Abstract
Objective
To assess hospital-level variability in diagnostic testing and outcomes for children with neurologic impairment hospitalized with pneumonia.
Study design
A retrospective cohort study of 27 455 children ages 1-18 years with neurologic impairment hospitalized with pneumonia at 39 children's hospitals. K-means clustering was used to assign each hospital to 1 of 3 groups (termed A, B, and C) based on similar diagnostic testing patterns. Outcomes of hospital-level median length of stay (LOS), 30-day readmissions, and pneumonia-associated complications were compared while controlling for patient differences.
Results
Overall, 48.5% had comorbid complex chronic conditions, and 25.4% were assisted with medical technology. Outcomes and diagnostic testing varied across hospitals: median hospital-level LOS, 3.2 days (IQR 2.8-3.8); median readmission, 8.4% (IQR 6.8,-10.0); and median pneumonia-associated complication rate, 23.1% (IQR 18.7-26.8). Despite similar populations, hospitals in group A tended to perform fewer tests than those in groups B and C. Across hospital groups, there was a significant difference in adjusted readmission rates (group A 7.2%, group B 9.0%, group C 7.7%, P = .003). There was no significant difference in adjusted median LOS (group A 3.4 days, group B 3.2 days, group C 3.3 days, P = .3) or adjusted pneumonia-associated complication rates (group A 22.5%, group B 22.5%, group C 25.0%, P = .6).
Conclusions
For children with neurologic impairment hospitalized with pneumonia, across hospital differences in diagnostic testing were not associated with clinically meaningful differences in outcomes. High-utilizing hospitals may be able to decrease diagnostic testing for children with neurologic impairment hospitalized with pneumonia without adversely impacting outcomes.
Children with neurologic impairment have functional and or intellectual impairments that result from a variety of neurologic diseases. These children experience frequent, lengthy, and expensive hospitalizations and account for an increasing and disproportionate amount of inpatient hospital resources.1 Pneumonia is one of the most common reasons for hospitalization, the most common reason for admission to an intensive care unit (ICU), and the most common cause of death in this population.1-4
Unlike pneumonia in otherwise healthy children, sparse evidence exists to guide diagnostic testing or management for children with neurologic impairment hospitalized with pneumonia. Clinicians instead use personal experience, local practice culture, and parental preference to guide their decision-making.5 Children with complex chronic conditions hospitalized with pneumonia receive more intensive management, yet have worse outcomes compared with otherwise healthy children.6 Little is known about current hospital management and outcomes of pneumonia in the population of children with neurologic impairment. Prior studies in otherwise healthy children hospitalized with pneumonia have demonstrated variation in diagnostic testing, treatment, and outcomes across hospitals.7,8 In fact, increased diagnostic testing has been associated with increased hospitalization rates among those evaluated in the emergency department8 and longer hospital length of stay (LOS) among those requiring admission.7 The objectives in this study of children with neurologic impairment hospitalized with bacterial pneumonia were to assess the variability in outcomes and diagnostic testing across hospitals, and to determine the association between hospital-level diagnostic test utilization and outcomes.
Methods
This multicenter, retrospective, cohort study included data from the Pediatric Health Information System (PHIS), an administrative database of 45 not-for-profit, tertiary care, US pediatric hospitals affiliated with the Children's Hospital Association (Overland Park, Kansas). PHIS contains data regarding patient demographics, diagnoses, and procedures (with International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes), and daily billed resource utilization, which include laboratory studies and radiologic imaging. Encrypted medical record numbers permit identification of patients across multiple visits to the same hospital. Data quality and reliability are assured through Children's Hospital Association and participating hospitals. The 39 hospitals that provided data to PHIS throughout the entire study period were included in this study.
