a searchlight method to locally control the false discovery rate in the entire image space at 0.05.28 Targeted brain volumes (measured by FreeSurfer) were driven by the TBM analyses and a priori hypotheses (e.g., deep grey matter structures, hippocampus, corpus callosum, and total grey and white matter) and tested using logistic models predicting ApoE ε4 carrier status with intracranial volume included as a covariate. An overall p<0.05 was used for testing statistical significance with covariates meeting p<0.20 retained in all models (e.g., CD4 count, ICV, and plasma HIV RNA). Structural volume statistical analyses were performed in Stata v 13.2.
The statistical approach for DTI analyses mirrored that of TBM, including a voxelwise approach. We corrected for multiple comparisons across all voxels using the false discovery rate procedure and controlling the rate of false discoveries at 0.05.29 Areas of significance in DTI analyses led us to calculate scalar metrics for structures involved, using a structural overlay of regions of interest. These were used in separate statistical models to calculate effect sizes.
RESULTS
The median age of the sample was 64 years; 96% of participants were men. On average, the group reported about two decades duration since initial diagnosis of HIV infection. ApoE ε4 carriers and non-carriers were well matched on all major demographic and clinical parameters (Table 1).
Table 1.
Participant Demographic characteristics
| Apo ε4 Non-carrier |
Apo ε4 Carrier |
p-value | |
|---|---|---|---|
| Sample size | 57 | 19 | - |
| Age, years (median, range) | 64 (60–84) | 64 (60–80) | 0.931 |
| Gender (% male) | 96% | 95% | 1.000 |
| Education, mean years (SD) | 16.2 (2.28) | 16.0 (2.41) | 0.692 |
| Ethnicity, (% Caucasian) | 93% | 88% | 0.624 |
| Risk for HIV, (% MSM1) | 82% | 95% | 0.271 |
| CD4 count, mean (SD) | 516 (206) | 595 (275) | 0.435 |
| Nadir CD4, mean (SD) | 200 (137) | 222 (198) | 0.940 |
| HIV duration, mean years (SD) | 20.2 (6.41) | 19.6 (5.85) | 0.433 |
| Plasma HIV RNA (log10, mean (SD) | 2.1 (0.66) | 2.0 (0.68) | 0.252 |
| Undetectable plasma HIV RNA (%) | 88% | 89% | 0.654 |
| On cART (%) | 95% | 89% | 0.599 |
| APOE Genotype (n, %) | |||
| e2/e3 | 13 (17%) | - | - |
| e3/e3 | 44 (58%) | - | - |
| e2/e4 | - | 1 (1%) | - |
| e3/e4 | - | 16 (21%) | - |
| e4/e4 | - | 2 (3%) | - |
| Geriatric Depression Scale, mean (SD) | 7.77 (6.23) | 7.18 (4.68) | 0.891 |
| HAND2 diagnosis (n, %) | |||
| Normal (NL) | 27 (47%) | 10 (53%) | |
| Asymptomatic impairment (ANI)3 | 13 (23%) | 2 (11%) | |
| Symptomatic impairment (SNI)4 | 17 (30%) | 7 (37%) | |
Risk factor for acquiring HIV self-reported as only having sex with men among male enrollees (men who have sex with men, MSM);
HAND = HIV-associated Neurocognitive Disorder;
Asymptomatic Neurocognitive Impairment (ANI);
Includes participants with Mild Neurocognitive Disorder (MND, n=23) and HIV-associated Dementia (HAD, n=1)
Associations between ApoE ε4 carrier status and cognitive measures
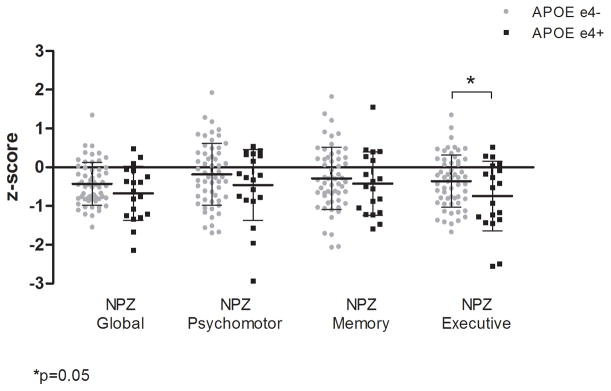
About one-half of ApoE ε4carriers and non-carriers met HAND criteria and there was no significant difference in distribution of diagnoses by group (p=0.449). However, a pattern of worse performance on neuropsychological testing associated with ApoE ε4 carrier status was found in analyses of domain-specific summary scores (Figure 1). Differences in mean domain scores met significance only in the NPZ-executive summary score composed of a modified trail making test, Trails B, Stroop Interference, Lexical Fluency (D words), and digits backwards [mean (SD) of −0.741 (0.898) and −0.358 (0.675) for ApoE ε4+ and ApoE ε4-, respectively, p=0.045]. The comparison for the NPZ-global summary score did not reach statistical significance, but the direction of trend was consistent with the NPZ-executive [mean (SD) of −0.674 (0.698) and −0.428 (0.550) for ApoE ε4+ and ApoE ε4-, respectively, p=0.092].
Figure 1. Mean neuropsychological summary scores (NPZ) by cognitive domain and ApoE ε4 carrier status.
Executive functioning domain was significantly worse for ApoE ε4 carriers (p=0.045) and a pattern of poorer performance is suggested on global (p=0.092), psychomotor (p=0.397), and memory (p=0.535) domains.
In sensitivity that included only participants with undetectable plasma HIV RNA (n=68), the NPZ-executive score remained significant (p=0.032) and the NPZ-global score reached significance (p=0.040) whereas the NPZ-psychomotor and NPZ-memory scores remained non-significant (p=0.177 and 0.246, respectively).
Brain volumetric analyses
Tensor Based Morphometry (TBM) analyses identified local ventricular expansion and areas of tissue loss in the cerebellum, brainstem, and frontal and subcortical regions associated with ApoE ε4 carriers compared to non-carriers (Figure 2).
Figure 2. Tensor based morphometry.
Two-dimensional representation demonstrating regions of ventricular expansion (top panel) and tissue shrinkage (lower panel) associated with ApoE ε4 carrier status. Warmer colors indicate greater differences between ε4 carriers and non-carriers.
Guided by the TBM results, we used FreeSurfer analyses to analyze individual structures in regions of notable atrophy. Results revealed significant differences in posterior corpus callosum (19.4% reduced in ApoE ε4 carriers compared to non-carriers, p=0.007) in a model that included ICV, age, and current CD4 count; but we did not find differences in total bilateral thalamic volume (p=0.313), total brainstem volume (p=0.156), or ventricular expansion (p=0.623). In sensitivity analyses that included only participants with suppressed plasma HIV RNA (n= 50), significance remained in the posterior corpus callosum (25.3% reduction, p=0.028). No new areas of significance emerged in the other structures. No significant difference was seen in hippocampal volume between ApoE ε4 carriers and non-carriers overall or upon examining the participants with suppressed plasma HIV RNA, separately.