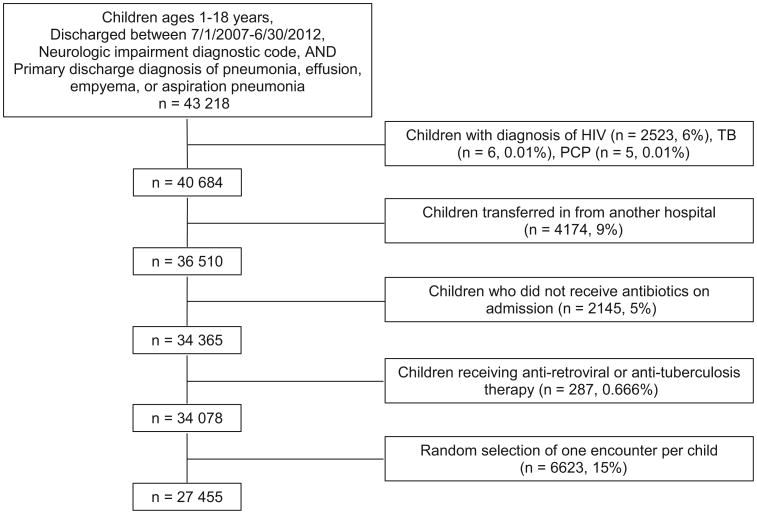
Hospitalizations of children 1-18 years of age who were discharged between July 1, 2007, and June 30, 2012, were included if they had a neurologic impairment ICD-9-CM diagnosis code1 and a principal discharge diagnosis indicative of bacterial pneumonia.9 Neurologic impairment was defined as functional and/or intellectual impairment resulting from a neurologic disease (eg, cerebral palsy, epilepsy) using a previously defined set of 606 ICD-9-CM diagnosis codes.1 Infants <1 year of age were excluded as many neurologic impairment diagnoses (eg, cerebral palsy) are not assigned until an older age. As in our prior work,10 hospitalizations for pneumonia were identified based on previously validated methods using principal ICD-9-CM diagnosis codes for pneumonia (480.0-2, 480.8-9, 481, 482.0, 482.30-2, 482.41-2, 482.83, 482.89-90, 483.8, 484.3, 485, 486, 487.0) and pulmonary effusion/empyema (510.0, 510.9, 511.0-1, 511.8-9, 513),9 as well as ICD-9-CM codes for aspiration pneumonia (507.x). For children with multiple hospitalizations, 1 admission was randomly selected for inclusion to minimize the chance of biasing the findings with a small group of children who experienced a large number of admissions.
We excluded children who did not receive an antibiotic in the first 2 calendar days of admission to minimize the likelihood of including children with nonbacterial pneumonia. This approach also minimized the inclusion of children who were admitted for reasons other than pneumonia, but then were treated and coded for pneumonia acquired during their hospitalization. Children transferred in from another hospital were excluded as records from their initial presentation including testing, treatment, and outcomes were not available in PHIS. Finally, children with a diagnosis of HIV, Pneumocystis pneumonia, or tuberculosis and children who received antiretroviral or antituberculosis therapy during hospitalization were excluded given expected differences in presentation, management, treatment, and outcomes (Figure 1; available at www.jpeds.com).11
Figure 1.
Cohort diagram. TB, tuberculosis; PCP, Pneumocystis pneumonia.
Outcome Measures
Outcome measures in this study were hospital-level LOS measured in hospital days, all-cause 30-day hospital readmission (ie, readmission for any cause and for any admission type, including observation),12 and pneumonia-associated complication rate. Pneumonia-associated complications (local [eg, effusion], systemic [eg, acute respiratory failure], and meta-static [eg, meningitis]) were examined using previously described ICD-9-CM codes.13
Diagnostic Test Utilization
As we aimed to examine only testing performed in the initial diagnosis and management, we examined only those tests obtained in the first 2 calendar days of admission. Diagnostic tests examined include laboratory studies and radiologic imaging, and were based on billing data in PHIS. Hospital-level diagnostic test utilization was defined as the percent of patients at each hospital who had each test ordered in the first 2 calendar days of admission. Laboratory studies included complete blood count (CBC), C-reactive protein, blood gas, blood chemistry profile, and microbiologic studies of viral testing, blood culture, and respiratory culture. Imaging studies included chest radiograph.
Patient Case-Mix
To compare patient case-mix across hospitals, we examined underlying neurologic disease, as well as medical comorbidities associated with severity of neurologic impairment and severity of acute illness. Nine neurologic impairment categories were assessed: (1) static neurologic disease; (2) progressive neurologic disease; (3) anatomic abnormality; (4) epilepsy; (5) genetic or metabolic condition; (6) cerebrovascular disease; (7) peripheral neurologic disease; (8) behavioral; and (9) not otherwise specified/other.1,10 These neurologic impairment categories are not mutually exclusive (ie, patients may have diagnoses in multiple categories). Underlying medical comorbidities included the number of non-neurologic complex chronic conditions (CCCs)14 endured by each patient and assistance with medical technology. CCCs are “any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or one system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.”15 The neurologic and neuromuscular CCC category was not included in the CCC count as all patients in our cohort had diagnoses of neurologic impairment. Medical technology assistance (eg, tracheostomy) was defined using the medical technology or device subcategory within relevant CCC categories.14 Severity of acute illness was examined by the percent of patients who required mechanical ventilation, vasopressor use, or ICU admission.
Statistical Analyses
Continuous data were described with median and IQR attributable to non-normal distribution. Categorical data were described with frequencies and percentages. Outcomes of LOS, 30-day all-cause hospital readmission rate, and pneumonia-associated complication rate were compared across hospitals. Correlation of LOS and 30-day all-cause hospital readmission was examined using Spearman rank correlation coefficient. Variation in diagnostic test utilization across hospitals was examined by calculating and comparing the rate of subjects at each hospital receiving each test.7,8,16
K-means clustering was used to assign each hospital to 1 of 3 groups based on similar diagnostic testing habits. K-means clustering was used to partition hospitals into k clusters (where k represents the number of clusters), such that each hospital belongs to the cluster with the nearest mean testing pattern for each diagnostic test. Clustering is a methodology used to identify comparable groupings of observations (eg, hospitals) based on multiple characteristics (eg, testing patterns). Distinct groups (ie, clusters) are identifiable when there is significant similarity in the characteristics among observations within a group and differences across groups. Observations within a group can display variability on a particular characteristic but should be well grouped across all characteristics included. The number of clusters (or groups) was determined through examination of the Scree plot. Scree plots allowed for visual demonstration of the number of factors that explain most of the variability. In this case, variability in diagnostic testing was explained in 3 factors (ie, 3 hospital groups). Addition of further factors, or groups, explained less and less variability.
Patient case-mix variables, including medical comorbidities associated with neurologic impairment severity, as well as markers of acute illness severity, were compared across hospital groups using the Kruskal-Wallis test. We used generalized linear mixed-effects models to compare the log transformed LOS, readmission rates, and pneumonia-associated complication rates across the 3 hospital groups while adjusting for patient case-mix variables, and accounted for patient clustering within hospitals using a random intercept term for hospital. Results from the log transformed LOS model were back transformed onto the original LOS scale.
All analyses were performed with SAS v 9.4 (SAS Institute, Cary, North Carolina), and P values of <.05 were considered statistically significant. By the policies of the Cincinnati Children's Hospital Medical Center Institutional Review Board, this research using a deidentified dataset was not considered human subjects research.
Results
A total of 27 455 patients met eligibility criteria at the 39 hospitals included in this study. The median age was 4 years (IQR 2-8). Most subjects were male (52.5%), non-Hispanic white (47.4%), and had a government payor (54.6%). Nearly one-half of patients had comorbid CCCs (48.5%) with one-quarter of patients assisted by medical technology (25.4%). ICU admission occurred in 17.3% of patients, with 8.8% receiving mechanical ventilation and 3.6% receiving vasopressors.
Variation in Outcomes
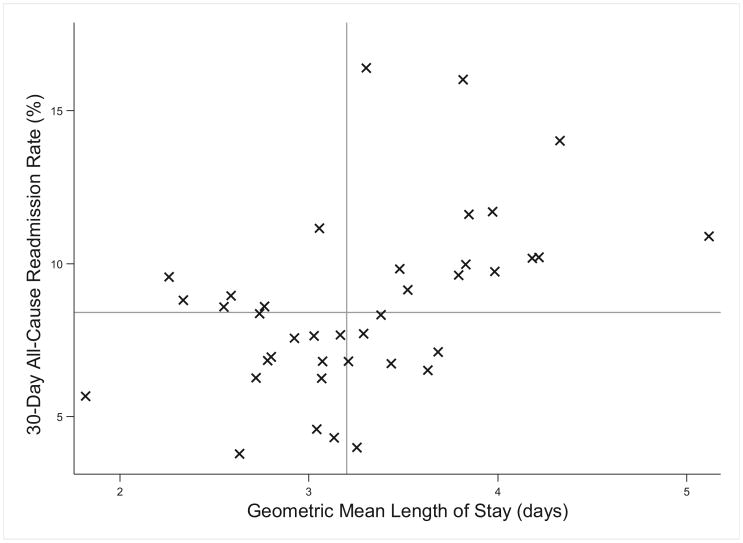
LOS and hospital readmission rates differed across hospitals (Table I and Figure 2). The geometric mean LOS by hospital ranged from 1.8 to 5.1 days, with a median of 3.2 days (IQR 2.7-3.8). Readmission rates ranged from 3.8% to 16.4% across hospitals, with a median of 8.4% (IQR 6.8-10.0%). There was a statistically significant positive correlation between LOS and readmission (r = 0.525, P value <.001).