Diffusion Tensor Imaging
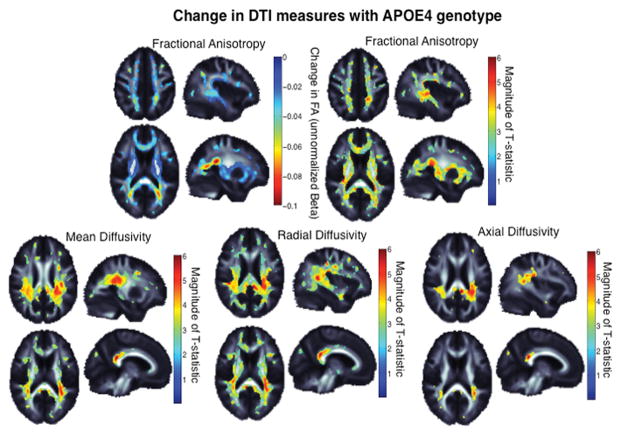
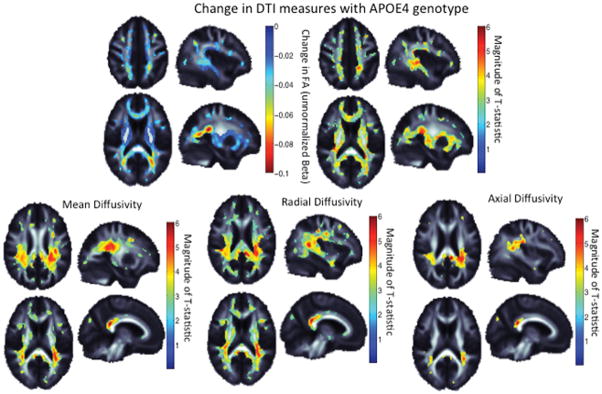
Voxelwise analyses of DTI measures revealed widespread patterns of reduced FA and increased diffusivity in regions of major white matter tracts in ApoE ε4 carriers compared to non-carriers, indicating decreased integrity of white matter (Figure 3). The greatest differences in FA were seen in temporal white matter regions including the thalamic radiations and the corona radiata. ApoE ε4 carriers had increased MD throughout many of the major white matter tracts with the greatest MD differences in the splenium of the corpus callosum and the corona radiata. Visual inspection of individual maps identified a more widespread area of abnormality in RD compared to AD (Figure 3, lower panels).
Figure 3. Diffusion Tensor Imaging by ApoE ε4 carrier status.
Upper panel, left: ApoE ε4 carriers demonstrate lower fractional anisotropy (FA) in broad regions; warmer colors represent more significant associations. Upper panel, right: The effect size, or unstandardized difference in FA value, where FA can be reduced between 0.02 and 0.1 in ApoE ε4 carriers. The magnitude of the normalized T-statistic is shown for comparison. Lower panel: The magnitude of the normalized T-statistic for MD, AD, and RD. The ApoE ε4 allele carriers had more diffusivity in all significant regions. *All colored regions are areas that retain significance after correction for multiple comparisons using the false discovery rate procedure to control the rate of false positives at 0.05. Warmer colors represent greater effect sizes.
To provide information on the magnitude of the effect, we measured FA and MD in the splenium of the corpus callosum. We noted a 5.1% increase in MD (p=0.016) and a 2.5% decrease in FA (p=0.063) in ApoE ε4 carriers compared to non-carriers. In sensitivity analyses that included only participants with suppressed plasma HIV RNA, the magnitude of association was generally unchanged although significance was lost (3.0% increase in MD (p=0.0912) and 2.5% decrease in FA (p=0.098)).
DISCUSSION
This sample of HIV-infected individuals aged 60 and older demonstrated associations between ApoE ε4 carrier status and clinical measures of cognitive performance, brain atrophy, and white matter integrity. Such associations have not been universally seen in the setting of HIV, and several large studies failed to find such detrimental effects.16 This may represent the influence of age modulating the associations.18 Our findings complement results from an investigation of younger HIV-infected cohort (group average age <30 years) in South Africa where ApoE ε4 was linked to memory impairments and decreased FA in the frontal and parietal regions of the corpus callosum.17 Unlike the current sample, the South African cohort was recently diagnosed (i.e., within 6 months) and naïve to cART. By using sensitivity analyses to assess cases with suppressed plasma HIV RNA only, we strengthened the contemporary importance of our findings by generally confirming associations among groups on cART, adherent and suppressed, although significance was sometimes lost, likely due to sample size limitations.