Table I. Unadjusted and adjusted hospital-level outcomes compared across hospital groups.
| All hospitals (n = 43) median (IQR) | Group A hospitals (n = 4) median (IQR) | Group B hospitals (n = 19) median (IQR) | Group C hospitals (n = 16) median (IQR) | P value* | |
|---|---|---|---|---|---|
| Unadjusted LOS (d) | 3.21 (2.78, 3.79) | 2.87 (2.19, 3.15) | 3.29 (2.72, 3.85) | 3.34 (3.06, 3.66) | .19 |
| Adjusted LOS (d)† | 3.28 (3.03, 3.50) | 3.39 (2.67, 3.76) | 3.24 (2.90, 3.41) | 3.32 (3.18, 3.59) | .34 |
| Unadjusted 30-d readmission rate | 8.4 (6.8, 9.9) | 4.9 (4.1, 6.7) | 8.9 (8.3, 11.6) | 6.9 (6.6, 9.7) | .003 |
| Adjusted 30-d readmission rate† | 8.5 (7.3, 9.3) | 7.2 (5.7, 8.7) | 9.0 (8.4, 10.5) | 7.7 (6.8, 9.2) | .03 |
| Unadjusted pneumonia-associated complications rate | 23.1 (18.7, 26.8) | 20.2 (15.9, 27.1) | 23.8 (18.7, 27.2) | 23.1 (19.9, 24.1) | .81 |
| Adjusted pneumonia-associated complications rate† | 23.0 (19.9, 25.7) | 22.5 (20.3, 27.4) | 22.45 (19.9, 24.4) | 24.78 (19.9, 26.2) | .58 |
Models for LOS and readmission outcomes additionally included pneumonia-associated complications as a covariate.
P value represents comparison of group A, group B, and group C hospital outcomes from generalized linear mixed-effects regression model for LOS and as a binary response in a generalized mixed-effects model with logit link for readmission rate and pneumonia-associated complications rate.
Models adjusted for non-neurologic CCC count, assistance with medical technology, respiratory technology, neuromuscular/neurologic technology, ICU-level care, ventilation, and vasopressor use.
Figure 2.
Variation in hospital-level outcomes of LOS and 30-day all-cause readmission rate. Each × represents data from 1 hospital. The vertical line represents median readmission rate (8.4%), and the horizontal reference line represents median LOS (3.2 days). There was a statistically significant positive correlation between LOS and readmission (r = 0.525, P value <.001).
The pneumonia-associated complication rates also varied across hospitals, ranging from 6.4% to 42.0% with a median of 23.1% (IQR 18.7-26.8; Table I).
Variation in Diagnostic Testing
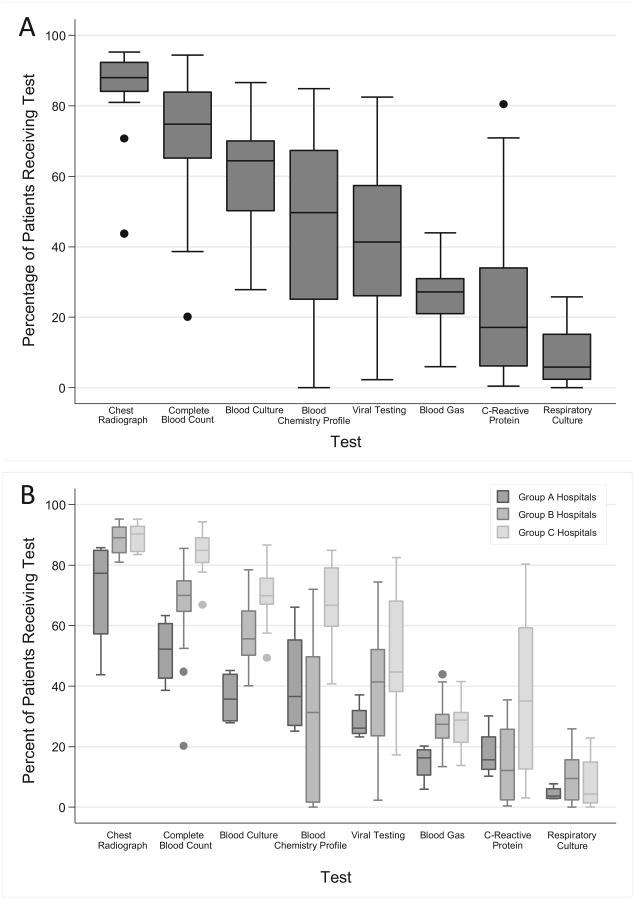
There was marked variability in laboratory testing across hospitals (Figure 3, A). The large variability occurred in almost every laboratory test evaluated as noted by wide IQRs and large numbers of outliers (ie, hospitals that performed tests outside 1.5 times the 25th and 75th percentiles for testing). For example, CBCs were almost always obtained at some hospitals (94.4%) and infrequently at other hospitals (20.1%).
Figure 3.
Variation in diagnostic testing across A, all hospitals and B, hospital groups. Line intersecting each box represents the median; the ends of the box represent the IQR. Whiskers represent values 1.5 times the IQR. Circles represent extreme outliers.
Hospital Clustering Based on Diagnostic Testing Patterns
Using K-means clustering, hospitals were clustered into one of 3 groups (for simplicity referred to as group A, B, or C) based on similar diagnostic testing patterns. Group A hospitals tended to perform fewer tests than hospitals in groups B and C (Figure 3, B). For example, a median of 52% of patients in group A hospitals had a CBC compared with medians with 70% and 85% in groups B and C hospitals, respectively. Groups B and C hospitals had similarly high performance of some tests (eg, chest radiograph, blood gas, and respiratory culture), but group B hospitals performed fewer tests of some types (eg, CBC, blood culture, blood chemistry profile).
Patient Case-Mix Across Hospital Groups
We examined patient case-mix across hospital groups to determine whether similarities or differences in patient populations could account for testing patterns. The only statistically significant differences in patient-level factors across hospital groups were in respiratory and neurologic technologies. Group A hospitals had fewer patients assisted by respiratory technology (median 3%, IQR 1.5-4.2) than hospitals in groups B and C (group B median 8%, IQR 5.8-10.2; group C median 7.2%, IQR 5.4-9.9). Similarly, group A hospitals had fewer patients assisted by neuromuscular or neurologic technology (median 1.7%, IQR 1.5-3.5) than hospitals in group B or C (group B median 4.4%, IQR 3.0-6.7; group C median 5.2%, IQR 3.7-6.7). Although there were no statistically significant differences in other factors examined across hospital groups, some of these differences across hospital groups might be clinically meaningful (Table II). For example, the median of patients requiring ICU-level care varied from 11.8% in group A hospitals to 20.3% in group B hospitals and 17.0% in group C hospitals. We included all examined markers of acute illness severity as covariates in models examining the relationship between diagnostic testing and outcomes.
Table II. Comparison of patient case-mix across hospital groups.
| All hospitals (n = 43) percent median (IQR) | Group A Hospitals (n = 4) percent median (IQR) | Group B Hospitals (n = 19) percent median (IQR) | Group C Hospitals (n = 16) percent median (IQR) | P value* | |
|---|---|---|---|---|---|
| Category of neurologic impairment | |||||
| Static neurologic disease | 76.3 (64.7, 94.0) | 86.3 (71.0, 94.0) | 76.3 (62.1, 85.5) | 71.8 (64.8, 82.9) | .42 |
| Epilepsy | 19.7 (15.9, 26.9) | 11.8 (7.7, 18.1) | 20.3 (16.8, 27.4) | 18.9 (16.1, 27.8) | .08 |
| Genetic/metabolic condition | 15.8 (12.0, 19.3) | 10.8 (7.3, 16.6) | 15.4 (13.9, 22.2) | 17 (11.6, 18.5) | .29 |
| Anatomic abnormality | 11.2 (10.2, 18.5) | 9.0 (6.4, 10.9) | 11.9 (10.2, 18.5) | 12.1 (10.9, 20.8) | .09 |
| Not otherwise sepcified/other | 8.9 (3.6, 12.2) | 3.2 (2.7, 6.2) | 9.7 (6.2, 12.0) | 8.5 (4.2, 18.3) | .23 |
| Peripheral neurologic disease | 5.7 (4.0, 7.4) | 2.7 (1.8, 5.3) | 6.3 (4.2, 7.6) | 5.5 (3.8, 7.5) | .14 |
| Behavioral | 2.6 (1.5, 4.5) | 1.5 (0.8, 4.0) | 2.7 (1.6, 3.4) | 2.6 (1.7, 4.6) | .48 |
| Cerebrovascular disease | 1.1 (0.7, 1.5) | 1.1 (0.7, 1.7) | 1.1 (0.7, 1.8) | 1.0 (0.6, 1.3) | .66 |
| Progressive neurologic disease | 0.9 (0.5, 1.3) | 0.9 (0.5, 1.7) | 1.1 (0.7, 1.4) | 0.7 (0.4, 1.1) | .35 |
| CCC count | |||||
| 0 | 54.4 (41.3, 59.2) | 62.7 (54.4, 72.7) | 53.3 (34.1, 58.6) | 54.2 (44.0, 60.0) | .20 |
| 1 | 15.5 (13.4, 18.1) | 14.9 (12.5, 15.4) | 15.5 (14.8, 20) | 16.9 (12.5, 17.9) | .39 |
| 2+ | 30.0 (25.9, 42.7) | 22.4 (14.5, 30.5) | 31.7 (26.5, 44.3) | 28.8 (24.5, 40.1) | .17 |
| Assistance with technology | 25.4 (21.9, 37.1) | 22.0 (15.6, 24.6) | 27.1 (22.9, 37.6) | 25.0 (21.3, 36.7) | .18 |
| Gastrointestinal | 21.9 (18.7, 31.5) | 15 (9.8, 21.0) | 23.1 (19.2, 31.9) | 20.2 (17.4, 31.4) | .15 |