The pattern of neuropsychological testing abnormality provides a hint about the mechanism, particularly when considered in the context of our complementary imaging data. Although ApoE ε4 is a well-known risk factor for AD and the age of our sample increases the risk that some subjects may suffer from the early stages of this neurodegenerative disorder, we failed to identify effects on the memory domain, as would be typical of AD. In contrast, ApoE ε4 carriers demonstrated deficits in executive functioning, congruent with the pattern of mostly central atrophy and broad detrimental measures of brain integrity by DTI. A prior paper by our group used this same sample of participants, and linked these DTI abnormalities to neuropsychological measures, demonstrating their clinical relevance.30
Together, these findings are less supportive of AD-related mechanisms and more indicative that ApoE ε4 impacts mechanisms of injury related to HIV itself; although central atrophy is found in both HIV and AD.31 We note that these conclusions need to be considered within the context of our study limitations (e.g. cross-sectional design and small sample); although our findings are similar to another study where executive functioning and information processing were more detrimental in ApoE ε4 carriers in older but not younger age.14 Other notable volumetric differences in our cohort by TBM (e.g. shrinkage in the region of the thalamus and brainstem), are not typical of AD nor that described in ApoE ε4-carriers.13,32 The failure to identify structural differences by FreeSurfer could suggest that the differences seen in TBM may not impact the full underlying structure or that the effect was smaller than we had power to identify. Overall, these findings suggest an exacerbation of HIV-related neuropathology rather than the emergence of age-related neurodegenerative disorders.32 Our TBM finding identifies areas of shrinkage within the brainstem, consistent with this hypothesis.33
Similarly, the differences in white matter integrity mirror that seen in HIV.34 Other groups have shown increased diffusivity in healthy uninfected ApoE ε4 carriers compared to non-carriers.35 Our DTI results revealed not only decreases in FA but also increased diffusivity in ApoE ε4 carriers with more widespread effects in radial compared to axial diffusivity. Others have argued that this pattern of greater radial than axial diffusivity marks greater myelin damage.36 Our TBM and DTI data are congruent, as ApoE ε4 carriers also have atrophy in areas that contain key white matter tracts.
Our sample size was small; a larger sample might have identified broader abnormalities in clinical and imaging findings. This issue may also underlie the lack of difference in HAND classification by ApoE ε4 carrier status despite differences in neuropsychological summary scores. On average, our participants have been living with HIV for more than two decades. Consequently, many were likely exposed to older antiretroviral and combinations (e.g. single drug therapy, higher doses of zidovudine). These factors inform generalizability to other HIV-infected populations. Other factors to consider include known associations between ApoE ε4 and AIDS mortality (e.g., survivorship bias)16 and the likelihood of substantial cerebrovascular co-pathology.37 The ApoE ε4 allele may exacerbate the effect of cerebrovascular disease on white matter integrity, particularly since ApoE ε4 has been linked to cardiovascular disease.8,38
The neuropathology of Aβ accumulation may contribute to the effects we observed. ApoE ε4 has a well-established role in the processing and clearance of amyloid-beta (A-beta) in the brain with pathological evidence for increased plaque deposition in ApoE ε4 carriers.39 Some researchers postulate an interaction between HIV and A-beta with HIV viral proteins promoting A-beta accumulation.40 One group reported increased likelihood of HAND in ApoE ε4 carriers with A-beta plaque deposition.41 In that study, non-carriers did not evidence increased risk for HAND with A-beta accumulation. ApoE is critical to neuronal health including synaptic plasticity, neuroprotection, and transport of cholesterol and other lipids crucial to myelin integrity and may play a role in mediating inflammation in the CNS.8,39 Future studies of A-beta imaging or levels of CSF A-beta might elucidate whether effects of ApoE on brain health and cognition in older HIV patients is mediated via A-beta-dependent or independent mechanisms.