| Respiratory | 7.4 (5.2, 10.2) | 3.0 (1.5, 4.2) | 8.0 (5.8, 10.2) | 7.2 (5.4, 9.9) | .02 |
| Neuromuscular/neurologic | 4.4 (3.2, 6.6) | 1.7 (1.5, 3.5) | 4.4 (3.0, 6.7) | 5.2 (3.7, 6.7) | .04 |
| Cardiovascular | 0.6 (0.4, 1.4) | 0.4 (0.3, 1.0) | 1.0 (0.5, 1.5) | 0.4 (0.3, 0.7) | .18 |
| Renal | 0.5 (0.3, 0.7) | 0.4 (0.2, 0.8) | 0.6 (0.4, 0.7) | 0.5 (0.2, 0.7) | .66 |
| Other | 1.8 (0.5, 2.8) | 3.7 (0.6, 10) | 2.1 (0.9, 4.2) | 1.0 (0.5, 2.1) | .20 |
| ICU-level care | 17.9 (12.9, 24.8) | 11.8 (9.4, 15.9) | 20.3 (14.4, 29.1) | 17.0 (13.6, 24.0) | .17 |
| Ventilation | 9.3 (6.8, 11.1) | 5.1 (3.8, 7.9) | 10.8 (7.0, 14) | 9.3 (7.1, 10.5) | .11 |
| Vasopressor use | 2.8 (1.9, 5.0) | 1.6 (1.2, 6.1) | 3.8 (1.9, 6.5) | 2.5 (2.1, 3.9) | .34 |
P value represents comparison of characteristics across group A, group B, and group C hospitals from Kruskal-Wallis test.
Association of Diagnostic Testing Patterns and Outcomes
Group A hospitals had a median unadjusted geometric mean LOS of 2.9 days (IQR 2.2-3.2), a median unadjusted readmission rate of 5% (IQR 4.1-6.7), and a median pneumonia-associated complication rate of 20.2% (IQR 15.9-27.1%). Group B hospitals had a median unadjusted geometric mean LOS of 3.3 days (IQR 2.7-3.9), a median unadjusted readmission rate of 9.0% (IQR 8.3-11.6), and a median pneumonia-associated complication rate of 23.8% (IQR 18.7-27.2%). Group C hospitals had a median unadjusted geometric mean LOS of 3.3 days (IQR 3.0-3.7), a median unadjusted readmission rate of 6.9% (IQR 6.6-9.7), and a median pneumonia-associated complication rate of 23.1% (IQR 19.9-24.1%). In unadjusted analysis, the difference in LOS and pneumonia-associated complication rates across the 3 hospital groups were not statistically significant, whereas differences in readmission rates were significant (Table I). After controlling for case-mix, the difference across hospital groups for LOS and pneumonia-associated complication rates remained insignificant, and the difference across hospital groups for 30-day readmission rates remained statistically significant (Table I).
Discussion
We found substantial variability in hospital outcomes and diagnostic testing among children with neurologic impairment hospitalized with bacterial pneumonia. When examining difference in outcomes between hospital groups based on diagnostic testing patterns, the overall adjusted LOS difference of 0.15 days, adjusted readmission difference of 1.83%, and adjusted pneumonia-associated complication rate difference of 2.33% are unlikely to be clinically relevant. Hospitals where less diagnostic testing was performed had outcomes clinically comparable with hospitals where more diagnostic testing was performed, regardless of case mix. These findings suggest that high-utilizing hospitals may be able to decrease diagnostic test utilization without compromising outcomes.
Hospital outcomes varied vastly across hospitals with nearly 2-fold variation in LOS, greater than 3-fold variation in readmission rates, and greater than 5-fold variation in pneumonia-associated complication rates. The variation in 30-day all cause readmission in children with neurologic impairment hospitalized with pneumonia (ranging from 3.8% to 16.4%) is an exaggeration of the previously described variation in readmission rates across hospitals for all children admitted with pneumonia (ranging from 5% to 13%).17 This is likely a reflection of the underlying complexity of children with neurologic impairment who frequently have multiple chronic, and often complex, conditions beyond their neurologic disease. Although children with chronic conditions have been found to be more likely to experience readmission following pneumonia hospitalization, the readmission rate increased with increasing number of chronic conditions.17 Future work focused on identifying patient and hospital characteristics associated with readmissions in children with neurologic impairment hospitalized with pneumonia may allow for the development of interventions to reduce unnecessary reutilization.
Diagnostic testing examined in this study varied widely by institution; some diagnostic tests (eg, blood chemistry tests) were almost always performed at some institutions and never performed at other institutions. In a study examining diagnostic testing in children hospitalized with pneumonia who were otherwise healthy, similar variability existed in diagnostic test performance across hospitals.7 However, overall testing rates were greater in our study of children with neurologic impairment. For example, hospital median performance of viral studies in children with neurologic impairment hospitalized with pneumonia was 41.4%, but just 22.6% in healthy children hospitalized with pneumonia.7 Similar to diagnostic testing for pneumonia in otherwise healthy children,7 we found more variation in the performance of some diagnostic tests than others. We believe this to be a reflection of the quality of evidence supporting different tests for the diagnosis of pneumonia in otherwise healthy children.18 Although there is high-quality evidence to support obtaining a chest radiograph for diagnosis of pneumonia, the poor specificity of other tests (eg, blood culture, CBC, erythrocyte sedimentation rate, and C-reactive protein) for a pneumonia diagnosis may amplify the variation of test performance.19 Indeed, hospitals have drastically different approaches (algorithms) for clinical decisions of obtaining blood cultures for otherwise healthy children with pneumonia. Published approaches include aiming for obtaining blood cultures in 100% of children admitted with community-acquired pneumonia,20 as well as obtaining blood cultures only in the subset of patients identified as high risk for bacteremia.21 Such differences in local practice guidelines certainly affect variability of test performance.
The underlying medical complexity of these children is likely an important factor in the high level of diagnostic testing in our cohort. Yet, it is unclear if there is value added in this additional testing or if this testing impacts the clinical care of these patients.
In our study, hospitals where more diagnostic testing was performed for children with neurologic impairment hospitalized with pneumonia did not have clinically meaningful improvement in hospitalization outcomes of LOS, readmission rates, or pneumonia-associated complication rates compared with hospitals where less diagnostic testing was performed. These results indicate that some of the diagnostic testing performed for children with neurologic impairment hospitalized with pneumonia may be unwarranted.22,23 A growing body of evidence in health care reveals that more care is not equivalent to better care.24-26 In fact, overuse in medical care has been associated with increased cost without improved outcomes.27
Our work should be interpreted in the context of several limitations. First, clinical data may be preferable to administrative data to identify children with neurologic impairment diagnosed with pneumonia. The use of diagnostic codes to identify children with neurologic impairment may have resulted in selection bias; it is possible that children with less severe neurologic impairment may not have had a diagnostic code indicative of their neurologic impairment diagnosis during an acute hospitalization. As it is unclear how severity of neurologic impairment may influence diagnostic testing but reasonable to suspect that it influences outcomes, we controlled for diagnostic categories of neurologic impairment, as well as medical comorbidities that frequently occur with severe neurologic impairment in the hospital-level analysis of relationship between diagnostic testing and outcomes. However, PHIS administrative data are not equipped to distinguish granular grades of functional status (eg, ability to cough, chest wall strength) that might influence pneumonia hospitalization outcomes of children with neurologic impairment. We attempted to limit the inclusion of patients with viral or hospital-acquired pneumonia through adaptation of validated approaches to identify patients hospitalized with pneumonia and by restricting our cohort to children who received antibiotics on first 2 calendar days of admission. Second, our data are limited to the free-standing children's hospitals included in PHIS and may not reflect the extent of variation occurring in other settings. However, children with neurologic impairment predominately are cared for at hospitals similar to those included in this study.1 Finally, given the use of administrative data, we were not able to examine system-level factors (eg, policies regarding management of children with neurologic impairment) that might influence diagnostics testing and/or outcomes. Future work focused on illuminating best practices should consider differences in such factors, including presence of inpatient complex care programs or pneumonia care pathways, as these may have a substantial effect on the standardization of care at individual institutions.
Our data suggest that hospitals may be able to decrease diagnostic testing without compromising care. We further believe that this study highlights the need for more clinical research on the optimal diagnostic and treatment approach for pneumonia in children with neurologic impairment. Such research may provide evidence for the development of guidelines and an opportunity to standardize care, reduce unnecessary resource utilization, and optimize outcomes for children with neurologic impairment hospitalized with pneumonia.
Acknowledgments
J.T. was supported by the Academic Pediatric Association Young Investigator Award and from National Research Service Award (T32HP10027-14). J.B. was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23 HD058092) and the Agency for Healthcare Research and Quality (R21 HS023092).