Supplementary Material
Acknowledgments
Conflicts of Interest and Sources of Funding: This project was supported by NIH grants K24-MH098759 (V.V.), K23-AG032872 (V.V.), P50-AG023501, UL1 RR024131, and P30-AI027763; and The Larry L. Hillblom Foundation; U54 EB020403, R01 NS080655 (P.T.). Dr. Victor Valcour has served as a consultant for ViiV Healthcare and Merck on topics related to HIV and aging and has been compensated for lecturing at a local nursing home. For the remaining authors none were declared.
Footnotes
Protection of Human Subjects: All procedures were carried out in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1.Mateen FJ, Mills EJ. Aging and HIV-related cognitive loss. JAMA : the journal of the American Medical Association. 2012;308(4):349–350. doi: 10.1001/jama.2012.8538. [DOI] [PubMed] [Google Scholar]
- 2.CDC Centers for Disease Control and Prevention. [Accessed October 2015];HIV Surveillance Report. 2015 25 http://www.cdc.gov/hiv/pdf/g-l/hiv_surveillance_report_vol_25.pdf; Published February 2015. [Google Scholar]
- 3.CDC. Centers for Disease Control and Prevention. [Accessed August 2011];HIV Surveillance Report. 2009 21 http://www.cdc.gov/hiv/topics/surveillance/resources/reports/-surveillance; Published February 2011. [Google Scholar]
- 4.Antiretroviral Therapy Cohort C. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54(11):2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler-Graham K, Brookmeyer R, Johnson E, Arrighi HM. Worldwide variation in the doubling time of Alzheimer’s disease incidence rates. Alzheimers Dement. 2008;4(5):316–323. doi: 10.1016/j.jalz.2008.05.2479. [DOI] [PubMed] [Google Scholar]
- 8.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caselli RJ, Reiman EM, Osborne D, et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62(11):1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- 10.Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology. 2004;63(10):1898–1901. doi: 10.1212/01.wnl.0000144279.21502.b7. [DOI] [PubMed] [Google Scholar]
- 11.Horsburgh K, McCarron MO, White F, Nicoll JA. The role of apolipoprotein E in Alzheimer’s disease, acute brain injury and cerebrovascular disease: evidence of common mechanisms and utility of animal models. Neurobiology of aging. 2000;21(2):245–255. doi: 10.1016/s0197-4580(00)00097-x. [DOI] [PubMed] [Google Scholar]
- 12.Busch RM, Lineweaver TT, Naugle RI, et al. ApoE-epsilon4 is associated with reduced memory in long-standing intractable temporal lobe epilepsy. Neurology. 2007;68(6):409–414. doi: 10.1212/01.wnl.0000253021.60887.db. [DOI] [PubMed] [Google Scholar]
- 13.Lu PH, Thompson PM, Leow A, et al. Apolipoprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: a tensor-based morphometry study. Journal of Alzheimer’s disease : JAD. 2011;23(3):433–442. doi: 10.3233/JAD-2010-101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panos SE, Hinkin CH, Singer EJ, et al. Apolipoprotein-E genotype and human immunodeficiency virus-associated neurocognitive disorder: the modulating effects of older age and disease severity. Neurobehav HIV Med. 2013;5:11–22. doi: 10.2147/NBHIV.S39573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corder EH, Robertson K, Lannfelt L, et al. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med. 1998;4(10):1182–1184. doi: 10.1038/2677. [DOI] [PubMed] [Google Scholar]
- 16.Burt TD, Agan BK, Marconi VC, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(25):8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoare J, Westgarth-Taylor J, Fouche JP, et al. Relationship between apolipoprotein E4 genotype and white matter integrity in HIV-positive young adults in South Africa. European archives of psychiatry and clinical neuroscience. 2012 doi: 10.1007/s00406-012-0341-8. [DOI] [PubMed] [Google Scholar]
- 18.Valcour V, Shikuma C, Shiramizu B, et al. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. Journal of neuroimmunology. 2004;157(1–2):197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Morgan EE, Woods SP, Letendre SL, et al. Apolipoprotein E4 genotype does not increase risk of HIV-associated neurocognitive disorders. J Neurovirol. 2013 doi: 10.1007/s13365-013-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooley SA, Paul RH, Fennema-Notestine C, et al. Apolipoprotein E epsilon4 genotype status is not associated with neuroimaging outcomes in a large cohort of HIV+ individuals. Journal of neurovirology. 2016 doi: 10.1007/s13365-016-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker JT, Martinson JJ, Penugonda S, et al. No association between Apoε4 alleles, HIV infection, age, neuropsychological outcome, or death. Journal of neurovirology. 2015;21(1):24–31. doi: 10.1007/s13365-014-0290-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiao S, Rosen HJ, Nicolas K, et al. Deficits in Self-Awareness Impact the Diagnosis of Asymptomatic Neurocognitive Impairment in HIV. AIDS research and human retroviruses. 2013;29(6):949–956. doi: 10.1089/aid.2012.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewey J, Hana G, Russell T, et al. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. NeuroImage. 2010;51(4):1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leow AD, Yanovsky I, Chiang MC, et al. Statistical properties of Jacobian maps and the realization of unbiased large-deformation nonlinear image registration. IEEE Trans Med Imaging. 2007;26(6):822–832. doi: 10.1109/TMI.2007.892646. [DOI] [PubMed] [Google Scholar]
- 26.Gutman B, Svarer C, Leow A, Yanovsky I, Toga AW, Thompson P. Organization for Human Brain Mapping. Barcelona, Spain: 2010. Creating Unbiased Minimal Deformation Templates for Brain Volume Registration. [Google Scholar]
- 27.Jahanshad N, Valcour VG, Nir TM, et al. Disrupted brain networks in the aging HIV+ population. Brain Connect. 2012 doi: 10.1089/brain.2012.0105-Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langers DR, Jansen JF, Backes WH. Enhanced signal detection in neuroimaging by means of regional control of the global false discovery rate. Neuroimage. 2007;38(1):43–56. doi: 10.1016/j.neuroimage.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 1995;57(1):289–300. [Google Scholar]
- 30.Nir TM, Jahanshad N, Busovaca E, et al. Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nestor SM, Rupsingh R, Borrie M, et al. Ventricular enlargement as a possible measure of Alzheimer’s disease progression validated using the Alzheimer’s disease neuroimaging initiative database. Brain. 2008;131(Pt 9):2443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua X, Leow AD, Parikshak N, et al. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: an MRI study of 676 AD, MCI, and normal subjects. NeuroImage. 2008;43(3):458–469. doi: 10.1016/j.neuroimage.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallianpur KJ, Shikuma C, Kirk GR, et al. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology. 2013;80(19):1792–1799. doi: 10.1212/WNL.0b013e318291903f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gongvatana A, Schweinsburg BC, Taylor MJ, et al. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. Journal of neurovirology. 2009;15(2):187–195. doi: 10.1080/13550280902769756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westlye LT, Reinvang I, Rootwelt H, Espeseth T. Effects of APOE on brain white matter microstructure in healthy adults. Neurology. 2012;79(19):1961–1969. doi: 10.1212/WNL.0b013e3182735c9c. [DOI] [PubMed] [Google Scholar]
- 36.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 37.Soontornniyomkij V, Umlauf A, Chung SA, et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014 doi: 10.1097/QAD.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lahoz C, Schaefer EJ, Cupples LA, et al. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis. 2001;154(3):529–537. doi: 10.1016/s0021-9150(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 39.Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pulliam L. HIV Regulation of Amyloid Beta Production. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2009 doi: 10.1007/s11481-009-9151-9. [DOI] [PubMed] [Google Scholar]
- 41.Soontornniyomkij V, Moore DJ, Gouaux B, et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS. 2012;26(18):2327–2335. doi: 10.1097/QAD.0b013e32835a117c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.