Glossary
- CBC
Complete blood count
- CCC
Complex chronic condition
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- ICU
Intensive care unit
- LOS
Length of stay
- PHIS
Pediatric Health Information System
Footnotes
The authors declare no conflicts of interest.
References
- 1.Berry JG, Poduri A, Bonkowsky JL, Zhou J, Graham DA, Welch C, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med. 2012;9:e1001158. doi: 10.1371/journal.pmed.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plioplys AV, Kasnicka I, Lewis S, Moller D. Survival rates among children with severe neurologic disabilities. South Med J. 1998;91:161–72. doi: 10.1097/00007611-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Seddon PC, Khan Y. Respiratory problems in children with neurological impairment. Arch Dis Child. 2003;88:75–8. doi: 10.1136/adc.88.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham RJ, Dumas HM, O'Brien JE, Burns JP. Congenital neurodevelopmental diagnoses and an intensive care unit: defining a population. Pediatr Crit Care Med. 2004;5:321–8. doi: 10.1097/01.pcc.0000128892.38431.2b. [DOI] [PubMed] [Google Scholar]
- 5.Palmer RH, Miller MR. Methodologic challenges in developing and implementing measures of quality for child health care. Ambul Pediatr. 2001;1:39–52. doi: 10.1367/1539-4409(2001)001<0039:mcidai>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Management and outcomes of pneumonia among children with complex chronic conditions. Pediatr Infect Dis J. 2014;33:907–11. doi: 10.1097/INF.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brogan TV, Hall M, Williams DJ, Neuman MI, Grijalva CG, Farris RW, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31:1036–41. doi: 10.1097/INF.0b013e31825f2b10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132:237–44. doi: 10.1542/peds.2013-0179. [DOI] [PubMed] [Google Scholar]
- 9.Williams DJ, Shah SS, Myers A, Hall M, Auger K, Queen MA, et al. Identifying pediatric community-acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr. 2013;167:851–8. doi: 10.1001/jamapediatrics.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson J, Hall M, Ambroggio L, Stone B, Srivastava R, Shah SS, et al. Aspiration and non-aspiration pneumonia in hospitalized children with neurologic impairment. Pediatrics. 2016;137:1–10. doi: 10.1542/peds.2015-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickering LK. American Academy of Pediatrics. Red book: 2012 report of the Committee on Infectious Diseases. 29th. Elk Grove Village (IL): American Academy of Pediatrics; 2012. American Academy of Pediatrics. Committee on infectious diseases. [Google Scholar]
- 12.National Quality Measures Clearinghouse. Plan all-cause readmissions: the number of acute inpatient stays during the measurement year that were followed by an acute readmission for any diagnosis within 30 days and the predicted probability of an acute readmission, for members 18 years of age and older. [Accessed April 30, 2015]; https://www.qualitymeasures.ahrq.gov/summaries/summary/49833.
- 13.Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126:204–13. doi: 10.1542/peds.2009-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:e99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 16.Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. J Pediatr. 2009;154:789–96. doi: 10.1016/j.jpeds.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Neuman MI, Hall M, Gay JC, Blaschke AJ, Williams DJ, Parikh K, et al. Readmissions among children previously hospitalized with pneumonia. Pediatrics. 2014;134:100–9. doi: 10.1542/peds.2014-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korppi M, Heiskanen-Kosma T, Leinonen M. White blood cells, C-reactive protein and erythrocyte sedimentation rate in pneumococcal pneumonia in children. Eur Respir J. 1997;10:1125–9. doi: 10.1183/09031936.97.10051125. [DOI] [PubMed] [Google Scholar]
- 20.Murtagh Kurowski E, Shah SS, Thomson J, Statile A, Sheehan B, Iyer S, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135:e1052–9. doi: 10.1542/peds.2014-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3:92–6. doi: 10.1542/hpeds.2012-0050. [DOI] [PubMed] [Google Scholar]
- 22.Goodman DC. Unwarranted variation in pediatric medical care. Pediatr Clin North Am. 2009;56:745–55. doi: 10.1016/j.pcl.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wennberg JE. Forty years of unwarranted variation–and still counting. Health Policy (New York) 2014;114:1–2. doi: 10.1016/j.healthpol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–98. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 25.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–87. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 26.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ. Variations in the longitudinal efficiency of academic medical centers. Health Aff (Millwood) 2004 doi: 10.1377/hlthaff.var.19. Suppl Variation:VAR19-32. [DOI] [PubMed] [Google Scholar]
- 27.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307:1513–6. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]